An Efficient Support-Free Nanoporous Co Catalyst for Reverse Water–Gas Shift Reaction
Abstract
:1. Introduction
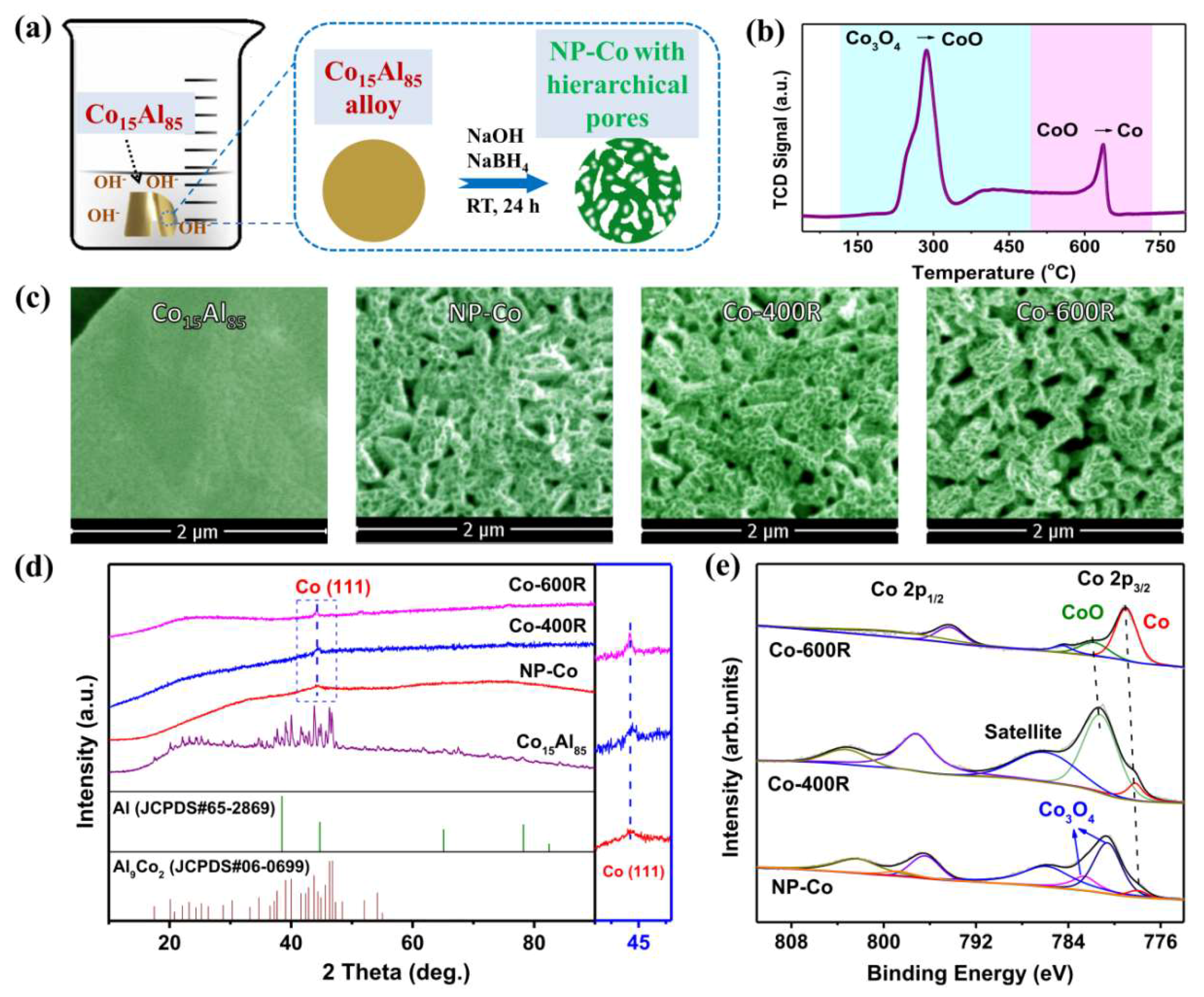
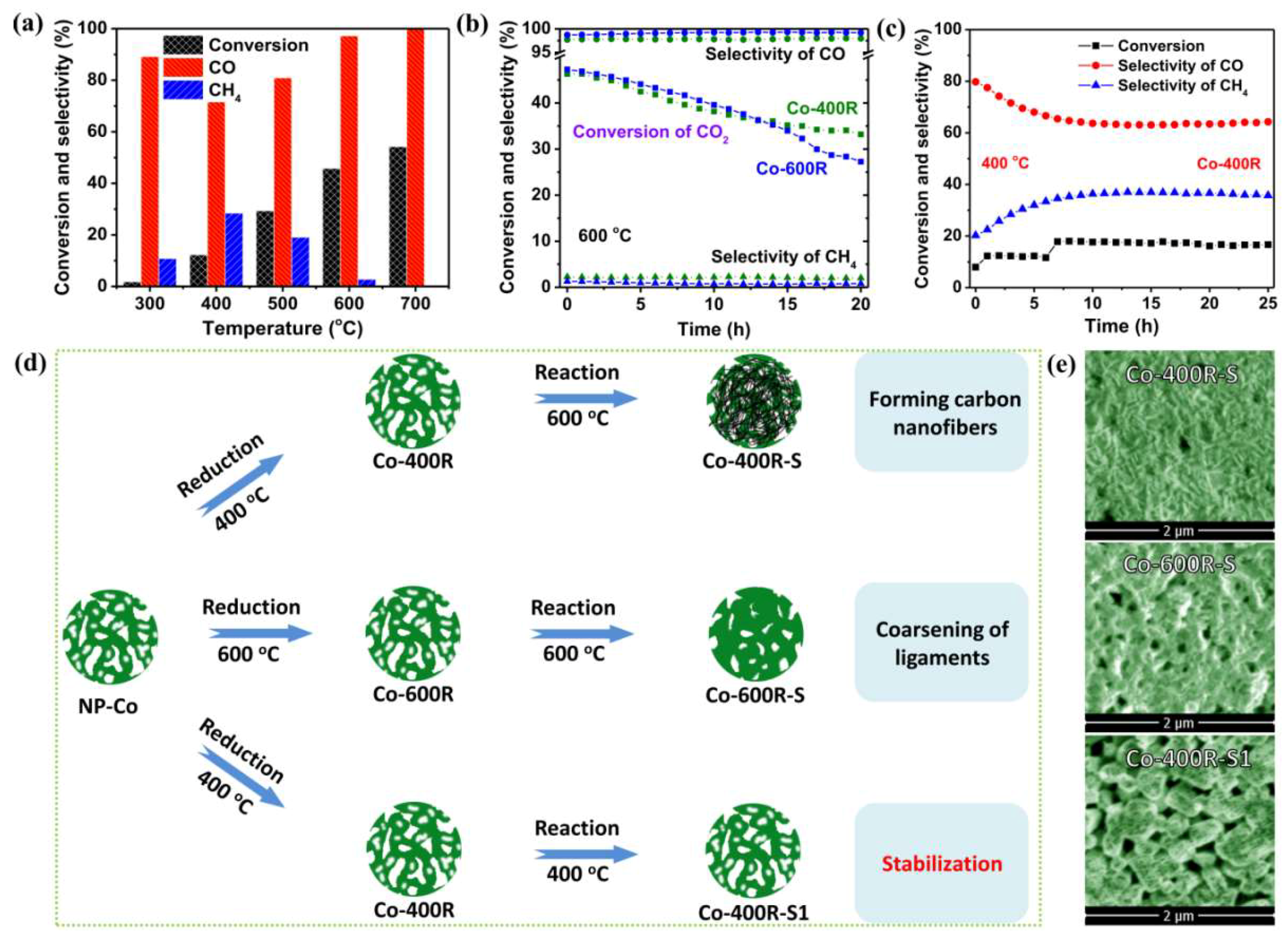
2. Results and Discussion
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Activity Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Porosoff, M.D.; Yan, B.; Chen, J.G. Catalytic reduction of CO2 by H2 for synthesis of CO, methanol and hydrocarbons: Challenges and opportunities. Energy Environ. Sci. 2016, 9, 62–73. [Google Scholar] [CrossRef]
- Daza, Y.A.; Kuhn, J.N. CO2 conversion by reverse water gas shift catalysis: Comparison of catalysts, mechanisms and their consequences for CO2 conversion to liquid fuels. RSC Adv. 2016, 6, 49675–49691. [Google Scholar] [CrossRef]
- Saeidi, S.; Amin, N.A.S.; Rahimpour, M.R. Hydrogenation of CO2 to value-added products—A review and potential future developments. J. CO2 Util. 2014, 5, 66–81. [Google Scholar] [CrossRef]
- Yang, X.; Su, X.; Chen, X.; Duan, H.; Liang, B.; Liu, Q.; Liu, X.; Ren, Y.; Huang, Y.; Zhang, T. Promotion effects of potassium on the activity and selectivity of Pt/zeolite catalysts for reverse water gas shift reaction. Appl. Catal. B Environ. 2017, 216, 95–105. [Google Scholar] [CrossRef]
- Kwak, J.H.; Kovarik, L.; Szanyi, J. Heterogeneous catalysis on atomically dispersed supported metals: CO2 reduction on multifunctional Pd catalysts. ACS Catal. 2013, 3, 2094–2100. [Google Scholar] [CrossRef]
- Xu, H.; Li, Y.; Luo, X.; Xu, Z.; Ge, J. Monodispersed gold nanoparticles supported on a zirconium-based porous metal-organic framework and their high catalytic ability for the reverse water-gas shift reaction. Chem. Commun. 2017, 53, 7953–7956. [Google Scholar] [CrossRef] [PubMed]
- Ronda-Lloret, M.; Rico-Francés, S.; Sepúlveda-Escribano, A.; Ramos-Fernandez, E.V. CuOx/CeO2 catalyst derived from metal organic framework for reverse water-gas shift reaction. Appl. Catal. A Gen. 2018, 562, 28–36. [Google Scholar] [CrossRef]
- Gonçalves, R.V.; Vono, L.L.R.; Wojcieszak, R.; Dias, C.S.B.; Wender, H.; Teixeira-Neto, E.; Rossi, L.M. Selective hydrogenation of CO2 into CO on a highly dispersed nickel catalyst obtained by magnetron sputtering deposition: A step towards liquid fuels. Appl. Catal. B Environ. 2017, 209, 240–246. [Google Scholar] [CrossRef]
- Wang, L.; Liu, H.; Chen, Y.; Yang, S. Reverse water-gas shift reaction over co-precipitated Co-CeO2 catalysts: Effect of Co content on selectivity and carbon formation. Int. J. Hydrog. Energy 2017, 42, 3682–3689. [Google Scholar] [CrossRef]
- Fishman, Z.S.; He, Y.; Yang, K.R.; Lounsbury, A.W.; Zhu, J.; Tran, T.M.; Zimmerman, J.B.; Batista, V.S.; Pfefferle, L.D. Hard templating ultrathin polycrystalline hematite nanosheets: Effect of nano-dimension on CO2 to CO conversion: Via the reverse water-gas shift reaction. Nanoscale 2017, 9, 12984–12995. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Kunkel, C.; Ramírez De La Piscina, P.; Homs, N.; Viñes, F.; Illas, F. Effective and Highly Selective CO Generation from CO2 Using a Polycrystalline α-Mo2C Catalyst. ACS Catal. 2017, 7, 4323–4335. [Google Scholar] [CrossRef]
- Kim, D.H.; Park, J.L.; Park, E.J.; Kim, Y.D.; Uhm, S. Dopant Effect of Barium Zirconate-Based Perovskite-Type Catalysts for the Intermediate-Temperature Reverse Water Gas Shift Reaction. ACS Catal. 2014, 4, 3117–3122. [Google Scholar] [CrossRef]
- Dai, B.; Zhou, G.; Ge, S.; Xie, H.; Jiao, Z.; Zhang, G.; Xiong, K. CO2 reverse water-gas shift reaction on mesoporous M-CeO2 catalysts. Can. J. Chem. Eng. 2017, 95, 634–642. [Google Scholar] [CrossRef]
- Zhou, G.; Wu, T.; Xie, H.; Zheng, X. Effects of structure on the carbon dioxide methanation performance of Co-based catalysts. Int. J. Hydrog. Energy 2013, 38, 10012–10018. [Google Scholar] [CrossRef]
- Díez-Ramírez, J.; Sánchez, P.; Kyriakou, V.; Zafeiratos, S.; Marnellos, G.E.; Konsolakis, M.; Dorado, F. Effect of support nature on the cobalt-catalyzed CO2 hydrogenation. J. CO2 Util. 2017, 21, 562–571. [Google Scholar] [CrossRef]
- Wang, L.; Liu, H. Mesoporous Co-CeO2 catalyst prepared by colloidal solution combustion method for reverse water-gas shift reaction. Catal. Today 2018, 316, 155–161. [Google Scholar] [CrossRef]
- Chen, Q.; Ding, Y.; Chen, M. Nanoporous metal by dealloying for electrochemical energy conversion and storage. MRS Bull. 2018, 43, 43–48. [Google Scholar] [CrossRef]
- Prieto, G.; Martínez, A.; Concepción, P.; Moreno-Tost, R. Cobalt particle size effects in Fischer-Tropsch synthesis: Structural and in situ spectroscopic characterisation on reverse micelle-synthesised Co/ITQ-2 model catalysts. J. Catal. 2009, 266, 129–144. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, L.; Xu, L.; Deng, Z.; Shi, W. Low temperature CO oxidation and CH4 combustion over Co3O4 nanosheets. Fuel 2017, 203, 419–429. [Google Scholar] [CrossRef]
- Ibrahim, M.; Marcelot-Garcia, C.; Aït Atmane, K.; Berrichi, E.; Lacroix, L.M.; Zwick, A.; Warot-Fonrose, B.; Lachaize, S.; Decorse, P.; Piquemal, J.Y.; et al. Carbon coating, carburization, and high-temperature stability improvement of cobalt nanorods. J. Phys. Chem. C 2013, 117, 15808–15816. [Google Scholar] [CrossRef]
- Su, X.; Xu, J.; Liang, B.; Duan, H.; Hou, B.; Huang, Y. Catalytic carbon dioxide hydrogenation to methane: A review of recent studies. J. Energy Chem. 2016, 25, 553–565. [Google Scholar] [CrossRef]
- Panaritis, C.; Edake, M.; Couillard, M.; Einakchi, R.; Baranova, E.A. Insight towards the role of ceria-based supports for reverse water gas shift reaction over RuFe nanoparticles. J. CO2 Util. 2018, 26, 350–358. [Google Scholar] [CrossRef]
- Liang, B.; Duan, H.; Su, X.; Chen, X.; Huang, Y.; Chen, X.; Delgado, J.J.; Zhang, T. Promoting role of potassium in the reverse water gas shift reaction on Pt/mullite catalyst. Catal. Today 2017, 281, 319–326. [Google Scholar] [CrossRef]
- Ye, J.; Ge, Q.; Liu, C.J. Effect of PdIn bimetallic particle formation on CO2 reduction over the Pd-In/SiO2 catalyst. Chem. Eng. Sci. 2015, 135, 193–201. [Google Scholar] [CrossRef]
- Zhu, X.; Qu, X.; Li, X.; Liu, J.; Liu, J.; Zhu, B.; Shi, C. Selective reduction of carbon dioxide to carbon monoxide over Au/CeO2 catalyst and identification of reaction intermediate. Chin. J. Catal. 2016, 37, 2053–2058. [Google Scholar] [CrossRef]
- Chen, C.S.; Cheng, W.H.; Lin, S.S. Study of iron-promoted Cu/SiO2 catalyst on high temperature reverse water gas shift reaction. Appl. Catal. A Gen. 2004, 257, 97–106. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, X.; Lin, L.; Yao, S.; Zhang, M.; Liu, X.; Wang, X.; Li, Y.W.; Shi, C.; Ma, D. Highly Dispersed Copper over β-Mo2C as an Efficient and Stable Catalyst for the Reverse Water Gas Shift (RWGS) Reaction. ACS Catal. 2017, 7, 912–918. [Google Scholar] [CrossRef]
- Zugic, B.; Wang, L.; Heine, C.; Zakharov, D.N.; Lechner, B.A.J.; Stach, E.A.; Biener, J.; Salmeron, M.; Madix, R.J.; Friend, C.M. Dynamic restructuring drives catalytic activity on nanoporous gold-silver alloy catalysts. Nat. Mater. 2016, 16, 558–564. [Google Scholar] [CrossRef]
- Biener, M.M.; Biener, J.; Wichmann, A.; Wittstock, A.; Baumann, T.F.; Bäumer, M.; Hamza, A.V. ALD Functionalized Nanoporous Gold: Thermal Stability, Mechanical Properties, and Catalytic Activity. Nano Lett. 2011, 11, 3085–3090. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, G.; Patterson, P.M.; Das, T.K.; Luo, M.; Davis, B.H. Fischer-Tropsch synthesis: Effect of water on Co/Al2O3 catalysts and XAFS characterization of reoxidation phenomena. Appl. Catal. A Gen. 2004, 270, 65–76. [Google Scholar] [CrossRef]
- Yin, G.; Yuan, X.; Du, X.; Zhao, W.; Bi, Q.; Huang, F. Efficient Reduction of CO2 to CO Using Cobalt–Cobalt Oxide Core–Shell Catalysts. Chem. Eur. J. 2018, 24, 2157–2163. [Google Scholar] [CrossRef] [PubMed]
| Catalysts | H2:CO2 | Temp. (°C) | WHSV (mL/g/h) | Conv. (%) (μmolCO2/g/s) | Sel. of CO (%) | Ref. |
|---|---|---|---|---|---|---|
| PtK/Mullite | 1:1 | 550 | 30,000 | 30.9 (52) | 99.2 | [23] |
| Pd–In/SiO2 | 1:1 | 600 | 60,000 | 29.4 (44) | 100 | [24] |
| K80-Pt/L | 1:1 | 400 | 30,000 | 13 (21.9) | 100 | [4] |
| Au/CeO2 | 1:1 | 400 | 6000 | 21 (15.7) | 100 | [25] |
| Mn–CeO2 | 4:1 | 400 | 60,000 | 8.1 (6.1) | 100 | [13] |
| BaZr0.8Y0.16Zn0.04O3 | 1:1 | 600 | 2400 | 37.5 (5.6) | 97 | [12] |
| Cu–Fe/SiO2 | 1:1 | 600 | 120,000 | 15 (112) | N/A | [26] |
| Ni/nSiO2 | 4:1 | 400 | 400,000 | 25 (13) | 96 | [8] |
| Co–CeO2 | 1:1 | 600 | 300,000 | 38 (711) | 100 | [9] |
| Co–CeO2 | 4:1 | 400 | 60,000 | 35 (26.2) | 62 | [13] |
| Mesoporous Co–CeO2 | 1:1 | 600 | 600,000 | 34.5 (1749) | 99.8 | [16] |
| Fe2O3 | 1:1 | 510 | 120,000 | 28 (84) | 100 | [10] |
| Cu/β-Mo2C | 2:1 | 600 | 300,000 | −(477) | 99.2 | [27] |
| Co-400R | 1:1 | 400 | 300,000 | 12.3 (184) | 71.6 | This work |
| Co-400R | 1:1 | 500 | 300,000 | 29.4 (440) | 80.9 | This work |
| Co-400R | 1:1 | 600 | 300,000 | 45.8 (686) | 97.2 | This work |
| Co-400R | 1:1 | 700 | 300,000 | 54.2 (812) | 99.9 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Y.; Cao, Z.; Xiao, Z. An Efficient Support-Free Nanoporous Co Catalyst for Reverse Water–Gas Shift Reaction. Catalysts 2019, 9, 423. https://doi.org/10.3390/catal9050423
Shen Y, Cao Z, Xiao Z. An Efficient Support-Free Nanoporous Co Catalyst for Reverse Water–Gas Shift Reaction. Catalysts. 2019; 9(5):423. https://doi.org/10.3390/catal9050423
Chicago/Turabian StyleShen, Yongli, Zhen Cao, and Zihui Xiao. 2019. "An Efficient Support-Free Nanoporous Co Catalyst for Reverse Water–Gas Shift Reaction" Catalysts 9, no. 5: 423. https://doi.org/10.3390/catal9050423





