Mass and Heat Transfer Coefficients in Automotive Exhaust Catalytic Converter Channels
Abstract
:1. Introduction
2. Models
2.1. 2-D Computational Fluid Dynamic (CFD) Models
2.1.1. Isothermal 2-D CFD Model
2.1.2. Non-Isothermal 2-D CFD Model
2.2. 1-D Thin Film Model
2.3. Conditions of Calculations
3. Mass Transfer Coefficients
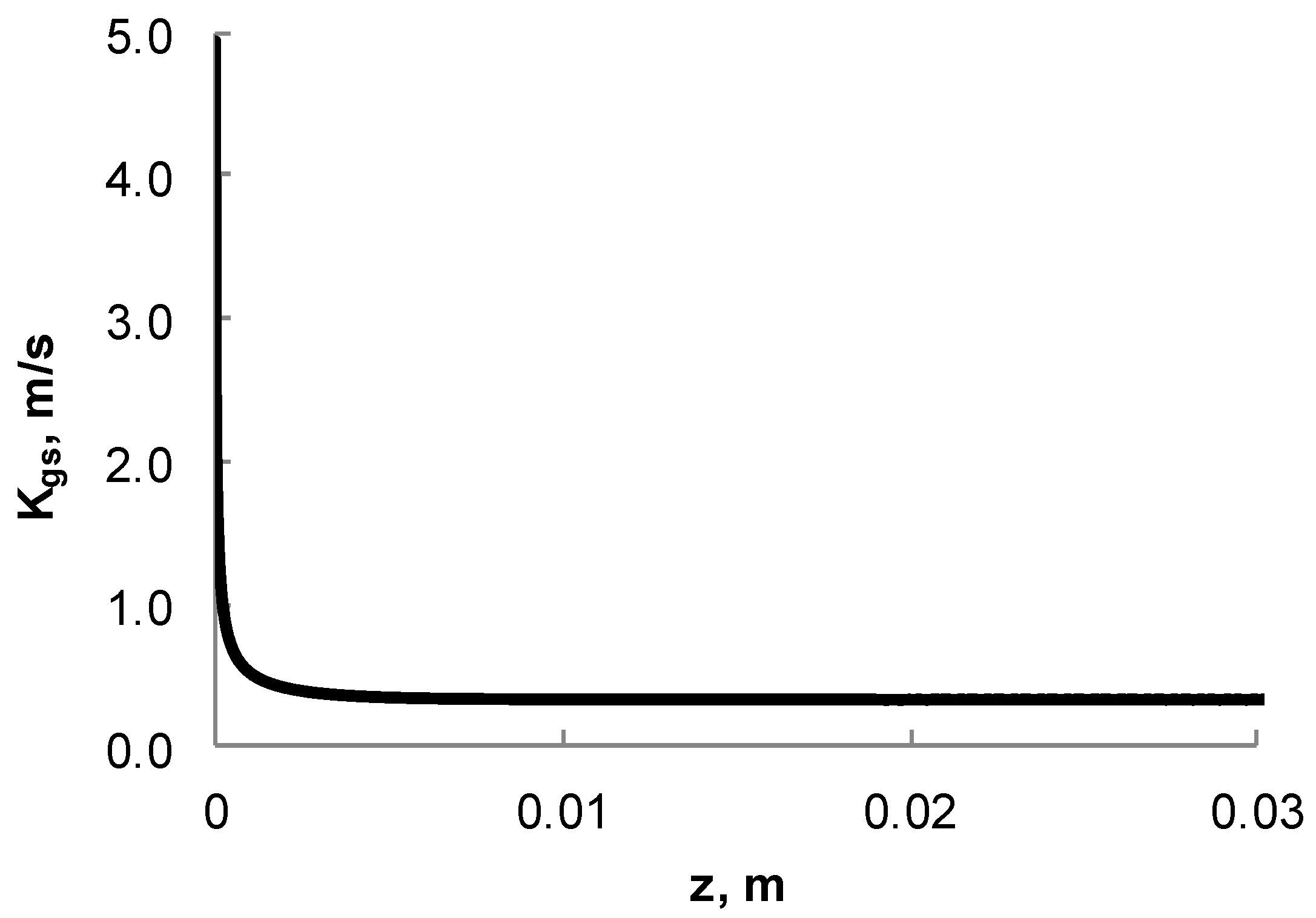
3.1. Calculation of Effective Gas–Solid Mass Transfer Coefficient Profiles Along the Channel
3.2. Correlation of the Effective Gas–Solid Mass Transfer Kgs,i
3.2.1. Correlation Parameters
Mass Transfer Correlation for Species CO, NO and O2
Mass Transfer Correlation for Hydrocarbons CH4, C3H6 and C3H8
3.2.2. Mass Transfer Correlation Fitting
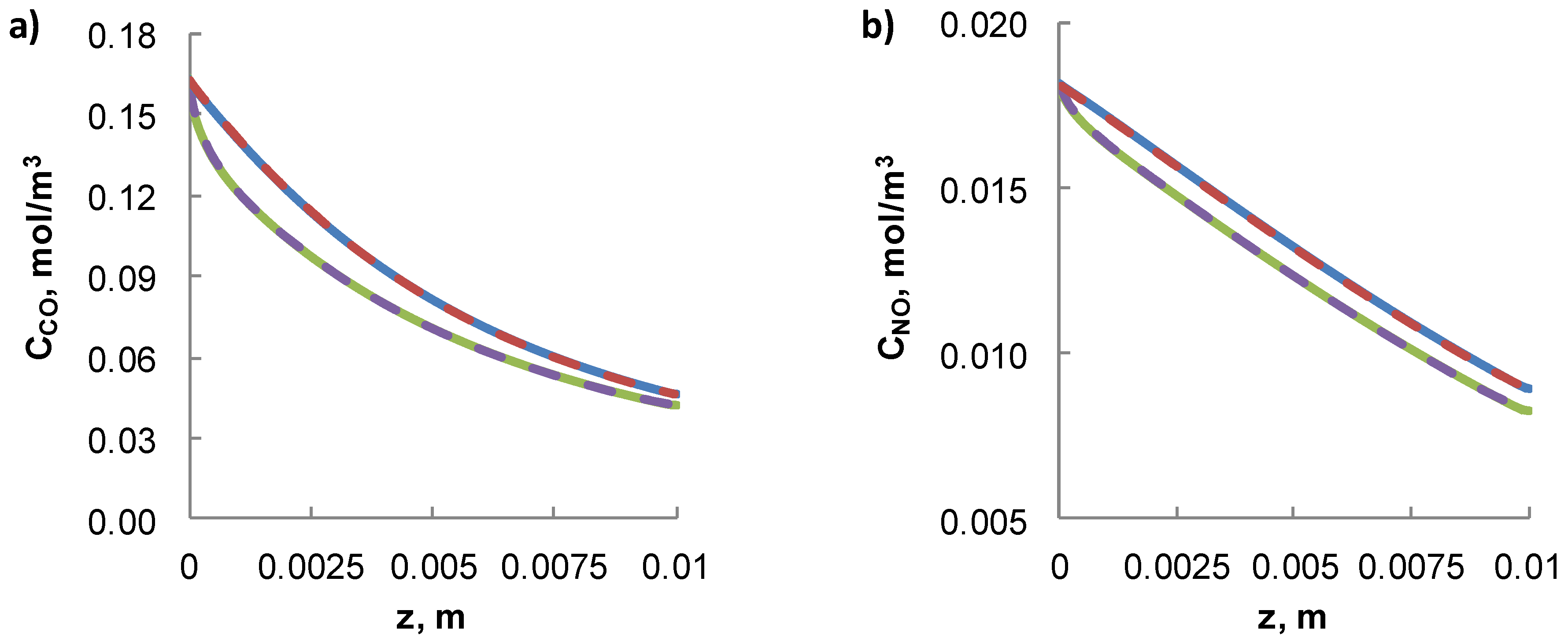
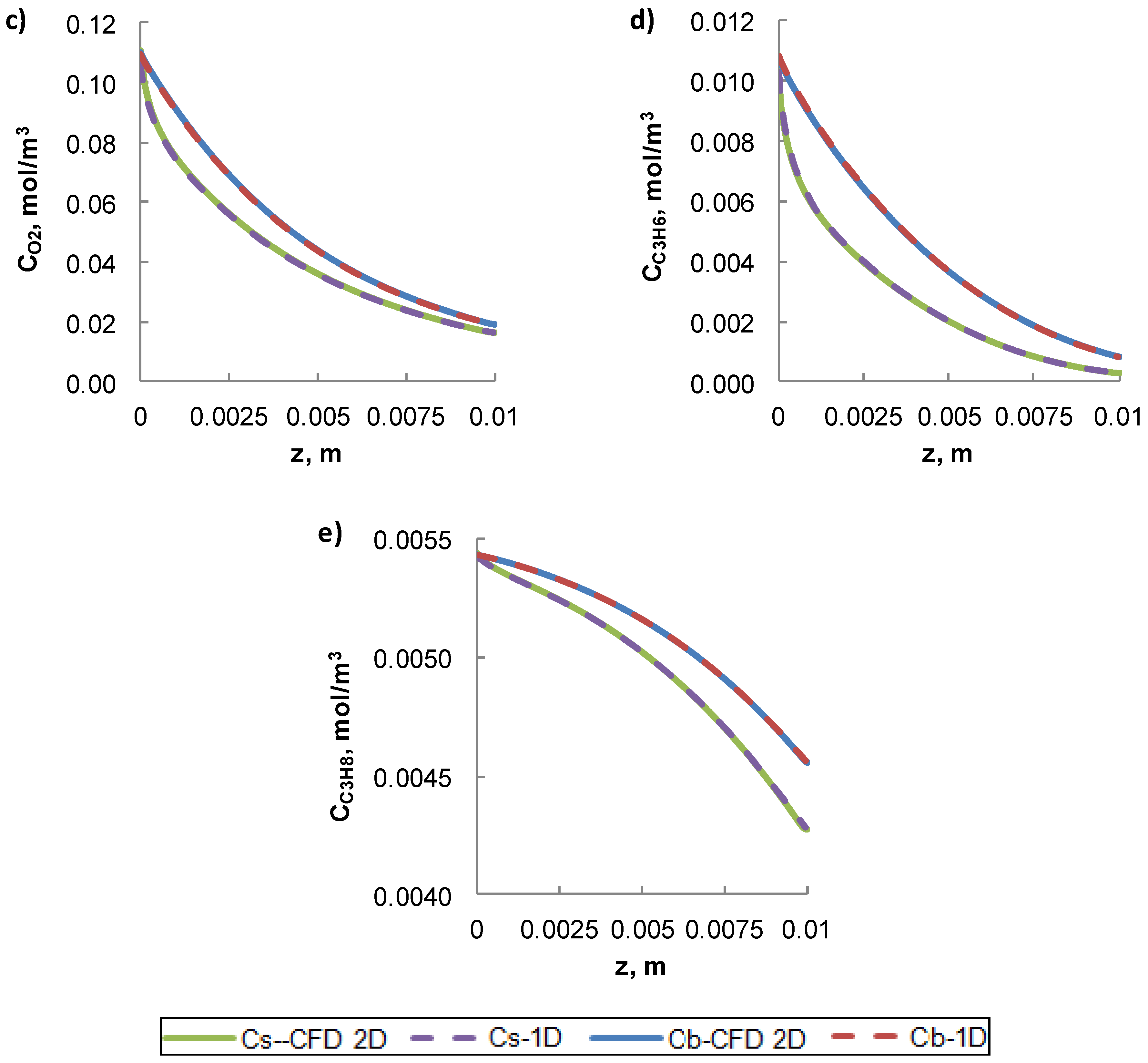
3.3. 2-D Isothermal CFD Model vs 1-D Isothermal Simplified Model
3.4. Comparison with Other Published Correlations
4. Heat Transfer Coefficients
4.1. Calculation of Effective Heat Transfer Coefficient Profiles Along the Cell
4.2. Correlation of the Heat Transfer Coefficient Kt
4.2.1. Heat Transfer Correlation Parameters
4.2.2. Heat Transfer Correlation Fitting
4.3. Non-Isothermal 2-D CFD Model vs Non-Isothermal 1-D Simplified Model
4.4. Comparison with Other Published Correlations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| a,b,c,e | Heat and mass transfer correlation coefficients |
| Ci | The concentration of component i in the gas phase (mol/m3) |
| Ci,in | The concentration of component i in the gas phase at cell inlet (mol/m3) |
| Ci,s | The concentration of component i in the gas phase over the catalytic surface (mol/m3) |
| Ci,b | The concentration of component i in the bulk of the gas phase (mol/m3) |
| Cp | Thermal capacity of the gas phase (J/K) |
| d | Diameter of the monolith cell (m) |
| Di | The diffusion coefficient of component i in the gas phase (m2/s) |
| Eaj | Activation energy of reaction j (J/mol) |
| I | Unit vector |
| Kji | Adsorption constant of component i at reaction j (m3/mol) |
| KRj | Reaction rate constant of reaction j (m4/mol/s) |
| Kgs,i | Gas solid mass transfer coefficient for the component i (m/s) |
| k | Thermal conductivity of the gas phase (W/m/K) |
| Kt | Heat transfer coefficient (W/m2/K) |
| L | Channel length (m) |
| Mn | Molar mass of the gas phase (Kg/mol) |
| n | The vector direction normal to the boundary surface (-) |
| Nu | Nusselt number (-) |
| NuF | Nusselt number for constant wall flux (-) |
| NuT | Nusselt number constant wall temperature (-) |
| q | Heat released due to reactions take place on the washcoat wall (W/m2) |
| p | Pressure (Pa) |
| The mean radial property prop at each z ordinate of the length | |
| Pr | Prandtl number (-) |
| R | Radius of monolith cell (m) |
| r | Radius coordinate (m) |
| Rg | Universal constant of gas (= 8.314 J/mol/K) |
| Rj | Reaction rate of reaction j = 1,2,... (mol/m2/s) |
| Ri | Reaction rate of component i = NO,CO,O2,C3H6,C3H8,CH4 (mol/m2/s) |
| Re | Reynolds number (-) |
| Sh | Sherwood number (-) |
| ShF | Sherwood number for constant wall flux (-) |
| ShT | Sherwood number constant wall concentration (-) |
| Sc | Schmidt number (-) |
| Sv | External surface for gas solid mass transfer per void volume (m2/m3) |
| T | Temperature (K) |
| Tb | Temperature of the bulk of the gas phase (K) |
| Ts | Temperature of the gas phase over the catalytic surface (K) |
| u | The vector of velocity field (m/s) |
| u | Velocity (m/s) |
| uin | Velocity at inlet (m/s) |
| z | Length coordinate, distance from the cell inlet (m) |
| Greek | |
| Standard enthalpy of reaction (KJ/mol) | |
| ΔHRj | Enthalpy of reaction j at temperature T (KJ/mol) |
| μ | Viscosity (Pas) |
| ρ | Density of the gas phase (Kg/m3) |
References
- Bertrand, F.; Devals, C.; Vidal, D.; de Préval, C.S.; Hayes, R.E. Towards the simulation of the catalytic monolith converter using discrete channel-scale models. Catal. Today 2012, 188, 80–86. [Google Scholar] [CrossRef]
- Shamim, T. Effect of heat and mass transfer coefficients on the performance of automotive catalytic converters. Int. J. Engine Res. 2003, 4, 129–141. [Google Scholar] [CrossRef]
- Hayes, R.E.; Kolaczkowski, S.T. A study of Nusselt and Sherwood numbers in a monolith reactor. Catal. Today 1999, 47, 295–303. [Google Scholar] [CrossRef]
- Koltsakis, G.C.; Stamatelos, A.M. Catalytic automotive exhaust aftertreatment. Prog. Energy Combust. Sci. 1997, 23, 1–39. [Google Scholar] [CrossRef]
- Iwaniszyn, M.; Piatek, M.; Gancarczyk, A.; Jodłowski, P.J.; Łojewska, J.; Kołodziej, A. Flow resistance and heat transfer in short channels of metallic monoliths: Experiments versus CFD. Int. J. Heat Mass Transf. 2017, 109, 778–785. [Google Scholar] [CrossRef]
- Santos, H.; Costa, M. Analysis of the mass transfer controlled regime in automotive catalytic converters. Int. J. Heat Mass Transf. 2008, 51, 41–51. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Harold, M.P.; Balakotaiah, V. Mass-Transfer Coefficients in Washcoated Monoliths. AIChE J. 2004, 50, 2939–2955. [Google Scholar] [CrossRef]
- Holmgren, A.; Andersson, B. Mass transfer in monolith catalysts-CO oxidation experiments and simulations. Chem. Eng. Sci. 1998, 53, 2285–2298. [Google Scholar] [CrossRef]
- Balakotaiah, V.; Gupta, N.; West, D. A simplified model for analyzing catalytic reactions in short monoliths. Chem. Eng. Sci. 2000, 55, 5367–5383. [Google Scholar] [CrossRef]
- Canu, P.; Vecchi, S. CFD Simulation of Reactive Flows: Catalytic Combustion in a Monolith. AIChE J. 2002, 48, 2921–2935. [Google Scholar] [CrossRef]
- Arrighetti, C.; Cordiner, S.; Mulone, V. Heat and Mass Transfer Evaluation in the Channels of an Automotive Catalytic Converter by Detailed Fluid-Dynamic and Chemical Simulation. J. Heat Transf. 2007, 129, 536–547. [Google Scholar] [CrossRef]
- Ramanathan, K.; Balakotaiah, V.; West, D.H. Light-off criterion and transient analysis of catalytic monoliths. Chem. Eng. Sci. 2003, 58, 1381–1405. [Google Scholar] [CrossRef]
- Depcik, C.; Assanis, D. One-dimensional automotive catalyst modelling. Prog. Energy Combust. Sci. 2005, 31, 308–369. [Google Scholar] [CrossRef]
- Holder, R.; Bollig, M.; Anderson, D.R.; Hochmuth, J.K. A discussion on transport phenomena and three-way kinetics of monolithic converters. Chem. Eng. Sci. 2006, 61, 8010–8027. [Google Scholar] [CrossRef]
- Gupta, N.; Balakotaiah, V. Heat and mass transfer coefficients in catalytic monoliths. Chem. Eng. Sci. 2001, 56, 4771–4786. [Google Scholar] [CrossRef]
- Hayes, R.E.; Kolaczkowski, S.T. Mass and heat transfer effects in catalytic monolith reactors. Chem. Eng. Sci. 1994, 49, 3587–3599. [Google Scholar] [CrossRef]
- Hawthorn, R.D. Afterburner catalysts-effects of heat and mass transfer between gas and catalyst surface. Am. Inst. Chem. Eng. 1974, 21, 849–853. [Google Scholar]
- Votruba, J.; Mikus, O.; Nguen, K.; Hlavacek, V.; Skrivanek, J. Heat and mass transfer in monolithic honeycomb catalysts—II. Chem. Eng. Sci. 1975, 30, 201–206. [Google Scholar] [CrossRef]
- Bennett, C.J.; Kolaczkowski, S.T.; Thomas, W.J. Determination of heterogeneous reaction kinetics and reaction rates under mass transfer controlled conditions for a monolith reactor. Trans. Inst. Chem. Eng. Part B 1991, 69, 209–220. [Google Scholar]
- Ullah, U.; Waldram, S.P.; Bennett, C.J.; Truex, T. Monolithic reactors: Mass transfer measurements under reacting conditions. Chem. Eng. Sci. 1992, 47, 2413–2418. [Google Scholar] [CrossRef]
- Uberoi, M.; Pereira, C.J. Mass transfer coefficients for monolith catalysts. Ind. Eng. Chem. Res. 1996, 35, 113–116. [Google Scholar] [CrossRef]
- Grigull, U.; Tratz, H. Thermischer Einlauf in ausgebildeter laminarer Rohrstromung. Int. J. Heat Mass Transf. 1965, 8, 669–678. [Google Scholar] [CrossRef]
- Shah, R.K.; London, A.L. Laminar Flow Forced Convection in Ducts; Academic Press: New York, NY, USA, 1978. [Google Scholar]
- Tronconi, E.; Forzatti, P. Adequacy of lumped parameter models for SCR reactors with monolith structures. AIChE J. 1992, 38, 201–210. [Google Scholar] [CrossRef]
- Kirchner, T.; Eigenberger, G. Optimization of the cold-start behavior of automotive catalysts using an electrically heated pre-catalyst. Chem. Eng. Sci. 1996, 51, 2409–2418. [Google Scholar] [CrossRef]
- Gundlapally, S.R.; Balakotaiah, V. Heat and mass transfer correlations and bifurcation analysis of catalytic monoliths with developing flows. Chem. Eng. Sci. 2011, 66, 1879–1892. [Google Scholar] [CrossRef]
- Groppi, G.; Belloli, A.; Tronconi, E.; Forzatti, P. A comparison of lumped and distributed models of monolith catalytic reactors. Chem. Eng. Sci. 1995, 50, 2705–2715. [Google Scholar] [CrossRef]
- Heck, R.H.; Wei, J.; Katzer, J.R. Mathematical modelling of monolithic catalysts. AIChE J. 1976, 22, 477–483. [Google Scholar] [CrossRef]







| Boundary | Momentum Transport | Mass transport |
|---|---|---|
| Symmetry boundary | Axial Symmetry r = 0 | Axial Symmetry r = 0 |
| Substrate wall | No Slip I. u = 0 | Mass Flux (Reaction Rate Ri) |
| Inlet | Normal Inflow Velocity: u = −uinn | Feed Composition Ci = Ci,in |
| Outlet | Ambient Pressure, No Viscous Stress p = 101323 Pa | Convective Flux (No Diffusion in the Normal Direction) |
| Boundary | Momentum Transport | Mass transport | Heat transport |
|---|---|---|---|
| Symmetry boundary | Axial Symmetry r = 0 | Axial Symmetry r = 0 | Axial Symmetry r = 0 |
| Substrate wall | No Slip II. u = 0 | Mass Flux (Reaction Rate Ri) | Heat flux |
| Inlet | Normal Inflow Velocity: u = –uinn | Feed Composition Ci = Ci,in | Feed Temperature T = Tin |
| Outlet | Ambient Pressure, No Viscous Stress p = 101323 Pa | Convective Flux (No Diffusion in the Normal Direction) | Convective Flux (No Conduction in the Normal Direction) |
| Inlet Temperatures (°C) | 200, 300, 400 and 500 |
| Velocities uin (m/s) | 0.028–7.2 |
| Inlet Compositions | Rich, Lean |
| Cell length (cm) | 1–12 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Templis, C.C.; Papayannakos, N.G. Mass and Heat Transfer Coefficients in Automotive Exhaust Catalytic Converter Channels. Catalysts 2019, 9, 507. https://doi.org/10.3390/catal9060507
Templis CC, Papayannakos NG. Mass and Heat Transfer Coefficients in Automotive Exhaust Catalytic Converter Channels. Catalysts. 2019; 9(6):507. https://doi.org/10.3390/catal9060507
Chicago/Turabian StyleTemplis, Chrysovalantis C., and Nikos G. Papayannakos. 2019. "Mass and Heat Transfer Coefficients in Automotive Exhaust Catalytic Converter Channels" Catalysts 9, no. 6: 507. https://doi.org/10.3390/catal9060507





