Enhanced Photocatalytic Degradation of 2-Butanone Using Hybrid Nanostructures of Gallium Oxide and Reduced Graphene Oxide Under Ultraviolet-C Irradiation
Abstract
1. Introduction
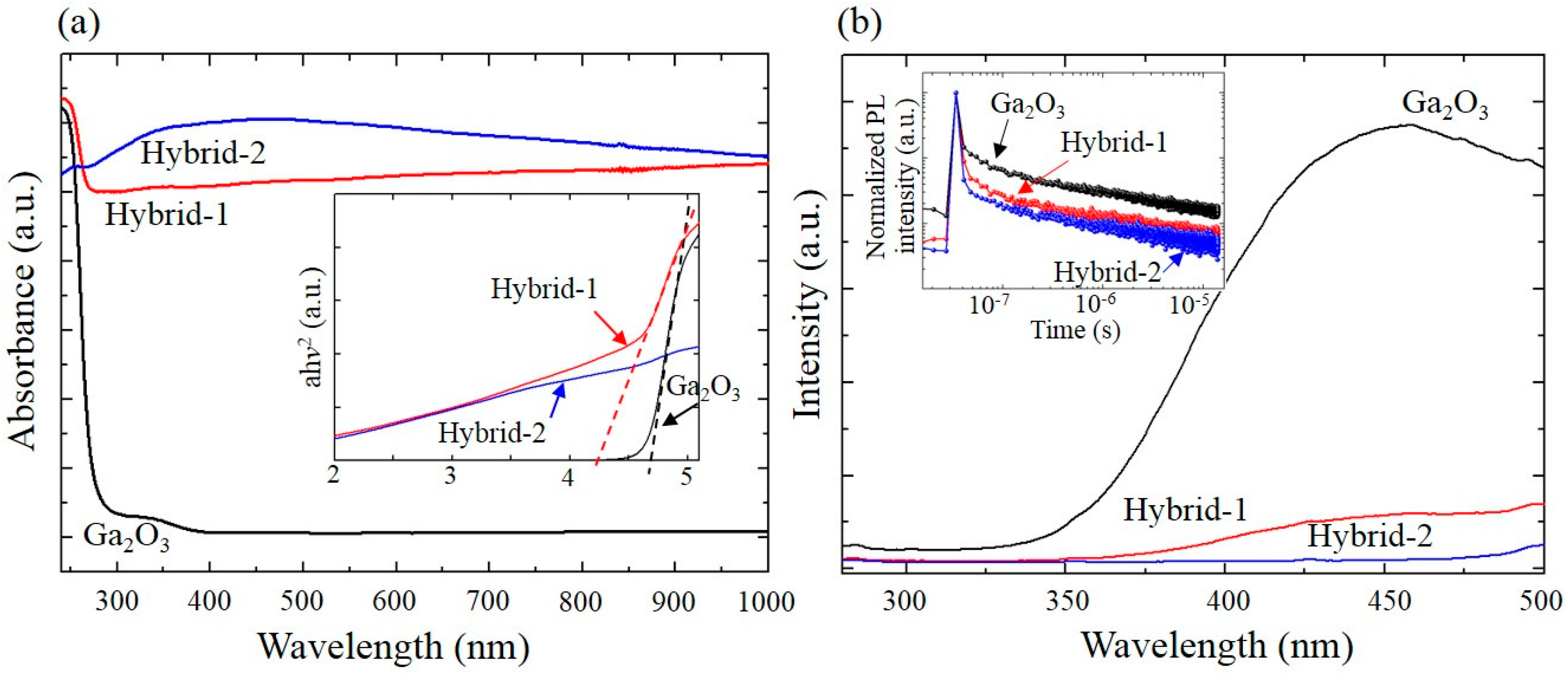
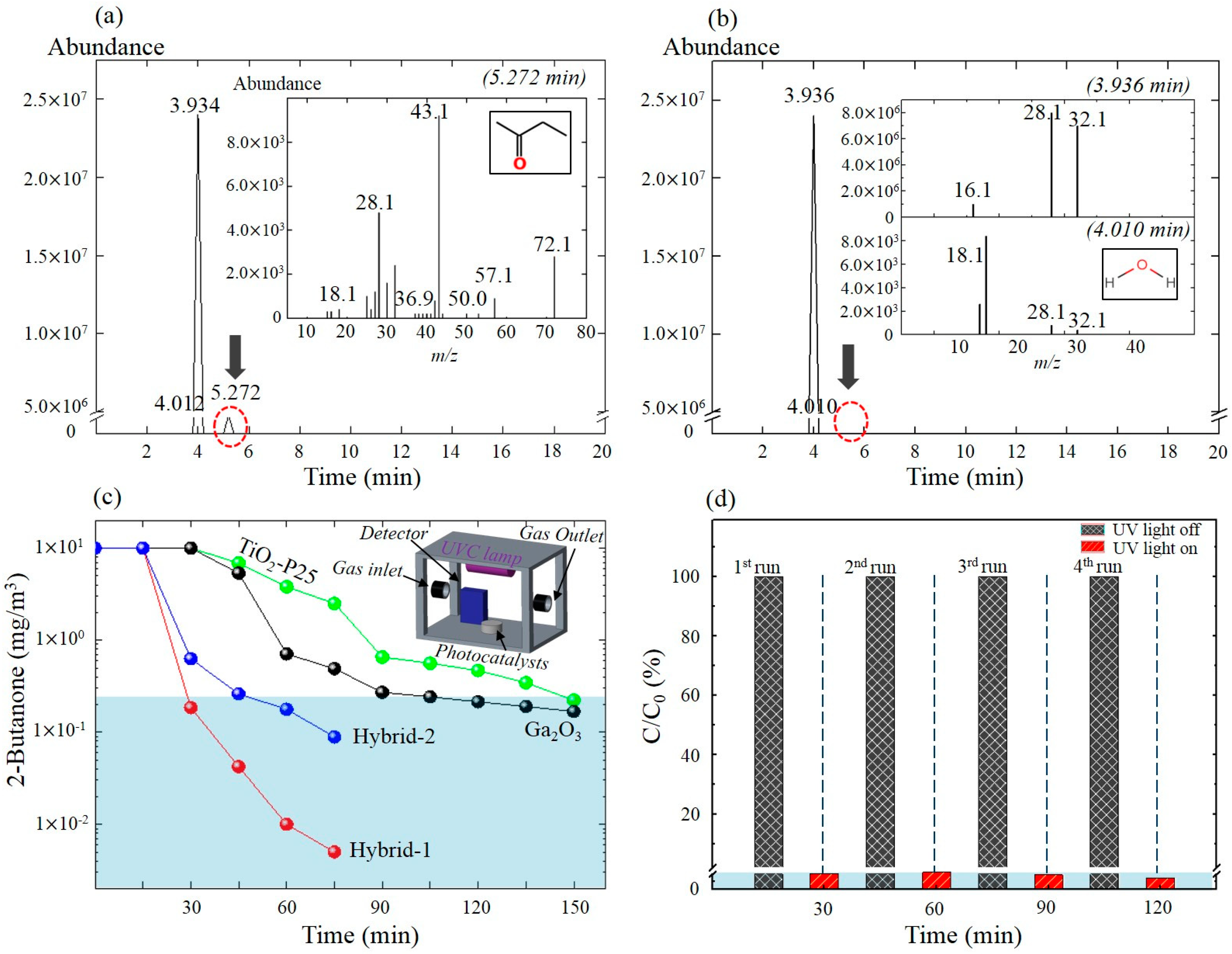
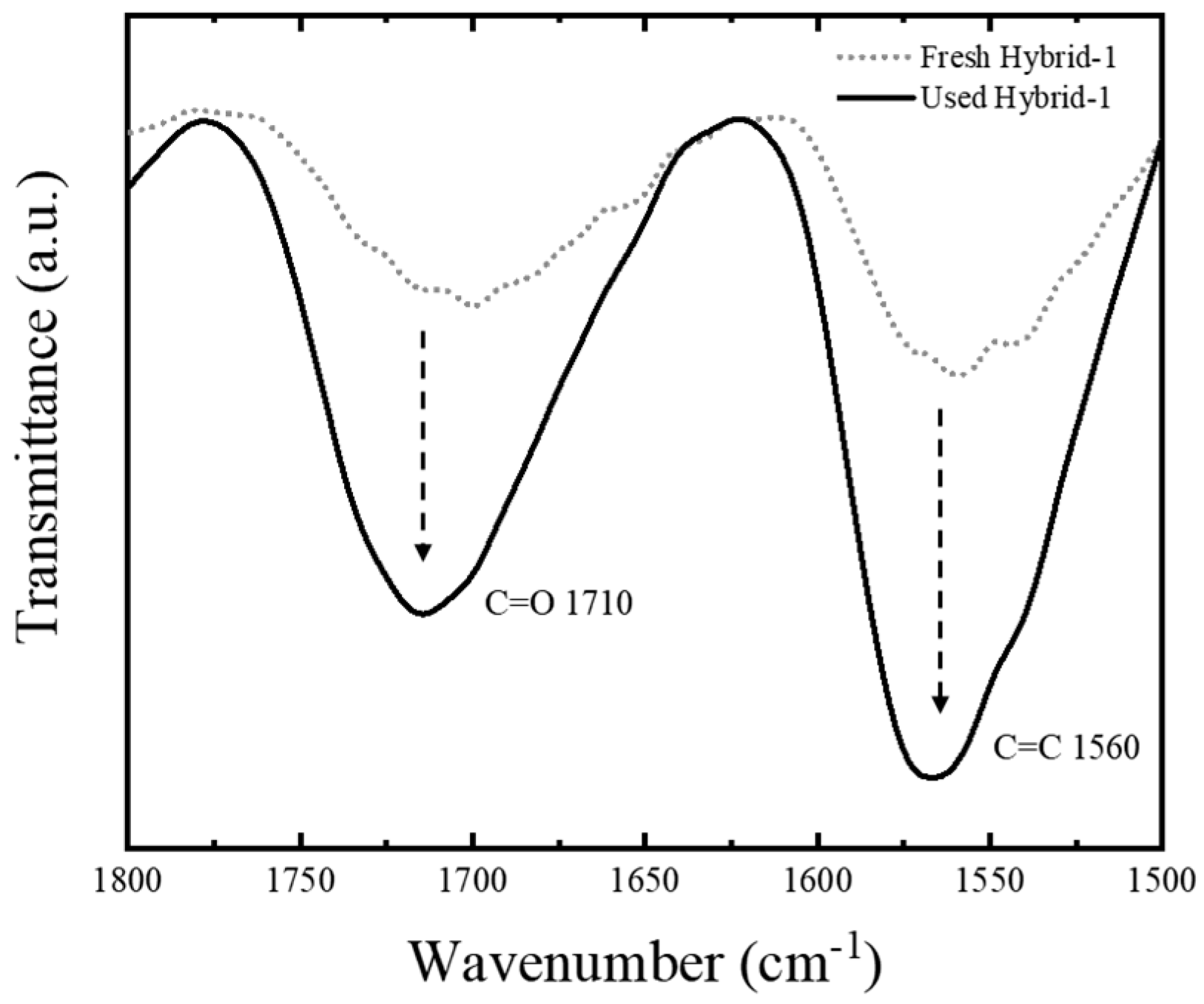
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of Hybrids
3.2. Materials Characterization
3.3. Photocatalytic Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dolai, S.; Bhunia, S.K.; Beglaryan, S.S.; Kolusheva, S.; Zeiri, L.; Jelinek, R. Colorimetric Polydiacetylene−Aerogel Detector for Volatile Organic Compounds (VOCs). ACS Appl. Mater. Interfaces 2017, 9, 2891–2898. [Google Scholar] [CrossRef] [PubMed]
- Konvalina, G.; Haick, H. Sensors for Breath Testing: From Nanomaterials to Comprehensive Disease Detection. Acc. Chem. Res. 2014, 47, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.M.; Sohn, W.B.; Shim, Y.S.; Choi, J.S.; Song, Y.G.; Kim, T.M.; Jeon, J.M.; Kwon, K.C.; Choi, K.S.; Kang, C.Y.; et al. p−p Heterojunction of Nickel Oxide-Decorated Cobalt Oxide Nanorods for Enhanced Sensitivity and Selectivity toward Volatile Organic Compounds. ACS Appl. Mater. Interfaces 2018, 10, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- McCurdy, T.; Glen, G.; Smith, L.; Lakkadi, Y. The National Exposure Research Laboratory’s Consolidated Human Activity Database. J. Expo. Anal. Environ. Epidemiol. 2000, 10, 566–578. [Google Scholar] [CrossRef]
- Kim, S.D.; Han, K.I.; Lee, I.G.; Park, W.K.; Yoon, Y.J.; Yoo, C.S.; Yang, W.S.; Hwang, W.S. A Gallium Oxide-Graphene Oxide Hybrid Composite for Enhanced Photocatalytic Reaction. Nanomaterials 2016, 6, 127. [Google Scholar] [CrossRef]
- Devahasdin, S.; Fan, C., Jr.; Li, K.; Chen, D.H. TiO2 photocatalytic oxidation of nitric oxide: Transient behavior and reaction kinetics. J. Photochem. Photobiol. A 2003, 156, 161170. [Google Scholar] [CrossRef]
- Kamal, M.S.; Razzak, S.A.; Hossain, M.M. Catalytic oxidation of volatile organic compounds (VOCs)—A review. Atmos. Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- Tatsuma, T.; Saitoh, S.; Ngaotrakanwiwat, P.; Ohko, Y.; Fujishima, A. Energy Storage of TiO2-WO3 Photocatalysis Systems in the Gas Phase. Langmuir 2002, 18, 7777–7779. [Google Scholar] [CrossRef]
- Martra, G.; Coluccia, S.; Marchese, L.; Augugliaro, V.; Loddo, V.; Palmisano, L.; Schiavello, M. The Role of H2O in the Photocatalytic Photocatalytic Oxidation of Toluene in Vapour Phase on Anatase TiO2 Catalyst: A FTIR Study. Catal. Today 1999, 53, 695–702. [Google Scholar] [CrossRef]
- Mendez-Roman, R.; Cardona-Martinez, N. Relationship between the Formation of Surface Species and Catalyst Deactivation during the Gas-Phase Photocatalytic Oxidation of Toluene. Catal. Today 1998, 40, 353–365. [Google Scholar] [CrossRef]
- Girija, K.; Thirumalairajan, S.; Patra, A.K.; Mangalaraj, D.; Ponpandian, N.; Viswanathan, C. Enhanced photocatalytic performance of novel self-assembled floral β-Ga2O3 nanorods. Curr. Appl. Phys. 2013, 13, 652–658. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, X.; Wu, L.; Ding, Z.; Fu, X. Efficient Decomposition of Benzene over a β-Ga2O3 Photocatalyst under Ambient Conditions. Environ. Sci. Technol. 2006, 40, 5799–5803. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wu, L.; Wang, X.; Ding, Z.; Li, Z.; Fu, X. Photocatalytic performance of α-, β-, and γ -Ga2O3 for the destruction of volatile aromatic pollutants in air. J. Catal. 2007, 250, 12–18. [Google Scholar] [CrossRef]
- Hou, Y.; Zhang, J.S.; Ding, Z.X.; Wu, L. Synthesis, characterization and photocatalytic activity of β-Ga2O3 nanostructures. Powder Technol. 2010, 203, 440–446. [Google Scholar] [CrossRef]
- Sun, M.; Li, D.; Zhang, W.; Fu, X.; Shao, Y.; Li, W.; Xiao, G.; He, Y. Rapid microwave hydrothermal synthesis of GaOOH nanorods with photocatalytic activity toward aromatic compounds. Nanotechnology 2010, 21, 355601. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yu, Z.; Li, M.; Hou, Y.; Sun, L.; Wang, L.; Peng, Z.; Chen, D.; Liu, Y. Fabrication of Ag/AgBr/Ga2O3 heterojunction composite with efficient photocatalytic activity. Mol. Catal. 2017, 432, 57–63. [Google Scholar] [CrossRef]
- Ikarashi, K.; Sato, J.; Kobayashi, H.; Saito, N.; Nishiyama, H.; Inoue, Y. Photocatalysis for Water Decomposition by RuO2-Dispersed ZnGa2O4 with d10 Configuration. J. Phys. Chem. B 2002, 106, 9048–9053. [Google Scholar] [CrossRef]
- Sato, J.; Kobayashi, H.; Ikarashi, K.; Saito, N.; Nishiyama, H.; Inoue, Y. Photocatalytic Activity for Water Decomposition of RuO2-Dispersed Zn2GeO4 with d10 Configuration. J. Phys. Chem. B 2004, 108, 4369–4375. [Google Scholar] [CrossRef]
- Ge, M.Z.; Cao, C.Y.; Li, S.H.; Tang, Y.X.; Wang, L.N.; Qi, N.; Huang, J.Y.; Zhang, K.Q.; Al-Deyab, S.S.; Lai, Y.K. In situ plasmonic Ag nanoparticle anchored TiO2 nanotube arrays as visible-light-driven photocatalysts for enhanced water splitting. Nanoscale 2016, 8, 5226–5234. [Google Scholar] [CrossRef] [PubMed]
- Shengyan, P.; Rongxin, Z.; Hui, M.; Daili, D.; Xiangjun, P.; Fei, Q.; Wei, C. Facile in-situ design strategy to disperse TiO2 nanoparticles on graphene for the enhanced photocatalytic degradation of rhodamine 6G. Appl. Catal. B 2017, 218, 208–219. [Google Scholar] [CrossRef]
- Singh, A.; Sinha, A.S.K. Active CdS/rGO photocatalyst by a high temperature gas-solid reaction for hydrogen production by splitting of water. Appl. Surf. Sci. 2018, 430, 184–197. [Google Scholar] [CrossRef]
- Yang, N.; Zhai, J.; Wang, D.; Chen, Y.; Jiang, L. Two-Dimensional Graphene Bridges Enhanced Photoinduced Charge Transport in Dye-Sensitized Solar Cells. ACS Nano 2010, 4, 887–894. [Google Scholar] [CrossRef]
- Pan, X.; Yi, Z. Graphene Oxide Regulated Tin Oxide Nanostructures: Engineering Composition, Morphology, Band Structure, and Photocatalytic Properties. ACS Appl. Mater. Interfaces 2015, 7, 27167–27175. [Google Scholar] [CrossRef]
- Tan, L.L.; Ong, W.J.; Chai, S.P.; Mohamed, A.R. Reduced graphene oxide-TiO2 nanocomposite as a promising visible-light-active photocatalyst for the conversion of carbon dioxide. Nanoscale Res. Lett. 2013, 8, 465. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, J.; Wang, X.; Yu, H.; Yu, J.; Lei, M.; Wang, Y. One-step synthesis of easy-recycling TiO2-rGO nanocomposite photocatalysts with enhanced photocatalytic activity. Appl. Catal. B 2013, 132–133, 452–459. [Google Scholar] [CrossRef]
- Zeng, X.; Wang, Z.; Wang, G.; Gengenbach, T.R.; McCarthy, D.T.; Deletic, A.; Yu, J.; Zhang, X. Highly dispersed TiO2 nanocrystals and WO3 nanorods on reduced graphene oxide: Z-scheme photocatalysis system for accelerated photocatalytic water disinfection. Appl. Catal. B 2017, 218, 163–173. [Google Scholar] [CrossRef]
- Koss, A.R.; Gouw, J.D.; Warneke, C.; Gilman, J.B.; Lerner, B.M.; Graus, M.; Yuan, B.; Edwards, P.; Brown, S.S.; Wild, R.; et al. Photochemical aging of volatile organic compounds associated with oil and natural gas extraction in the Uintah Basin, UT, during a wintertime ozone formation event. Atmos. Chem. Phys. 2015, 15, 5727–5741. [Google Scholar] [CrossRef]
- Torpy, F.; Clements, N.; Pollinger, M.; Dengel, A.; Mulvihill, I.; He, C.; Irga, P. Testing the single-pass VOC removal efficiency of an active green wall using methyl ethyl ketone (MEK). Air Qual. Atmos. Health 2018, 11, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Mungse, H.P.; Sharma, O.P.; Sugimura, H.; Khatri, O.P. Hydrothermal deoxygenation of graphene oxide in sub- and supercritical water. RSC Adv. 2014, 4, 22589–22595. [Google Scholar] [CrossRef]
- Zhou, Y.; Bao, Q.; Tang LA, L.; Zhong, Y.; Loh, K.P. Hydrothermal Dehydration for the “Green” Reduction of Exfoliated Graphene Oxide to Graphene and Demonstration of Tunable Optical Limiting Properties. Chem. Mater. 2009, 21, 2950–2956. [Google Scholar] [CrossRef]
- Li, D.; Duan, X.; Qin, Q.; Fan, H.; Zheng, W. Ionic liquid-assisted synthesis of mesoporous α-Ga2O3 hierarchical structures with enhanced photocatalytic activity. J. Mater. Chem. A 2013, 1, 12417–12421. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, Y.; Hao, R.; Liu, F.; Wang, Y.; Tan, M.; Tang, J.; Ren, D.; Zhao, D. Synthesis of mesoporous β-Ga2O3 nanorods using PEG as template: Preparation, characterization and photocatalytic properties. J. Hazard. Mater. 2011, 192, 1548–1554. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.F.; Yan, B.; Shi, M.; Ma, H.; Li, N.; Ye, M. One step hydrothermal synthesis of TiO2-reduced graphene oxide sheets. J. Mater. Chem. 2011, 21, 3415–3421. [Google Scholar] [CrossRef]
- Hu, K.; Xie, X.; Szkopek, T.; Cerruti, M. Understanding Hydrothermally Reduced Graphene Oxide Hydrogels: From Reaction Products to Hydrogel Properties. Chem. Mater. 2016, 28, 1756–1768. [Google Scholar] [CrossRef]
- Xu, Y.; Sheng, K.; Li, C.; Shi, G. Self-Assembled Graphene Hydrogel via a One-Step Hydrothermal Process. ACS Nano 2010, 4, 4324–4330. [Google Scholar] [CrossRef]
- Bai, X.; Wang, L.; Zong, R.; Lv, Y.; Sun, Y.; Zhu, Y. Performance Enhancement of ZnO Photocatalyst via Synergic Effect of Surface Oxygen Defect and Graphene Hybridization. Langmuir 2013, 29, 3097–3105. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guo, B.; Yu, J.; Ran, J.; Zhang, B.; Yan, H.; Gong, J.R. Highly Efficient Visible-Light-Driven Photocatalytic Hydrogen Production of CdS-Cluster-Decorated Graphene Nanosheets. J. Am. Chem. Soc. 2011, 133, 10878–10884. [Google Scholar] [CrossRef]
- Soltani, T.; Lee, B.K. A benign ultrasonic route to reduced graphene oxide from pristine graphite. J. Colloid Interface Sci. 2017, 486, 337–343. [Google Scholar] [CrossRef]
- Gollu, S.R.; Sharma, R.; Srinivas, G.; Kundu, S.; Gupta, D. Incorporation of silver and gold nanostructures for performance improvement in P3HT: PCBM inverted solar cell with rGO/ZnO nanocomposite as an electron transport layer. Org. Electron. 2016, 29, 79–87. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, L.; Cheng, H.; Zhu, Y. Significantly enhanced photocatalytic performance of ZnO via graphene hybridization and the mechanism study. Appl. Catal. B 2011, 101, 382–387. [Google Scholar] [CrossRef]
- Williams, G.; Kamat, P.V. Graphene-Semiconductor Nanocomposites: Excited-State Interactions between ZnO Nanoparticles and Graphene Oxide. Langmuir 2009, 25, 13869–13873. [Google Scholar] [CrossRef]
- Wu, F.; Wang, X.; Li, M.; Xu, H. A high capacity NiFe2O4/RGO nanocomposites as superior anode materials for sodium-ion batteries. Ceram. Int. 2016, 42, 16666–16670. [Google Scholar] [CrossRef]
- Khatamian, M.; Khodakarampoor, N.; Oskoui, M.S.; Kazemian, N. Synthesis and characterization of RGO/zeolite composites for the removal of arsenic from contaminated water. RSC Adv. 2015, 5, 35352–35360. [Google Scholar] [CrossRef]
- Li, J.; Xing, X.; Li, J.; Shi, M.; Lin, A.; Xu, C.; Zheng, J.; Li, R. Preparation of thiol-functionalized activated carbon from sewage sludge with coal blending for heavy metal removal from contaminated water. Environ. Pollut. 2018, 234, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]


| A1 | τ1 (ns) | A2 | τ2 (ns) | A3 | τ3 (ns) | A4 | τ4 (ns) | ||
|---|---|---|---|---|---|---|---|---|---|
| Ga2O3 | 8973 | 2.5 | 0 | 603 | 110 | 201 | 2876 | 68.3 | |
| Hybrid-1 | 9538 | 2.4 | 0 | 236 | 126 | 81 | 3081 | 30.7 | |
| Hybrid-2 | 9597 | 1.6 | 186 | 43.6 | 59 | 419 | 49 | 4171 | 25.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, H.J.; Yoo, T.H.; Kim, S.; Choi, W.; Song, Y.S.; Kwon, D.-K.; Cho, B.J.; Hwang, W.S. Enhanced Photocatalytic Degradation of 2-Butanone Using Hybrid Nanostructures of Gallium Oxide and Reduced Graphene Oxide Under Ultraviolet-C Irradiation. Catalysts 2019, 9, 449. https://doi.org/10.3390/catal9050449
Bae HJ, Yoo TH, Kim S, Choi W, Song YS, Kwon D-K, Cho BJ, Hwang WS. Enhanced Photocatalytic Degradation of 2-Butanone Using Hybrid Nanostructures of Gallium Oxide and Reduced Graphene Oxide Under Ultraviolet-C Irradiation. Catalysts. 2019; 9(5):449. https://doi.org/10.3390/catal9050449
Chicago/Turabian StyleBae, Hyun Jeong, Tae Hee Yoo, Seungdu Kim, Wonhyeok Choi, Yo Seung Song, Do-Kyun Kwon, Byung Jin Cho, and Wan Sik Hwang. 2019. "Enhanced Photocatalytic Degradation of 2-Butanone Using Hybrid Nanostructures of Gallium Oxide and Reduced Graphene Oxide Under Ultraviolet-C Irradiation" Catalysts 9, no. 5: 449. https://doi.org/10.3390/catal9050449
APA StyleBae, H. J., Yoo, T. H., Kim, S., Choi, W., Song, Y. S., Kwon, D.-K., Cho, B. J., & Hwang, W. S. (2019). Enhanced Photocatalytic Degradation of 2-Butanone Using Hybrid Nanostructures of Gallium Oxide and Reduced Graphene Oxide Under Ultraviolet-C Irradiation. Catalysts, 9(5), 449. https://doi.org/10.3390/catal9050449




