Facet-Dependent Interfacial Charge Transfer in TiO2/Nitrogen-Doped Graphene Quantum Dots Heterojunctions for Visible-Light Driven Photocatalysis
Abstract
:1. Introduction
2. Results and Discussion
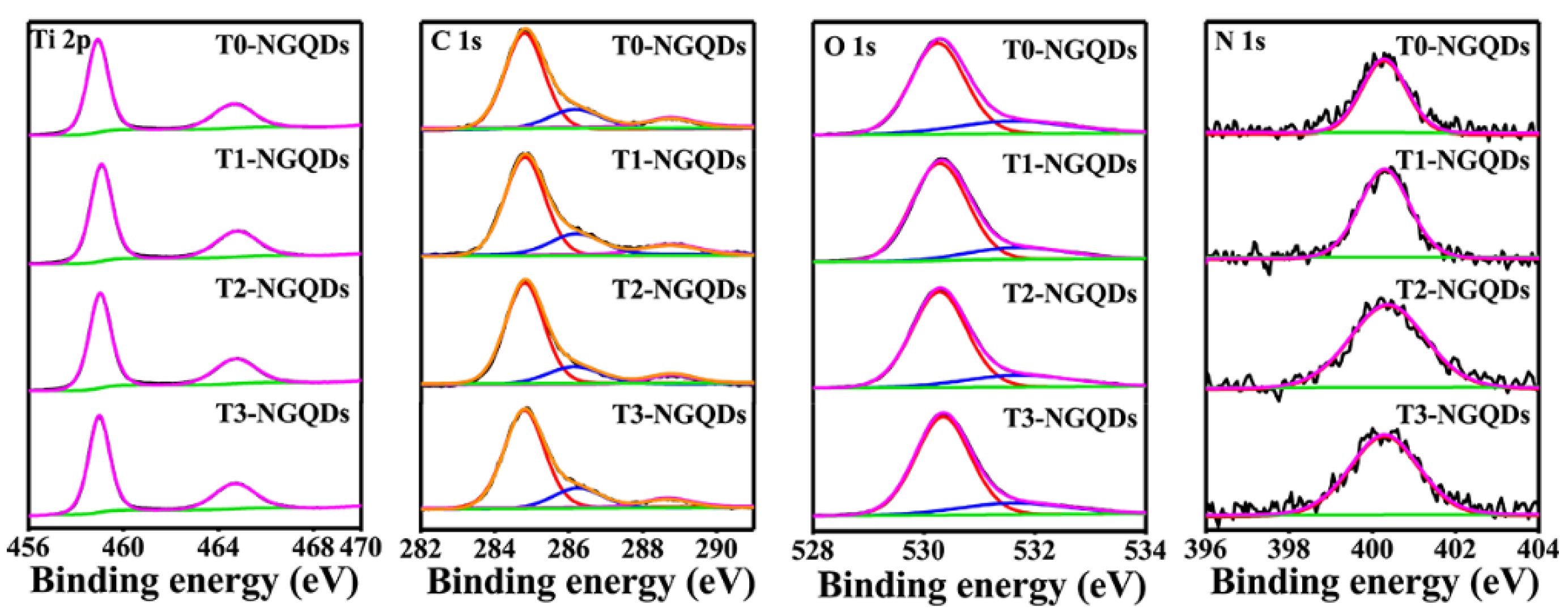
2.1. Structural Characterization
2.2. Morphology Characterization
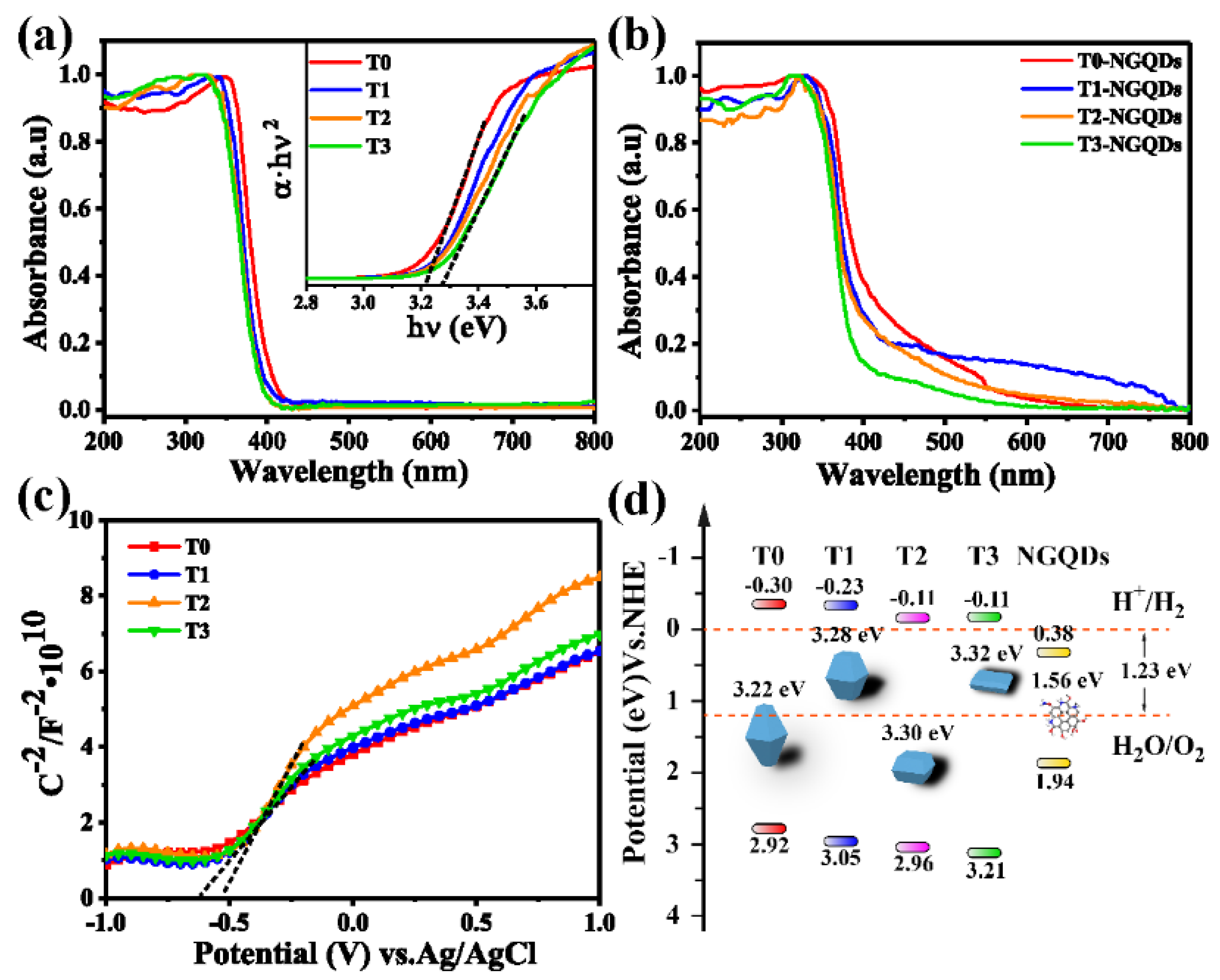
2.3. Optical and Electrical Properties
2.4. Photocatalytic Performance
2.5. Photocatalytic Mechanism
3. Materials and Methods
3.1. Synthesis of Anatase TiO2
3.2. Synthesis of NGQDs
3.3. Synthesis of TiO2/NGQDs Heterojunction Composites
3.4 Characterization
3.5 Photocatalytic Performance
3.6. Photoelectrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tang, H.; Prasad, K.; Sanjinès, R.; Schmid, P.E.; Lévy, F. Electrical and optical properties of TiO2 anatase thin films. J. Appl. Phys. 1994, 75, 2042–2047. [Google Scholar] [CrossRef]
- Xu, M.; Gao, Y.; Moreno, E.M.; Kunst, M.; Muhler, M.; Wang, Y.; Idriss, H.; Woll, C. Photocatalytic activity of bulk TiO2 anatase and rutile single crystals using infrared absorption spectroscopy. Phys. Rev. Lett. 2011, 106, 138302. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Low, J.; Xiao, W.; Zhou, P.; Jaroniec, M. Enhanced Photocatalytic CO2-Reduction Activity of Anatase TiO2 by Co-exposed {001} and {101} Facets. J. Am. Chem. Soc. 2014, 136, 8839–8842. [Google Scholar] [CrossRef]
- Tachikawa, T.; Yamashita, S.; Majima, T. Evidence for crystal-face-dependent TiO2 photocatalysis from single-molecule imaging and kinetic analysis. J. Am. Chem. Soc. 2011, 133, 7197–7204. [Google Scholar] [CrossRef] [PubMed]
- Selcuk, S.; Selloni, A. Facet-dependent trapping and dynamics of excess electrons at anatase TiO2 surfaces and aqueous interfaces. Nat. Mater. 2016, 15, 1107–1118. [Google Scholar] [CrossRef]
- Chen, Q.; Ma, W.; Chen, C.; Ji, H.; Zhao, J. Anatase TiO2 mesocrystals enclosed by (001) and (101) facets: Synergistic effects between Ti3+ and facets for their photocatalytic performance. Chem. Eur. J. 2012, 18, 12584–12589. [Google Scholar] [CrossRef]
- Hyam, R.S.; Lee, J.; Cho, E.; Khim, J.; Lee, H. Effect of Annealing Environments on Self-Organized TiO2 Nanotubes for Efficient Photocatalytic Applications. Nanosci. Nanotechnol. 2012, 12, 8908–8912. [Google Scholar] [CrossRef]
- Wang, X.; Sun, G.; Li, N.; Chen, P. Quantum dots derived from two-dimensional materials and their applications for catalysis and energy. Chem. Soc. Rev. 2016, 45, 2239–2262. [Google Scholar] [CrossRef]
- Wegner, K.D.; Hildebrandt, N. Quantum dots: Bright and versatile in vitro and in vivo fluorescence imaging biosensors. Chem. Soc. Rev. 2015, 44, 4792–4834. [Google Scholar] [CrossRef]
- Yuan, F.; Li, S.; Fan, Z.; Meng, X.; Fan, L.; Yang, S. Shining carbon dots: Synthesis and biomedical and optoelectronic applications. Nano Today 2016, 11, 565–586. [Google Scholar] [CrossRef]
- Crake, A.; Christoforidis, K.C.; Godin, R.; Moss, B.; Kafizas, A.; Zafeiratos, S.; Durrant, J.R.; Petit, C. Titanium dioxide/carbon nitride nanosheet nanocomposites for gas phase CO2 photoreduction under UV-visible irradiation. Appl. Catal. B 2019, 242, 369–378. [Google Scholar] [CrossRef]
- Olowoyo, J.O.; Kumar, M.; Jain, S.L.; Babalola, J.O.; Vorontsov, A.V.; Kumar, U. Insights into Reinforced Photocatalytic Activity of the CNT–TiO2 Nanocomposite for CO2 Reduction and Water Splitting. J. Phys. Chem. C 2018, 123, 367–378. [Google Scholar] [CrossRef]
- Rimoldi, L.; Giordana, A.; Cerrato, G.; Falletta, E.; Meroni, D. Insights on the photocatalytic degradation processes supported by TiO2/WO3 systems. The case of ethanol and tetracycline. Catal. Today 2019, 328, 210–215. [Google Scholar] [CrossRef]
- Zheng, X.T.; Ananthanarayanan, A.; Luo, K.Q.; Chen, P. Glowing graphene quantum dots and carbon dots: Properties, syntheses, and biological applications. Small 2015, 11, 1620–1655. [Google Scholar] [CrossRef]
- Freeman, R.; Finder, T.; Bahshi, L.; Gill, R.; Willner, I. Functionalized CdSe/ZnS QDs for the detection of nitroaromatic or RDX explosives. Adv. Mater. 2012, 24, 6416–6421. [Google Scholar] [CrossRef]
- Long, R.; Casanova, D.; Fang, W.-H.; Prezhdo, O.V. Donor-Acceptor Interaction Determines the Mechanism of Photo-Induced Electron Injection from Graphene Quantum Dots into TiO2: Stacking Supersedes Covalent Bonding. J. Am. Chem. Soc. 2017, 139, 2619–2629. [Google Scholar] [CrossRef]
- Wang, W.-S.; Wang, D.-H.; Qu, W.-G.; Lu, L.-Q.; Xu, A.-W. Large Ultrathin Anatase TiO2 Nanosheets with Exposed {001} Facets on Graphene for Enhanced Visible Light Photocatalytic Activity. J. Phys. Chem. C 2012, 116, 19893–19901. [Google Scholar] [CrossRef]
- Wang, F.; Wu, Y.; Wang, Y.; Li, J.; Jin, X.; Zhang, Q.; Li, R.; Yan, S.; Liu, H.; Feng, Y.; et al. Construction of novel Z-scheme nitrogen-doped carbon dots/{0 0 1} TiO2 nanosheet photocatalysts for broad-spectrum-driven diclofenac degradation: Mechanism insight, products and effects of natural water matrices. Chem. Eng. J. 2019, 356, 857–868. [Google Scholar] [CrossRef]
- Wang, W.; Ni, Y.; Xu, Z. One-step uniformly hybrid carbon quantum dots with high-reactive TiO2 for photocatalytic application. J. Alloys Compd. 2015, 622, 303–308. [Google Scholar] [CrossRef]
- Baker, D.R.; Kamat, P.V. Photosensitization of TiO2 Nanostructures with CdS Quantum Dots: Particulate versus Tubular Support Architectures. Adv. Funct. Mater. 2009, 19, 805–811. [Google Scholar] [CrossRef]
- Kenrick, J.; Williams, C.A.N.; Yan, X.; Li, L.-S.; Zhu, X. Hot Electron Injection from Graphene Quantum Dots to TiO2. ACS Nano 2013, 7, 1388–1394. [Google Scholar]
- Yang, N.; Liu, Y.; Wen, H.; Tang, Z.; Zhao, H.; Li, Y.; Wang, D. Photocatalytic Properties of Graphdiyne and Graphene Modified TiO2: From Theory to Experiment. ACS Nano 2013, 7, 1504–1512. [Google Scholar] [CrossRef]
- Pan, D.; Jiao, J.; Li, Z.; Guo, Y.; Feng, C.; Liu, Y.; Wang, L.; Wu, M. Efficient Separation of Electron–Hole Pairs in Graphene Quantum Dots by TiO2 Heterojunctions for Dye Degradation. ACS Sustain. Chem. Eng. 2015, 3, 2405–2413. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, A.Y.; Xie, X.; Wang, X.; Wang, D.; Wang, P.; Li, H.-J.; Yang, J.; Li, Y. Doping effect and fluorescence quenching mechanism of N-doped graphene quantum dots in the detection of dopamine. Talanta 2018, 196, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhong, Y.Q.; Yu, B.Q.; Cai, S.Y.; Wu, L.Z.; Zhou, Y. Graphene quantum dots to enhance the photocatalytic hydrogen evolution efficiency of anatase TiO2 with exposed {001} facet. Phys. Chem. Chem. Phys. 2016, 18, 20338–20381. [Google Scholar] [CrossRef]
- Zheng, L.; Su, H.; Zhang, J.; Walekar, L.S.; Vafaei Molamahmood, H.; Zhou, B.; Long, M.; Hu, Y.H. Highly selective photocatalytic production of H2O2 on sulfur and nitrogen co-doped graphene quantum dots tuned TiO2. Appl. Catal. B 2018, 239, 475–484. [Google Scholar] [CrossRef]
- Jiang, B.; Tian, C.; Zhou, W.; Wang, J.; Xie, Y.; Pan, Q.; Ren, Z.; Dong, Y.; Fu, D.; Han, J.; et al. In situ growth of TiO2 in interlayers of expanded graphite for the fabrication of TiO2-graphene with enhanced photocatalytic activity. Chem. Eur. J. 2011, 17, 8379–8387. [Google Scholar] [CrossRef]
- Liu, L.; Jiang, Y.; Zhao, H.; Chen, J.; Cheng, J.; Yang, K.; Li, Y. Engineering Coexposed {001} and {101} Facets in Oxygen-Deficient TiO2 Nanocrystals for Enhanced CO2 Photoreduction under Visible Light. ACS Catal. 2016, 6, 1097–1108. [Google Scholar] [CrossRef]
- Tian, F.; Zhang, Y.; Zhang, J.; Pan, C. Raman Spectroscopy: A New Approach to Measure the Percentage of Anatase TiO2 Exposed (001) Facets. J. Phys. Chem. C 2012, 116, 7515–7519. [Google Scholar] [CrossRef]
- Li, H.-J.; Sun, X.; Xue, F.; Ou, N.; Sun, B.-W.; Qian, D.-J.; Chen, M.; Wang, D.; Yang, J.; Wang, X. Redox Induced Fluorescence On–Off Switching Based on Nitrogen Enriched Graphene Quantum Dots for Formaldehyde Detection and Bioimaging. ACS Sustain. Chem. Eng. 2018, 6, 1708–1716. [Google Scholar] [CrossRef]
- Qu, A.; Xie, H.; Xu, X.; Zhang, Y.; Wen, S.; Cui, Y. High quantum yield graphene quantum dots decorated TiO2 nanotubes for enhancing photocatalytic activity. Appl. Surf. Sci. 2016, 375, 230–241. [Google Scholar] [CrossRef]
- Rajender, G.; Kumar, J.; Giri, P.K. Interfacial charge transfer in oxygen deficient TiO2-graphene quantum dot hybrid and its influence on the enhanced visible light photocatalysis. Appl. Catal. B 2018, 224, 960–972. [Google Scholar] [CrossRef]
- Shen, K.; Xue, X.; Wang, X.; Hu, X.; Tian, H.; Zheng, W. One-step synthesis of band-tunable N, S co-doped commercial TiO2/graphene quantum dots composites with enhanced photocatalytic activity. RSC Adv. 2017, 7, 23319–23327. [Google Scholar] [CrossRef]
- Wang, W.; Xu, D.; Cheng, B.; Yu, J.; Jiang, C. Hybrid carbon@TiO2 hollow spheres with enhanced photocatalytic CO2 reduction activity. J. Mater. Chem. A 2017, 5, 5020–5029. [Google Scholar] [CrossRef]
- Xu, C.; Han, Q.; Zhao, Y.; Wang, L.; Li, Y.; Qu, L. Sulfur-doped graphitic carbon nitride decorated with graphene quantum dots for an efficient metal-free electrocatalyst. J. Mater. Chem. A 2015, 3, 1841–1846. [Google Scholar] [CrossRef]
- Han, S.; Hu, X.; Wang, J.; Fang, X.; Zhu, Y. Novel Route to Fe-Based Cathode as an Efficient Bifunctional Catalysts for Rechargeable Zn-Air Battery. Adv. Energy Mater. 2018, 8, 1800955. [Google Scholar] [CrossRef]
- Li, H.-J.; Ou, N.-Q.; Sun, X.; Sun, B.-W.; Qian, D.-J.; Chen, M.; Wang, X.; Yang, J. Exploitation of the synergistic effect between surface and bulk defects in ultra-small N-doped titanium suboxides for enhancing photocatalytic hydrogen evolution. Catal. Sci. Technol. 2018, 8, 5515–5525. [Google Scholar] [CrossRef]
- Rimoldi, L.; Pargoletti, E.; Meroni, D.; Falletta, E.; Cerrato, G.; Turco, F.; Cappelletti, G. Concurrent role of metal (Sn, Zn) and N species in enhancing the photocatalytic activity of TiO2 under solar light. Catal. Today 2018, 313, 40–46. [Google Scholar] [CrossRef]
- Ding, H.; Yu, S.B.; Wei, J.S.; Xiong, H.M. Full-Color Light-Emitting Carbon Dots with a Surface-State-Controlled Luminescence Mechanism. ACS Nano 2016, 10, 484–491. [Google Scholar] [CrossRef]
- Ding, H.; Wei, J.S.; Zhang, P.; Zhou, Z.Y.; Gao, Q.Y.; Xiong, H.M. Solvent-Controlled Synthesis of Highly Luminescent Carbon Dots with a Wide Color Gamut and Narrowed Emission Peak Widths. Small 2018, 14, e1800612. [Google Scholar] [CrossRef]
- Yang, H.; Wang, P.; Wang, D.; Zhu, Y.; Xie, K.; Zhao, X.; Yang, J.; Wang, X. New Understanding on Photocatalytic Mechanism of Nitrogen-Doped Graphene Quantum Dots-Decorated BiVO4 Nanojunction Photocatalysts. ACS Omega 2017, 2, 3766–3773. [Google Scholar] [CrossRef]
- Deming, C.P.; Mercado, R.; Gadiraju, V.; Sweeney, S.W.; Khan, M.; Chen, S. Graphene Quantum Dots-Supported Palladium Nanoparticles for Efficient Electrocatalytic Reduction of Oxygen in Alkaline Media. ACS Sustain. Chem. Eng. 2015, 3, 3315–3323. [Google Scholar] [CrossRef]
- Safardoust-Hojaghan, H.; Salavati-Niasari, M. Degradation of methylene blue as a pollutant with N-doped graphene quantum dot/titanium dioxide nanocomposite. J. Clean. Prod. 2017, 148, 31–36. [Google Scholar] [CrossRef]
- Wang, S.; Cole, I.S.; Li, Q. Quantum-confined bandgap narrowing of TiO2 nanoparticles by graphene quantum dots for visible-light-driven applications. Chem. Commun. 2016, 52, 9208–9211. [Google Scholar] [CrossRef]
- Wang, D.; Chen, J.-F.; Dai, L. Recent Advances in Graphene Quantum Dots for Fluorescence Bioimaging from Cells through Tissues to Animals. Part. Part. Syst. Charact. 2015, 32, 515–523. [Google Scholar] [CrossRef]
- Zou, X.; Liu, M.; Wu, J.; Ajayan, P.M.; Li, J.; Liu, B.; Yakobson, B.I. How Nitrogen-Doped Graphene Quantum Dots Catalyze Electroreduction of CO2 to Hydrocarbons and Oxygenates. ACS Catal. 2017, 7, 6245–6250. [Google Scholar] [CrossRef]
- Li, H.-J.; Qian, D.J.; Chen, M. Templateless Infrared Heating Process for Fabricating Carbon Nitride Nanorods with Efficient Photocatalytic H2 Evolution. ACS Appl. Mater. Interfaces 2015, 7, 25162–25170. [Google Scholar] [CrossRef]
- Yan, M.; Zhu, F.; Gu, W.; Sun, L.; Shi, W.; Hua, Y. Construction of nitrogen-doped graphene quantum dots-BiVO4/g-C3N4Z-scheme photocatalyst and enhanced photocatalytic degradation of antibiotics under visible light. RSC Adv. 2016, 6, 61162–61174. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Sedhain, A.; Lin, J.; Jiang, H. Structure and Photoluminescence Study of TiO2 Nanoneedle Texture along Vertically Aligned Carbon Nanofiber Arrays. J. Phys. Chem. C 2008, 112, 17127–17132. [Google Scholar] [CrossRef]
- Nadica, D.; Abazovic, M.I.C.O.; Dramicanin, M.D. Photoluminescence of Anatase and Rutile TiO2 Particles. J. Phys. Chem. B 2006, 110, 25366–25370. [Google Scholar]
- Hu, C.; Zhang, X.; Li, X.; Yan, Y.; Xi, G.; Yang, H.; Bai, H. Au photosensitized TiO2 ultrathin nanosheets with {001} exposed facets. Chem. Eur. J. 2014, 20, 13557–13560. [Google Scholar] [CrossRef]
- Li, K.; Xu, Y.; He, Y.; Yang, C.; Wang, Y.; Jia, J. Photocatalytic fuel cell (PFC) and dye self-photosensitization photocatalytic fuel cell (DSPFC) with BiOCl/Ti photoanode under UV and visible light irradiation. Environ. Sci. Technol. 2013, 47, 3490–3497. [Google Scholar] [CrossRef]
- Wang, X.; Xia, R.; Muhire, E.; Jiang, S.; Huo, X.; Gao, M. Highly enhanced photocatalytic performance of TiO2 nanosheets through constructing TiO2/TiO2 quantum dots homojunction. Appl. Surf. Sci. 2018, 459, 9–15. [Google Scholar] [CrossRef]
- Qu, D.; Zheng, M.; Du, P.; Zhou, Y.; Zhang, L.; Li, D.; Tan, H.; Zhao, Z.; Xie, Z.; Sun, Z. Highly luminescent S, N co-doped graphene quantum dots with broad visible absorption bands for visible light photocatalysts. Nanoscale 2013, 5, 12272–12278. [Google Scholar] [CrossRef]







| Samples | Average thickness (nm) | Average length (nm) | Percentage of {001} |
|---|---|---|---|
| T0 | 8.9 | 8.1 | 12% |
| T1 | 8.5 | 13.8 | 56% |
| T2 | 6.1 | 21.6 | 71% |
| T3 | 4.3 | 25.5 | 85% |
| Bond | T0-NGQDs | T1-NGQDs | T2-NGQDs | T3-NGQDs |
|---|---|---|---|---|
| % of Ti 2p | 26.57 | 27.63 | 27.23 | 27.69 |
| % of C 1s | 21.18 | 19.4 | 20.65 | 18.99 |
| O–C=O/Ti–O–C 288.8 eV | 7.91 | 8.03 | 7.84 | 7.83 |
| C–O 286.1 eV | 19.53 | 20.30 | 19.05 | 19.26 |
| C=C 284.8 eV | 72.56 | 71.07 | 73.40 | 73.34 |
| % of O 1s | 51.69 | 52.33 | 51.53 | 52.85 |
| Ti–O–Ti 530.3 eV | 77.80 | 80.97 | 80.84 | 80.42 |
| Ti–O–C 531.6 eV | 22.20 | 19.03 | 19.16 | 19.58 |
| % of N 1s | 0.55 | 0.64 | 0.58 | 0.47 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ou, N.-Q.; Li, H.-J.; Lyu, B.-W.; Gui, B.-J.; Sun, X.; Qian, D.-J.; Jia, Y.; Wang, X.; Yang, J. Facet-Dependent Interfacial Charge Transfer in TiO2/Nitrogen-Doped Graphene Quantum Dots Heterojunctions for Visible-Light Driven Photocatalysis. Catalysts 2019, 9, 345. https://doi.org/10.3390/catal9040345
Ou N-Q, Li H-J, Lyu B-W, Gui B-J, Sun X, Qian D-J, Jia Y, Wang X, Yang J. Facet-Dependent Interfacial Charge Transfer in TiO2/Nitrogen-Doped Graphene Quantum Dots Heterojunctions for Visible-Light Driven Photocatalysis. Catalysts. 2019; 9(4):345. https://doi.org/10.3390/catal9040345
Chicago/Turabian StyleOu, Nan-Quan, Hui-Jun Li, Bo-Wen Lyu, Bo-Jie Gui, Xiong Sun, Dong-Jin Qian, Yanlin Jia, Xianying Wang, and Junhe Yang. 2019. "Facet-Dependent Interfacial Charge Transfer in TiO2/Nitrogen-Doped Graphene Quantum Dots Heterojunctions for Visible-Light Driven Photocatalysis" Catalysts 9, no. 4: 345. https://doi.org/10.3390/catal9040345
APA StyleOu, N.-Q., Li, H.-J., Lyu, B.-W., Gui, B.-J., Sun, X., Qian, D.-J., Jia, Y., Wang, X., & Yang, J. (2019). Facet-Dependent Interfacial Charge Transfer in TiO2/Nitrogen-Doped Graphene Quantum Dots Heterojunctions for Visible-Light Driven Photocatalysis. Catalysts, 9(4), 345. https://doi.org/10.3390/catal9040345





