Thermal and Catalytic Pyrolysis of Dodecanoic Acid on SAPO-5 and Al-MCM-41 Catalysts
Abstract
:1. Introduction
 | Reaction 1 |
 | Reaction 2 |
 | Reaction 3 |
2. Results and Discussion
2.1. X-ray Diffraction
2.2. Thermodesorption of Ammonia
2.3. Thermogravimetric Analysis
2.4. Differential Scanning Calorimetry
2.5. Fast Pyrolysis Reaction
2.6. Comparison between the SAPO-5 and Al-MCM-41 Catalytic Behavior
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.2.1. X-ray Diffraction
3.2.2. Temperature Programmed Desorption of Ammonia (TPD-NH3)
3.2.3. Thermogravimetric Analysis
3.3. Catalytic Test
4. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De M. Araújo, A.M.; de O. Lima, R.; Gondim, A.D.; Diniz, J.; Di Souza, L.; de Araujo, A.S. Thermal and catalytic pyrolysis of sunflower oil using AlMCM-41. Renew. Energy 2017, 101, 900–906. [Google Scholar]
- Badoga, S.; Ganesan, A.; Dalai, A.K.; Chand, S. Effect of synthesis technique on the activity of CoNiMo tri-metallic catalyst for hydrotreating of heavy gas oil. Catal. Today 2017, 291, 160–171. [Google Scholar] [CrossRef]
- Rodrigues, T.O.; Rousset, P.; do Vale, A.T.; Broust, F. Bioóleo: Uma Alternativa para Valorização Energética da Biomassa. Rev. Bras. Energ. 2011, 17, 39–56. [Google Scholar]
- Maher, K.D.; Bressler, D.C. Pyrolysis of Triglyceride Materials for the Production of Renewable Fuels and Chemicals. Bioresour. Technol. 2007, 98, 2351–2368. [Google Scholar] [CrossRef]
- Wu, P.; Jiang, X.; Jin, Q.; Liu, F.; Zhang, J.; Zhu, Y.; Xia, L.; Shao, T.; Wang, K.; Li, T. Production of jet fuel range biofuels by catalytic transformation of triglycerides based oils. Fuel 2017, 188, 205–211. [Google Scholar] [CrossRef]
- Sonthalia, A.; Kumar, N. Hydroprocessed vegetable oil as a fuel for transportation sector: A review. J. Energy Inst. 2019, 82, 1–17. [Google Scholar] [CrossRef]
- Bezergianni, S.; Dimitriadis, A.; Kikhtyanin, O.; Kubicka, D. Refinery co-processing of renewable feeds. Prog. Energy Comb. Sci. 2018, 68, 29–64. [Google Scholar] [CrossRef]
- Kubicka, D.; Kikhtyanin, O. Opportunities for zeolites in biomass up-grading: Lessons from the refining and petrochemical industry. Catal. Today 2015, 243, 10–22. [Google Scholar] [CrossRef]
- Merdun, H.; Sezgin, I.V. Products distribution of catalytic co-pyrolysis of greenhouse vegetable waste and coal. Energy 2018, 162, 953–963. [Google Scholar] [CrossRef]
- Jahromi, H.; Agblevor, F.A. Hydrodeoxygenation of Aqueous-Phase Catalytic Pyrolysis Oil to Liquid Hydrocarbons using Multifunctional Nickel catalysts. Ind. Eng. Chem. Res. 2018, 57, 13257–13268. [Google Scholar] [CrossRef]
- Castille, A.; Bessette, C.; Thomas, F.; Eternad, M. Sustainable hydrocarbons production via simultaneous condensation-hydrodeoxygenation of propionic acid with furfural over red mud-supported noble metal catalysts. Catal. Commun. 2019, 121, 5–10. [Google Scholar] [CrossRef]
- Wang, Y.; Dai, L.; Fan, L.; Cao, L.; Zhou, Y.; Zhao, Y.; Liu, Y.; Ruan, R. Catalytic co-pyrolysis of waste vegetable oil and high density polyethylene for hydrocarbon fuel production. Waste Manag. 2017, 61, 276–282. [Google Scholar] [CrossRef]
- Abdelfattah, M.S.H.; Abu-Elyazeed, O.S.M.; Abdelazeem, M.A. On biodiesels from castor raw oil using catalytic pyrolysis. Energy 2018, 143, 950–960. [Google Scholar] [CrossRef]
- Nash, C.P.; Ramanathan, A.; Ruddy, D.A.; Behl, M.; Gjersing, E.; Griffin, M.; Zhu, H.; Subramaniam, B.; Schaidle, J.A.; Hensley, J.E. Mixed alcohol dehydration over Brønsted and Lewis acidic catalysts. Appl. Catal. A Gen. 2016, 510, 110–124. [Google Scholar] [CrossRef]
- Karnjanakom, G.; Suriya-umporn, S.; Bayu, T.; Kongparakul, A.; Samart, S.; Fushimi, C.; Abudula, C.; Guan, A. High selectivity and stability of Mg-doped Al-MCM-41 for in-situ catalytic upgrading fast pyrolysis bio-oil. Energy Convers. Manag. 2017, 142, 272–285. [Google Scholar] [CrossRef]
- De Azevedo, A.F., Jr. Desenvolvimento de Catalisadores a Base de SAPO-5 Para Avaliação na Reação de Fast Pirólise de Compostos Graxos. Ph.D. Thesis, Universidade Federal da Bahia (UFBA), Salvador, Brazil, 24 Auguest 2014. [Google Scholar]
- Bandyopadhyay, R.; Bandyopadhyay, M.; Kubota, Y.; Sugi, Y. Synthesis of AlPO4 Molecular Sieves with AFI and AEL Structures by Dry-Gel Conversion Method and Catalytic Application of Their SAPO Counterparts on Isopropylation of Biphenyl. J. Porous Mat. 2002, 9, 83–95. [Google Scholar] [CrossRef]
- Vieira, S.S. Produção de Biodiesel via Esterificação de Ácidos Graxos Livres Utilizando Catalisadores Heterogêneos Ácidos. Ph.D. Thesis, Universidade Federal de Lavras (UFLA), Lavras, Brazil, 24 February 2011. [Google Scholar]
- Yu, F.; Gao, L.; Wang, W.; Zhang, G.; Ji, J. Bio-fuel production from the catalytic pyrolysis of soybean oil over Me-Al-MCM-41 (Me = La, Ni or Fe) mesoporous materials. J. Anal. Appl. Pyrolysis 2013, 104, 325–329. [Google Scholar] [CrossRef]
- Barbosa, F.A.; dos Santos, A.C.B.; da Silva, M.I.P.; Stumbo, A.M. Resistance to poisoning by nitrogen compounds of NiMo/Al-MCM-41 hydrocracking catalysts. Catal. Today 2004, 98, 109–113. [Google Scholar] [CrossRef]
- Liu, C.W.; Hu, W.W.; Yang, B.H.; Tong, Y.; Zhu, D.M.; Zhang, L.F.; Zhao, R. N Study on the effect of metal types in (Me)-Al-MCM-41 on the mesoporous structure and catalytic behavior during the vapor-catalyzed co-pyrolysis of pubescens and LDPE. Appl. Catal. B Environ. 2013, 129, 202–213. [Google Scholar] [CrossRef]
- Intana, T.; Föttinger, K.; Rupprechter, G.; Kongkachuichay, P. Physicochemical properties of Cu loaded onto core-shell Al-MCM-41: Effect of loading methods. Colloids Surf. A Physicochem. Eng. Asp. 2015, 467, 157–165. [Google Scholar] [CrossRef]
- Benítez-Guerrero, M.; López-Beceiro, J.; Sánchez-Jiménez, P.E.; Pascual-Cosp, J. Comparison of thermal behavior of natural and hot-washed sisal fibers based on their main components: Cellulose, xylan and lignin. TG-FTIR analysis of volatile products. Thermochim. Acta 2014, 581, 70–86. [Google Scholar] [CrossRef] [Green Version]
- Zafar, R.; Watson, J.S. Adsorption of tetradecanoic acid on kaolinite minerals: Using fast pyrolysis to characterize the catalytic efficiency of clay mineral adsorbed fatty acids. Chem. Geol. 2017, 471, 111–118. [Google Scholar] [CrossRef]
- Souza, M.J.B.; Marinkovic, B.A.; Jardim, P.M.; Araujo, A.S.; Pedrosa, A.M.G.; Souza, R.R. HDS of thiophene over CoMo/AlMCM-41 with different Si/Al ratios. Appl. Catal. A Gen. 2007, 316, 212–218. [Google Scholar] [CrossRef]





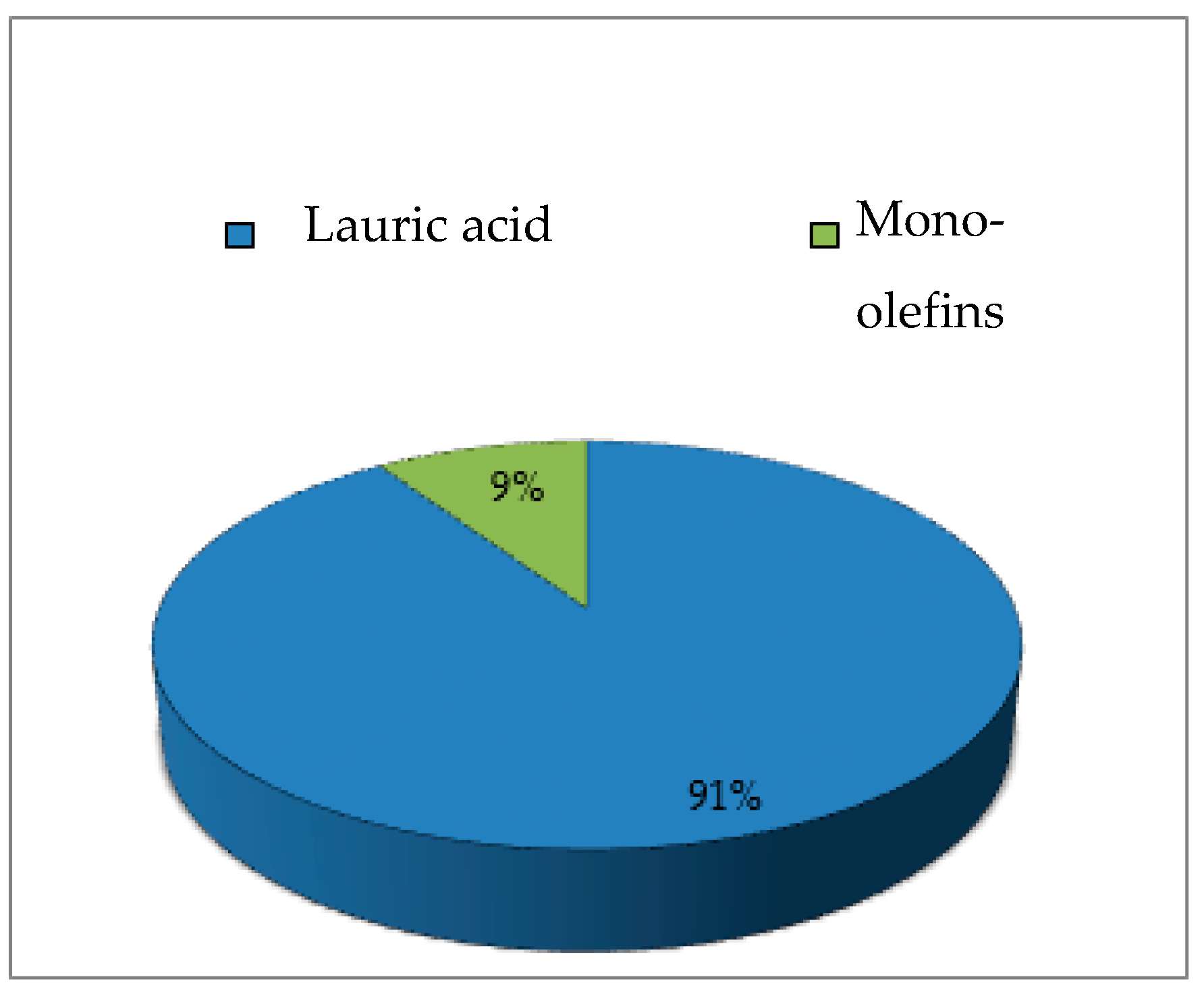
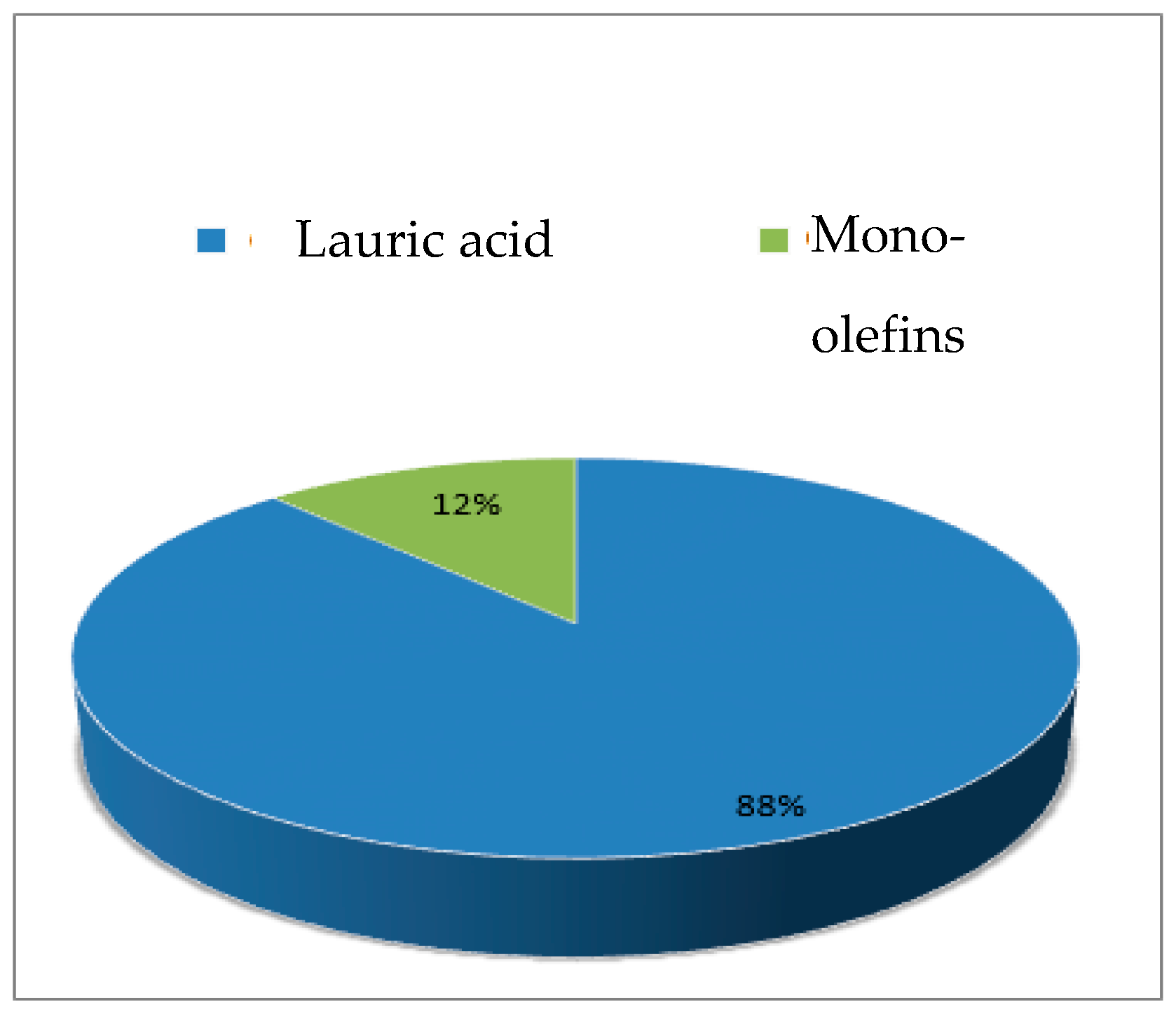



| Catalysts | Weak | Moderate | Strong |
|---|---|---|---|
| SAPO-5 | 1.04 | 1.94 | 0.54 |
| Al-MCM-41 | 0.11 | 0.55 | 0.41 |
| Carbon Number | Retention Time (min) | Mono-Unsaturated |
|---|---|---|
| C6 | 1.686 | 2-Pentene, 4-methyl- |
| 1.785 | 1-Hexene | |
| 1.883 | 3-Hexene, (E)- | |
| 1.918 | 3-Hexene, (Z)- | |
| 1.995 | 2-Pentene, 3-methyl-, (Z)- | |
| 2.268 | Cyclopentene, 1-methyl- | |
| C11 | 8.383 | 4-Decene, 3-methyl-, (E)- |
| 8.426 | 2-Decene, 4-methyl-, (Z)- | |
| 8.538 | 5-Undecene, (E)- | |
| 8.608 | 4-Undecene, (Z)- | |
| 8.671 | 2-Decene, 7-methyl-, (Z)- | |
| 8.699 | 5-Undecene, (Z)- | |
| 8.769 | 1-Decene, 5-methyl- | |
| 8.825 | 4-Undecene, (e)- | |
| 8.846 | 2-Decene, 6-methyl-, (Z)- | |
| 8.916 | 3-Undecene, (Z)- | |
| 8.973 | 3-Undecene, (E)- | |
| 9.064 | 2-Undecene, (Z)- | |
| 9.169 | 2-Undecene, (E)- |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freitas, C.; Pereira, M.; Souza, D.; Fonseca, N.; Sales, E.; Frety, R.; Felix, C.; Azevedo, A., Jr.; Brandao, S. Thermal and Catalytic Pyrolysis of Dodecanoic Acid on SAPO-5 and Al-MCM-41 Catalysts. Catalysts 2019, 9, 418. https://doi.org/10.3390/catal9050418
Freitas C, Pereira M, Souza D, Fonseca N, Sales E, Frety R, Felix C, Azevedo A Jr., Brandao S. Thermal and Catalytic Pyrolysis of Dodecanoic Acid on SAPO-5 and Al-MCM-41 Catalysts. Catalysts. 2019; 9(5):418. https://doi.org/10.3390/catal9050418
Chicago/Turabian StyleFreitas, Carolina, Marizania Pereira, Damari Souza, Noyala Fonseca, Emerson Sales, Roger Frety, Camila Felix, Aroldo Azevedo, Jr., and Soraia Brandao. 2019. "Thermal and Catalytic Pyrolysis of Dodecanoic Acid on SAPO-5 and Al-MCM-41 Catalysts" Catalysts 9, no. 5: 418. https://doi.org/10.3390/catal9050418
APA StyleFreitas, C., Pereira, M., Souza, D., Fonseca, N., Sales, E., Frety, R., Felix, C., Azevedo, A., Jr., & Brandao, S. (2019). Thermal and Catalytic Pyrolysis of Dodecanoic Acid on SAPO-5 and Al-MCM-41 Catalysts. Catalysts, 9(5), 418. https://doi.org/10.3390/catal9050418






