MoS2/CdS Heterostructure for Enhanced Photoelectrochemical Performance under Visible Light
Abstract
1. Introduction
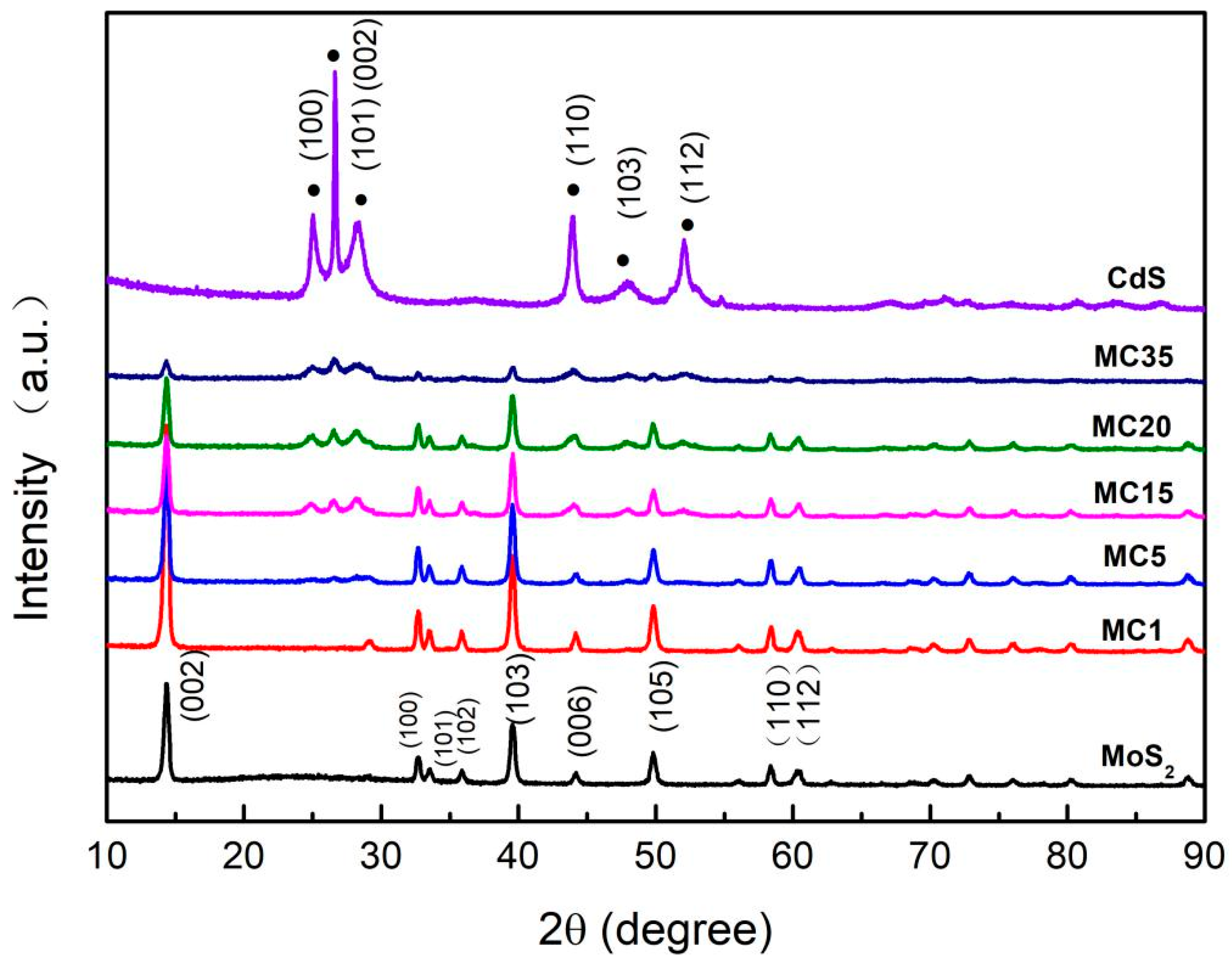
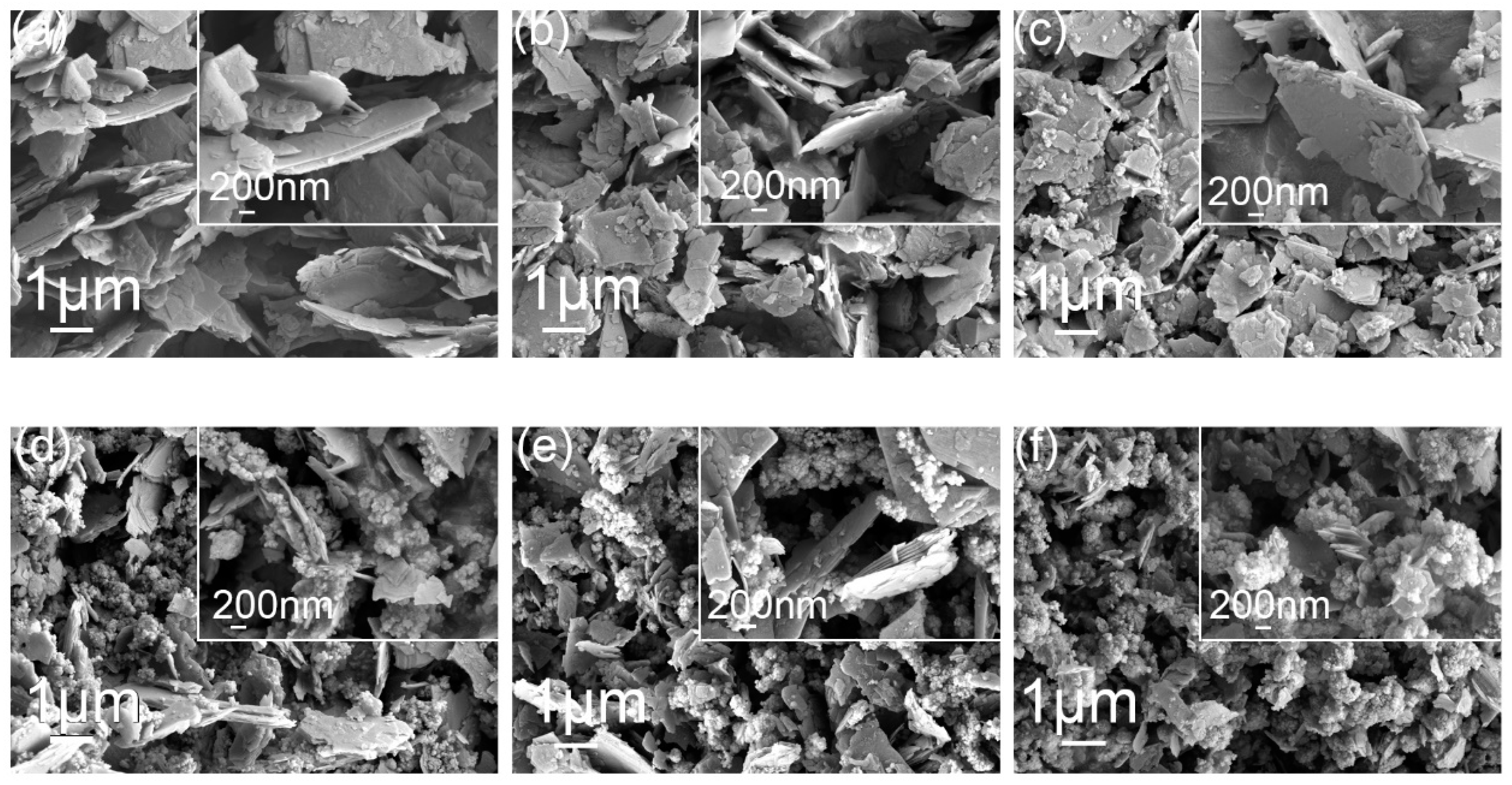
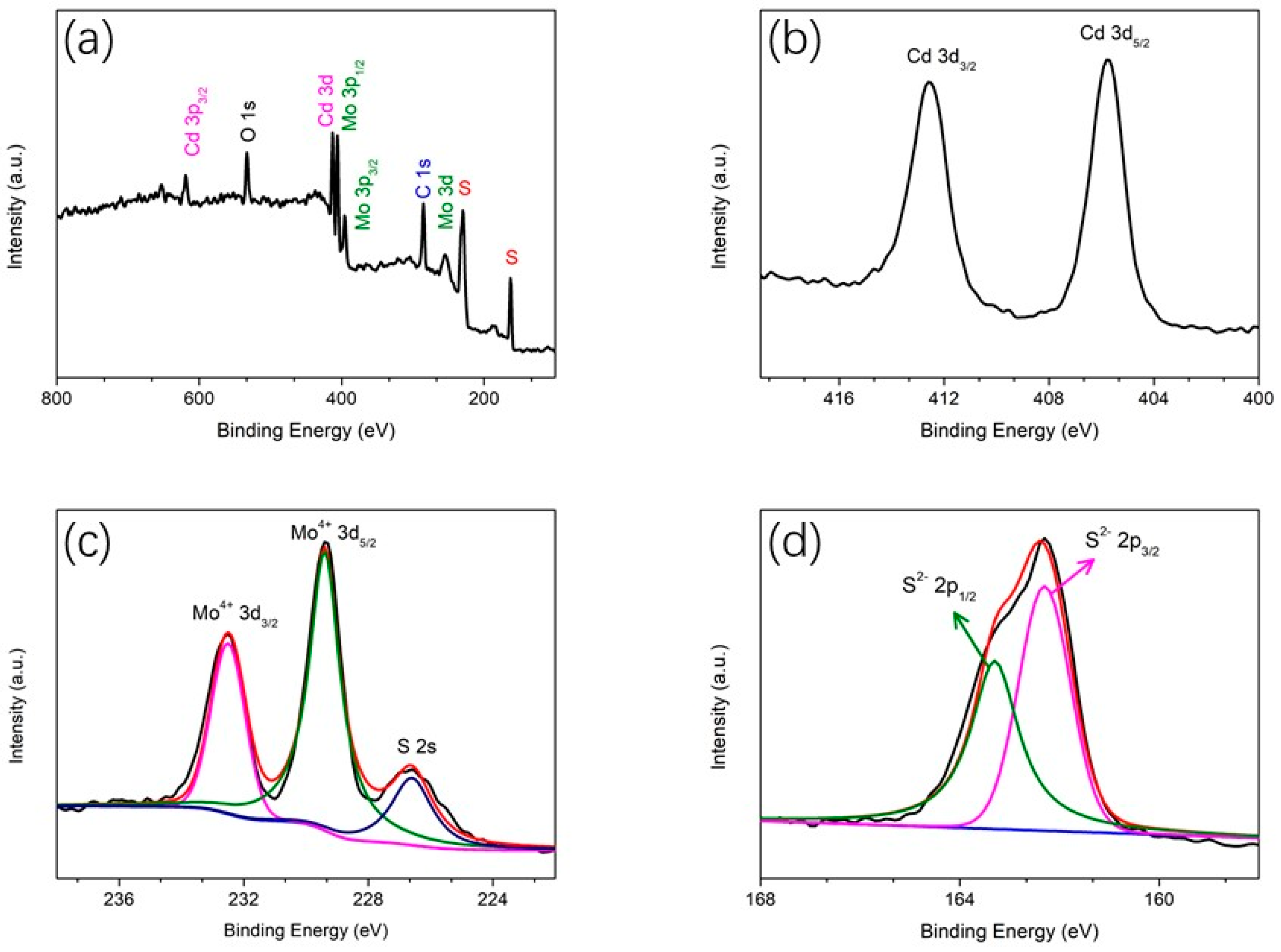
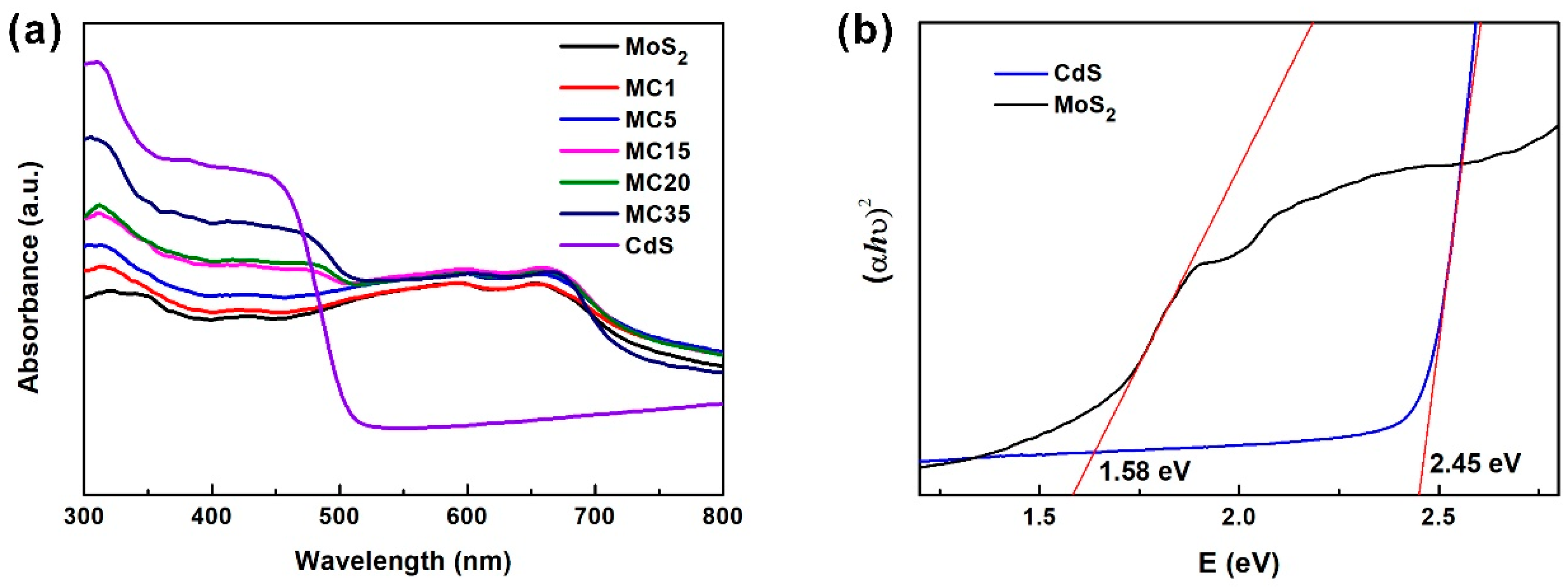
2. Results and Discussion
3. Experimental Section
3.1. Materials
3.2. Preparation of MoS2 Nanosheets
3.3. Synthesis of MoS2/CdS Nanocomposites
3.4. Synthesis of Pure CdS Nanoparticles
3.5. Characterization
3.6. Photoelectrochemical and Electrochemical Performance Measurements
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Han, J.S.; Dai, F.X.; Liu, Y.; Zhao, R.Y.; Wang, L.; Feng, S.H. Synthesis of CdSe/SrTiO3 nanocomposites with enhanced photocatalytic hydrogen production activity. Appl. Surf. Sci. 2019, 467, 1033–1039. [Google Scholar] [CrossRef]
- Zhu, Y.W.; Wang, L.L.; Liu, Y.T.; Shao, L.H.; Xia, X.N. In-situ hydrogenation engineering of ZnIn2S4 for promoted visible-light water splitting. Appl. Catal. B-Eviron. 2019, 241, 483–490. [Google Scholar] [CrossRef]
- Bian, T.; Shang, L.; Yu, H.J.; Perez, M.T.; Wu, L.Z.; Tung, C.H.; Nie, Z.H.; Tang, Z.Y.; Zhang, T.R. Spontaneous organization of inorganic nanoparticles into nanovesicles triggered by UV light. Advanced Mater. 2014, 26, 5613–5618. [Google Scholar] [CrossRef] [PubMed]
- Ruan, D.M.; Kim, S.; Fujitsuka, M.; Majima, T. Defects rich g-C3N4 with mesoporous structure for efficient photocatalytic H-2 production under visible light irradiation. Appl. Catal. B-Environ. 2018, 238, 638–646. [Google Scholar] [CrossRef]
- Yang, Y.; Luo, M.C.; Xing, Y.; Wang, S.T.; Zhang, W.Y.; Lv, F.; Li, Y.J.; Zhang, Y.L.; Wang, W.; Guo, S.J. A universal strategy for intimately coupled carbon nanosheets/MoM nanocrystals (M = P, S, C, and O) hierarchical hollow nanospheres for hydrogen evolution catalysis and sodium-ion storage. Adv. Mater. 2018, 30, 1706085. [Google Scholar] [CrossRef]
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef]
- Song, X.F.; Hu, J.L.; Zeng, H.B. Two-dimensional semiconductors: Recent progress and future perspectives. J. Mater. Chem. C 2013, 1, 2952–2969. [Google Scholar] [CrossRef]
- Xie, J.F.; Zhang, H.; Li, S.; Wang, R.X.; Sun, X.; Zhou, M.; Zhou, J.F.; Lou, X.W.; Xie, Y. Defect-rich MoS2 ultrathin nanosheets with additional active edge sites for enhanced electrocatalytic hydrogen evolution. Adv. Mater. 2013, 25, 5807–5813. [Google Scholar] [CrossRef] [PubMed]
- Anpo, M.; Takeuchi, M. The design and development of highly reactive titanium oxide photocatalysts operating under visible light irradiation. J. Catal. 2003, 216, 505–516. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef]
- Bu, Y.Y.; Chen, Z.Y.; Sun, C.J. Highly efficient Z-Scheme Ag3PO4/Ag/WO3-x photocatalyst for its enhanced photocatalytic performance. Appl. Catal. B-Environ. 2015, 179, 363–371. [Google Scholar] [CrossRef]
- Chang, K.; Mei, Z.W.; Wang, T.; Kang, Q.; Ouyang, S.X.; Ye, J.H. MoS2/Graphene cocatalyst for efficient photocatalytic H-2 evolution under visible light irradiation. ACS Nano 2014, 8, 7078–7087. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.P.; Li, D.M.; Li, F.; Fan, Y.Z.; Zhao, H.F.; Luo, Y.H.; Yu, R.C.; Meng, Q.B. Ball-milling combined calcination synthesis of MoS2/CdS photocatalysts for high photocatalytic H-2 evolution activity under visible light irradiation. Appl. Catal. A-Gen. 2012, 443, 138–144. [Google Scholar] [CrossRef]
- Feng, C.; Chen, Z.; Hou, J.; Li, J.; Li, X.; Xu, L.; Sun, M.; Zeng, R. Effectively enhanced photocatalytic hydrogen production performance of one-pot synthesized MoS2 clusters/CdS nanorod heterojunction material under visible light. Chem. Eng. J. 2018, 345, 404–413. [Google Scholar] [CrossRef]
- Habba, Y.G.; Capochichi-Gnambodoe, M.; Leprince-Wang, Y. Enhanced photocatalytic activity of iron-doped ZnO nanowires for water purification. Appl. Sci. 2017, 7, 1185. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef]
- Li, X.L.; Wang, X.J.; Zhu, J.Y.; Li, Y.P.; Zhao, J.; Li, F.T. Fabrication of two-dimensional Ni2P/ZnIn2S4 heterostructures for enhanced photocatalytic hydrogen evolution. Chem. Eng. J. 2018, 353, 15–24. [Google Scholar] [CrossRef]
- Radisavljevic, B.; Radenovic, A.; Brivio, J.; Giacometti, V.; Kis, A. Single-layer MoS2 transistors. Nat. Nanotechnol. 2011, 6, 147–150. [Google Scholar] [CrossRef]
- Splendiani, A.; Sun, L.; Zhang, Y.B.; Li, T.S.; Kim, J.; Chim, C.Y.; Galli, G.; Wang, F. Emerging Photoluminescence in Monolayer MoS2. Nano Lett. 2010, 10, 1271–1275. [Google Scholar] [CrossRef]
- Parzinger, E.; Miller, B.; Blaschke, B.; Garrido, J.A.; Ager, J.W.; Holleitner, A.; Wurstbauer, U. Photocatalytic stability of single- and few-layer MoS2. ACS Nano 2015, 9, 11302–11309. [Google Scholar] [CrossRef]
- Xu, S.J.; Li, D.; Wu, P.Y. One-pot, facile, and versatile synthesis of monolayer MoS2/WS2 quantum dots as bioimaging probes and efficient electrocatalysts for hydrogen evolution reaction. Adv. Funct. Mater. 2015, 25, 1127–1136. [Google Scholar] [CrossRef]
- Tan, Y.H.; Yu, K.; Li, J.Z.; Fu, H.; Zhu, Z.Q. MoS2@ZnO nano-heterojunctions with enhanced photocatalysis and field emission properties. J. Appl. Phys. 2014, 116, 064305. [Google Scholar] [CrossRef]
- Li, M.; Ruan, H.R.; Yuan, X.Q.; Chen, Y.S.; Wang, X.D.; Liu, Y.P.; Lu, Z.H.; Hai, J.F. Construction of 2D MoS2/PbS heterojunction nanocomposites with enhanced photoelectric property. Mater. Lett. 2018, 212, 82–85. [Google Scholar] [CrossRef]
- Fageria, P.; Sudharshan, K.Y.; Nazir, R.; Basu, M.; Pande, S. Decoration of MoS2 on g-C3N4 surface for efficient hydrogen evolution reaction. Electrochim. Acta 2017, 258, 1273–1283. [Google Scholar] [CrossRef]
- Yang, J.H.; Wang, D.G.; Han, H.X.; Li, C. Roles of cocatalysts in photocatalysis and photoelectrocatalysis. Accounts Chem. Res. 2013, 46, 1900–1909. [Google Scholar] [CrossRef]
- Yin, Y.X.; Jin, Z.G.; Hou, F. Enhanced solar water-splitting efficiency using core/sheath heterostructure CdS/TiO2 nanotube arrays. Nanotechnology 2007, 18, 495608. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.P.; Hong, S.; Reddy, D.A.; Kim, T.K. Ultrathin MoS2 layers anchored exfoliated reduced graphene oxide nanosheet hybrid as a highly efficient cocatalyst for CdS nanorods towards enhanced photocatalytic hydrogen production. Appl. Catal. B Environ. 2017, 212, 7–14. [Google Scholar] [CrossRef]
- Zheng, Z.X.; Qiao, Y.; Cai, Y.H.; He, Y.N.; Tang, Y.M.; Li, L.S. MoS2 decorated CdS hybrid heterojunction for enhanced photoelectrocatalytic performance under visible light irradiation. J. Colloid Interface Sci. 2019, 533, 561–568. [Google Scholar] [CrossRef]
- Bie, C.B.; Fu, J.W.; Cheng, B.; Zhang, L.Y. Ultrathin CdS nanosheets with tunable thickness and efficient photocatalytic hydrogen generation. Appl. Surf. Sci. 2018, 462, 606–614. [Google Scholar] [CrossRef]
- Liu, Z.M.; Liu, G.L.; Hong, X.L. Influence of surface defects and palladium deposition on the activity of CdS nanocrystals for photocatalytic hydrogen production. Acta Phys.-Chim. Sin. 2019, 35, 215–222. [Google Scholar]
- Zhao, Y.; Fang, Z.B.; Feng, W.H.; Wang, K.Q.; Huang, X.Y.; Liu, P. Hydrogen production from pure water via piezoelectric-assisted visible-light photocatalysis of CdS nanorod arrays. ChemCatChem 2018, 10, 3397–3401. [Google Scholar] [CrossRef]
- Kim, H.J.; Song, Y.W.; Namgung, S.D.; Song, M.K.; Yang, S.; Kwon, J.Y. Optical properties of the crumpled pattern of selectively layered MoS2. Opt. Lett. 2018, 43, 4590–4593. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hu, S.Z.; Fan, Z.P.; Liu, D.S.; Zhao, Y.F.; Ma, H.F.; Li, F.Y. Preparation of g-C3N4/ZnMoCdS hybrid heterojunction catalyst with outstanding nitrogen photofixation performance under visible light via hydrothermal post-treatment. Dalton Trans. 2016, 45, 3497–3505. [Google Scholar] [CrossRef]
- Premkumar, J. Development of super-hydrophilicity on nitrogen-doped TiO2 thin film surface by photoelectrochemical method under visible light. Chem. Mater. 2004, 16, 3980–3981. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Cao, X.H.; Wang, B.; Xia, M.; Lin, S.; Guo, Z.H.; Zhang, X.M.; Gao, S.Y. Coupling thermoelectricity and electrocatalysis for hydrogen production via PbTe-PbS/TiO2 heterojunction. J. Power Sources 2017, 342, 452–459. [Google Scholar] [CrossRef]
- Bai, J.; Li, J.H.; Liu, Y.B.; Zhou, B.X.; Cai, W.M. A new glass substrate photoelectrocatalytic electrode for efficient visible-light hydrogen production: CdS sensitized TiO2 nanotube arrays. Appl. Catal. B-Environ. 2010, 95, 408–413. [Google Scholar] [CrossRef]



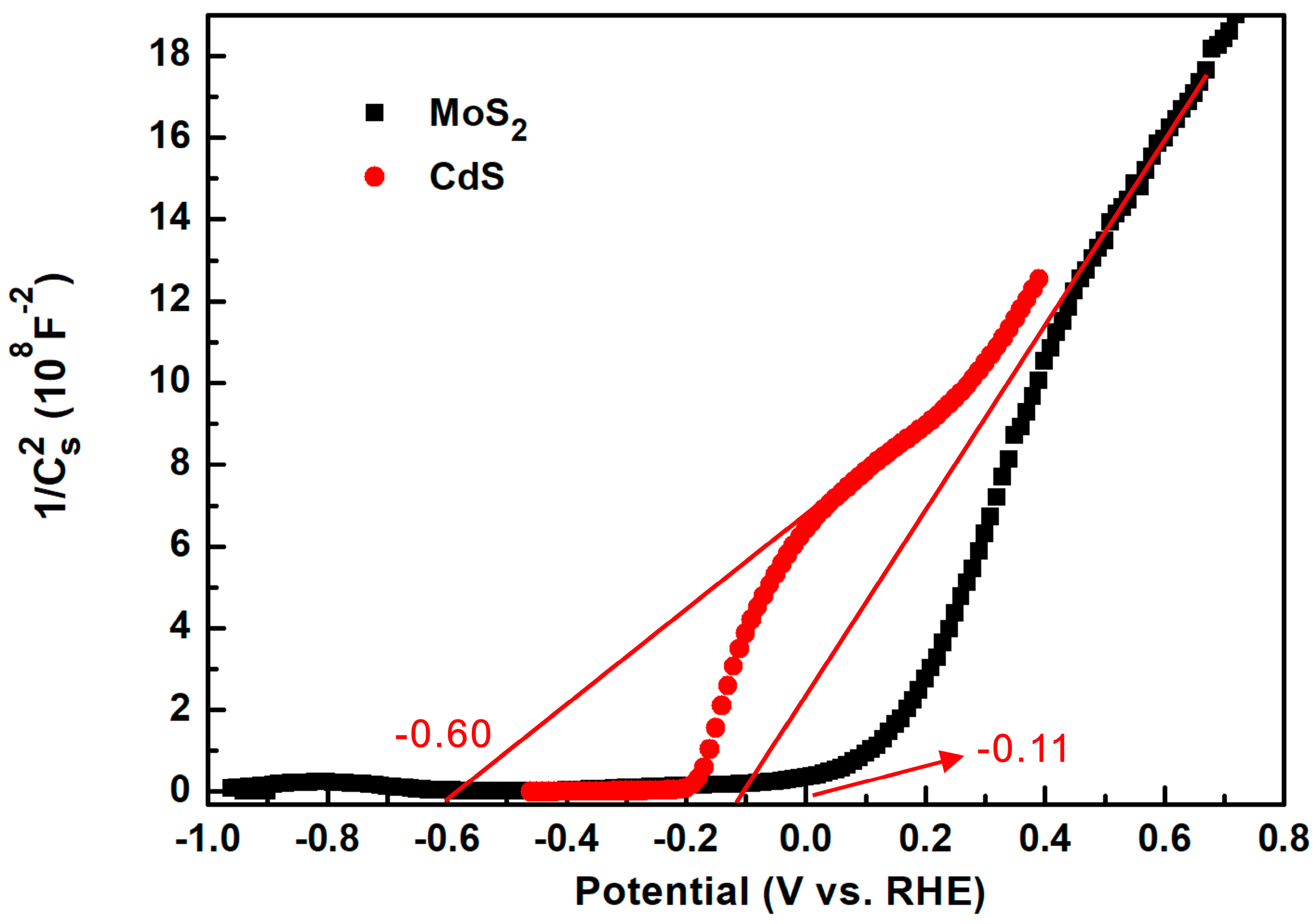
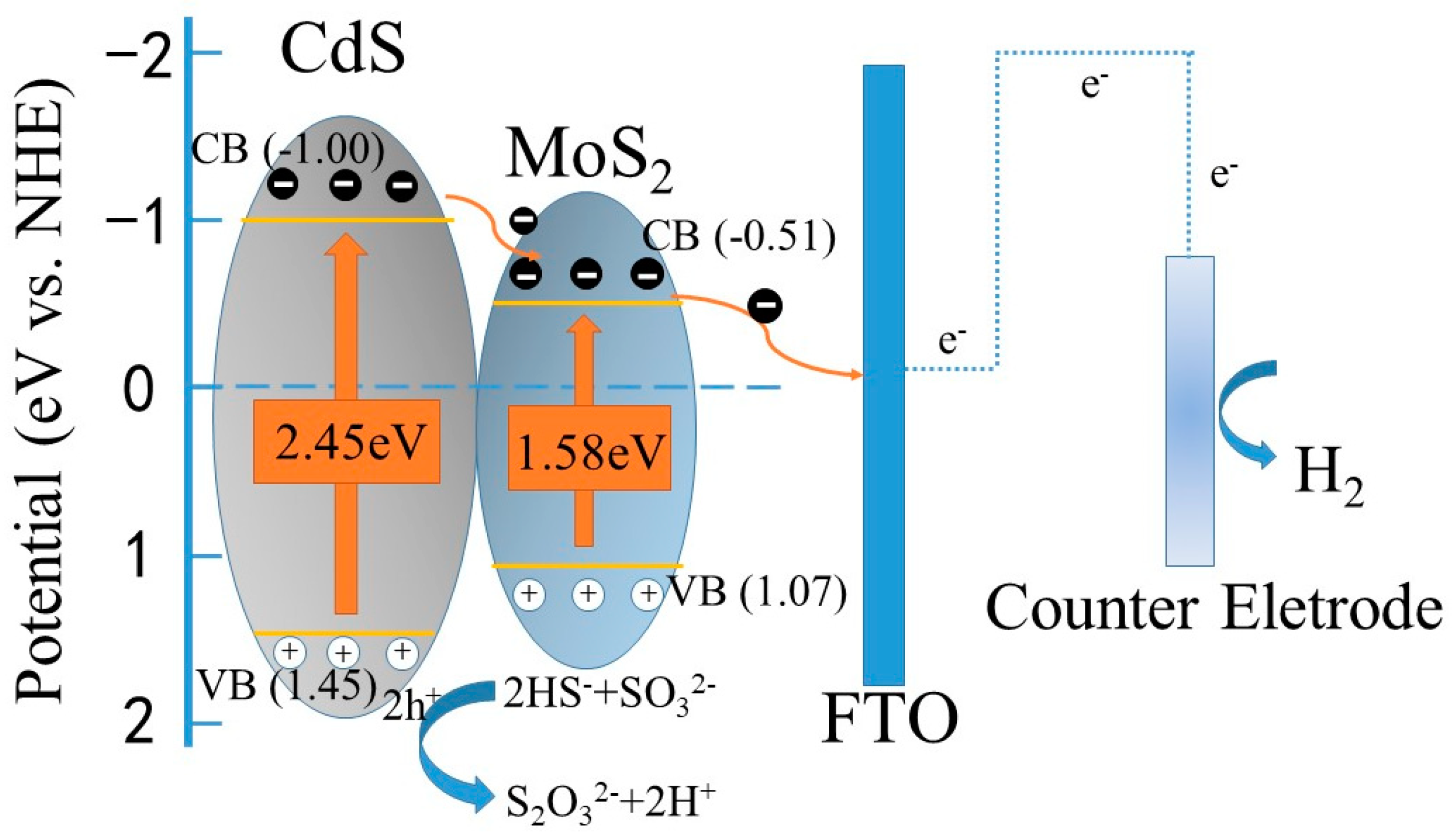
| Sample | CdS | MC1 | MC5 | MC15 | MC20 | MC35 |
|---|---|---|---|---|---|---|
| Rct/ | 6903 | 7389 | 8032 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, G.; Zhang, Y.; He, Q. MoS2/CdS Heterostructure for Enhanced Photoelectrochemical Performance under Visible Light. Catalysts 2019, 9, 379. https://doi.org/10.3390/catal9040379
He G, Zhang Y, He Q. MoS2/CdS Heterostructure for Enhanced Photoelectrochemical Performance under Visible Light. Catalysts. 2019; 9(4):379. https://doi.org/10.3390/catal9040379
Chicago/Turabian StyleHe, Guannan, Yimin Zhang, and Qinyu He. 2019. "MoS2/CdS Heterostructure for Enhanced Photoelectrochemical Performance under Visible Light" Catalysts 9, no. 4: 379. https://doi.org/10.3390/catal9040379
APA StyleHe, G., Zhang, Y., & He, Q. (2019). MoS2/CdS Heterostructure for Enhanced Photoelectrochemical Performance under Visible Light. Catalysts, 9(4), 379. https://doi.org/10.3390/catal9040379





