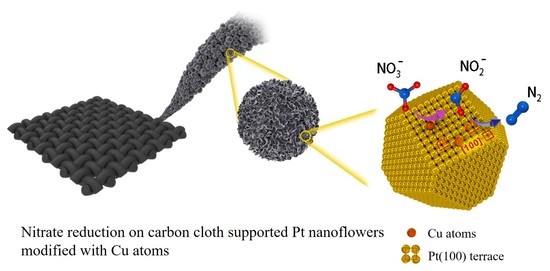
Cu Modified Pt Nanoflowers with Preferential (100) Surfaces for Selective Electroreduction of Nitrate
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterizations of Pt NFs/CC and Cu/Pt NFs/CC
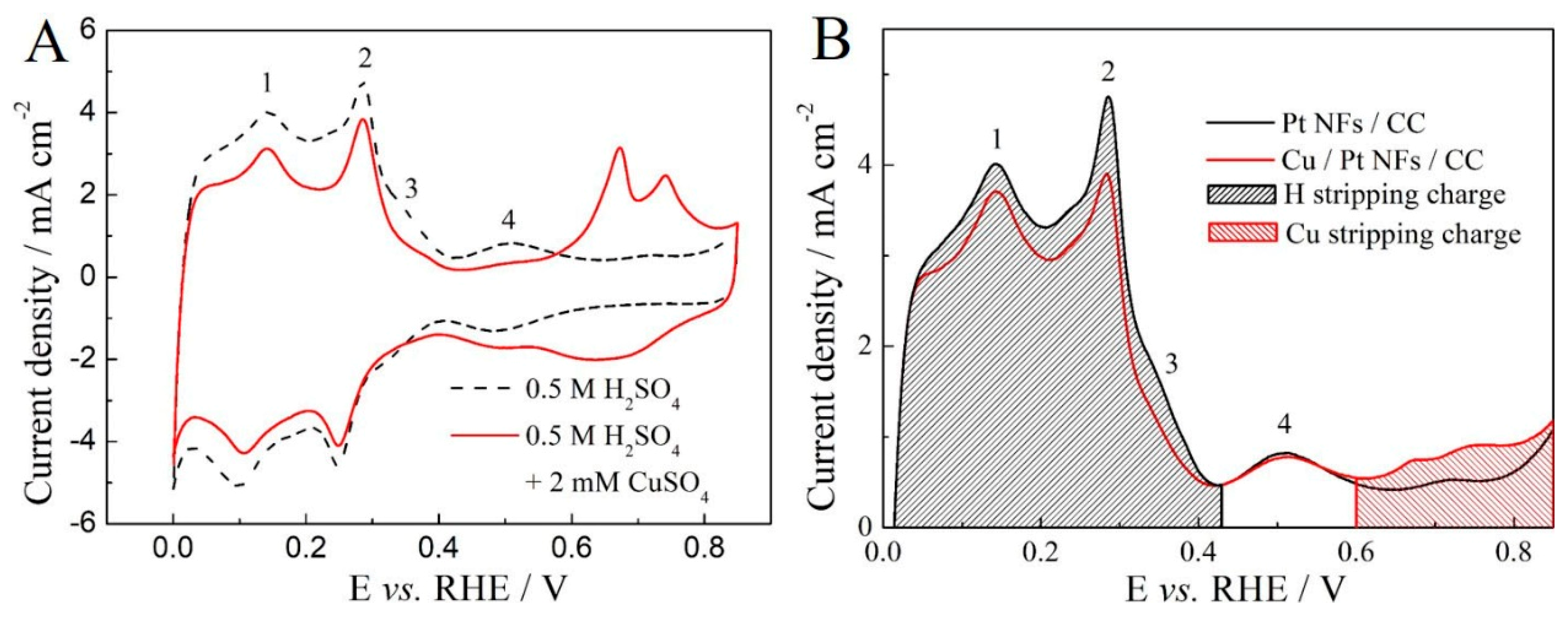
2.2. Assessment of Cu Sites and Coverage on Cu/Pt NFs/CC
2.3. Nitrate Reduction on Cu/Pt NFs/CC
3. Materials and Methods
3.1. Materials
3.2. Preparation of Cu/Pt NFs/CC
3.3. Characterizations and Electrochemical Measurements
3.4. Product Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Duca, M.; Koper, M.T.M. Powering denitrification: The perspectives of electrocatalytic nitrate reduction. Energy Environ. Sci. 2012, 5, 9726–9742. [Google Scholar] [CrossRef]
- Kuang, P.; Natsui, K.; Einaga, Y. Comparison of performance between boron-doped diamond and copper electrodes for selective nitrogen gas formation by the electrochemical reduction of nitrate. Chemosphere 2018, 210, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Ghafari, S.; Hasan, M.; Aroua, M.K. Bio-electrochemical removal of nitrate from water and wastewater-A review. Bioresour. Technol. 2008, 99, 3965–3974. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Segura, S.; Lanzarini-Lopes, M.; Hristovski, K.; Westerhoff, P. Electrocatalytic reduction of nitrate: Fundamentals to full-scale water treatment applications. Appl. Catal. B Environ. 2018, 236, 546–568. [Google Scholar] [CrossRef]
- Katsounaros, I.; Kyriacou, G. Influence of nitrate concentration on its electrochemical reduction on tin cathode: Identification of reaction intermediates. Electrochim. Acta 2008, 53, 5477–5484. [Google Scholar] [CrossRef]
- de Vooys, A.C.A.; van Santen, R.A.; van Veen, J.A.R. Electrocatalytic reduction of NO3− on palladium/copper electrodes. J. Mol. Catal. A Chem. 2000, 154, 203–215. [Google Scholar] [CrossRef]
- Duca, M.; Cucarella, M.O.; Rodriguez, P.; Koper, M.T.M. Direct reduction of nitrite to N2 on a Pt(100) electrode in alkaline media. J. Am. Chem. Soc. 2010, 132, 18042–18044. [Google Scholar] [CrossRef]
- Duca, M.; Figueiredo, M.C.; Climent, V.; Rodriguez, P.; Feliu, J.M.; Koper, M.T.M. Selective catalytic reduction at quasi-perfect Pt(100) domains: A universal low-temperature pathway from nitrite to N2. J. Am. Chem. Soc. 2011, 133, 10928–10939. [Google Scholar] [CrossRef] [PubMed]
- de Groot, M.T.; Koper, M.T.M. The influence of nitrate concentration and acidity on the electrocatalytic reduction of nitrate on platinum. J. Electroanal. Chem. 2004, 562, 81–94. [Google Scholar] [CrossRef]
- Rosca, V.; Duca, M.; de Groot, M.T.; Koper, M.T.M. Nitrogen cycle electrocatalysis. Chem. Rev. 2009, 109, 2209–2244. [Google Scholar] [CrossRef]
- Duca, M.; Sacre, N.; Wang, A.; Garbarino, S.; Guay, D. Enhanced electrocatalytic nitrate reduction by preferentially-oriented (100) PtRh and PtIr alloys: The hidden treasures of the ‘miscibility gap’. Appl. Catal. B Environ. 2018, 221, 86–96. [Google Scholar] [CrossRef]
- Duca, M.; Kightley, J.; Garbarino, S.; Guay, D. The art of decoration: Rhodium-modified platinum films with preferential (100) orientation as electrocatalysts for nitrate reduction and dimethyl ether oxidation. J. Phys. Chem. C 2017, 121, 15233–15247. [Google Scholar] [CrossRef]
- Ehrenburg, M.R.; Danilov, A.I.; Botryakova, I.G.; Molodkina, E.B.; Rudnev, A.V. Electroreduction of nitrate anions on cubic and polyoriented platinum nanoparticles modified by copper adatoms. J. Electroanal. Chem. 2017, 802, 109–117. [Google Scholar] [CrossRef]
- Katsounaros, I.; Figueiredo, M.C.; Chen, X.T.; Calle-Vallejo, F.; Koper, M.T.M. Interconversions of nitrogen-containing species on Pt(100) and Pt(111) electrodes in acidic solutions containing nitrate. Electrochim. Acta 2018, 271, 77–83. [Google Scholar] [CrossRef]
- Molodkina, E.B.; Danilov, A.I.; Ehrenburg, M.R.; Feliu, J.M. Regularities of nitrate electroreduction on Pt(S)[n(100)x(110)] stepped platinum single crystals modified by copper adatoms. Electrochim. Acta 2018, 278, 165–175. [Google Scholar] [CrossRef]
- Kato, M.; Okui, M.; Taguchi, S.; Yagi, I. Electrocatalytic nitrate reduction on well-defined surfaces of tin-modified platinum, palladium and platinum-palladium single crystalline electrodes in acidic and neutral media. J. Electroanal. Chem. 2017, 800, 46–53. [Google Scholar] [CrossRef]
- Souza-Garcia, J.; Ticianelli, E.A.; Climent, V.; Feliu, J.M. Nitrate reduction on Pt single crystals with Pd multilayer. Electrochim. Acta 2009, 54, 2094–2101. [Google Scholar] [CrossRef]
- Chen, T.; Li, H.J.; Ma, H.Y.; Koper, M.T.M. Surface modification of Pt(100) for electrocatalytic nitrate reduction to dinitrogen in alkaline solution. Langmuir 2015, 31, 3277–3281. [Google Scholar] [CrossRef]
- Duca, M.; van der Klugt, B.; Hasnat, M.A.; Machida, M.; Koper, M.T.M. Electrocatalytic reduction of nitrite on a polycrystalline rhodium electrode. J. Catal. 2010, 275, 61–69. [Google Scholar] [CrossRef]
- Reyter, D.; Belanger, D.; Roue, L. Study of the electroreduction of nitrate on copper in alkaline solution. Electrochim. Acta 2008, 53, 5977–5984. [Google Scholar] [CrossRef]
- Duca, M.; Rodriguez, P.; Yanson, A.I.; Koper, M.T.M. Selective electrocatalysis on platinum nanoparticles with preferential (100) orientation prepared by cathodic corrosion. Top. Catal. 2014, 57, 255–264. [Google Scholar] [CrossRef]
- Wang, Q.F.; Zhao, X.B.; Zhang, J.F.; Zhang, X.W. Investigation of nitrate reduction on polycrystalline Pt nanoparticles with controlled crystal plane. J. Electroanal. Chem. 2015, 755, 210–214. [Google Scholar] [CrossRef]
- Zhang, Y.; Shao, Z.W.; Shen, Q.; Li, M.Y.; Xu, L.; Luo, Z.M. Aqueous preparation of platinum nanoflowers on three-dimensional graphene for efficient methanol oxidation. Catalysts 2018, 8, 519. [Google Scholar] [CrossRef]
- Roy, C.; Deschamps, J.; Martin, M.H.; Bertin, E.; Reyter, D.; Garbarno, S.; Roue, L.; Guay, D. Identification of Cu surface active sites for a complete nitrate-to-nitrite conversion with nanostructured catalysts. Appl. Catal. B Environ. 2016, 187, 399–407. [Google Scholar] [CrossRef]
- Ponrouch, A.; Garbarino, S.; Bertin, E.; Andrei, C.; Botton, G.A.; Guay, D. Highly porous and preferentially oriented {100} platinum nanowires and thin films. Adv. Funct. Mater. 2012, 22, 4172–4181. [Google Scholar] [CrossRef]
- Solla-Gullón, J.; Rodríguez, P.; Herrero, E.; Aldaz, A.; Feliu, J.M. Surface characterization of platinum electrodes. Phys. Chem. Chem. Phys. 2008, 10, 1359–1373. [Google Scholar] [CrossRef] [PubMed]
- Roy, C.; Bertin, E.; Martin, M.H.; Garbarino, S.; Guay, D. Hydrazine oxidation at porous and preferentially oriented {100} Pt thin films. Electrocatalysis 2013, 4, 76–84. [Google Scholar] [CrossRef]
- Buller, L.J.; Herrero, E.; Gómez, R.; Feliu, J.M.; Abruna, H.D. Induced adsorption of sulfate/bisulfate anions by submonolayer amounts of copper on deliberately stepped Pt surfaces. J. Chem. Soc. Faraday Trans. 1996, 92, 3757–3762. [Google Scholar] [CrossRef]
- Nishihara, C.; Nozoye, H. Underpotential deposition of copper on Pt(S)-[n(111) × (100)] electrodes in sulfuric acid solution. J. Electroanal. Chem. 1995, 386, 75–82. [Google Scholar] [CrossRef]
- Francke, R.; Climent, V.; Baltruschat, H.; Feliu, J.M. Electrochemical deposition of copper on stepped platinum surfaces in the [0 1 1¯] zone vicinal to the (1 0 0) plane. J. Electroanal. Chem. 2008, 624, 228–240. [Google Scholar] [CrossRef]
- Rudnev, A.V.; Molodkina, E.B.; Ehrenburg, M.R.; Fedorov, R.G.; Danilov, A.I.; Polukarov, Y.M.; Feliu, J.M. Methodical aspects of studying the electroreduction of nitrate on modified single crystal Pt(hkl) + Cu electrodes. Russ. J. Electrochem. 2009, 45, 1052–1063. [Google Scholar] [CrossRef]
- Dima, G.E.; de Vooys, A.C.A.; Koper, M.T.M. Electrocatalytic reduction of nitrate at low concentration on coinage and transition-metal electrodes in acid solutions. J. Electroanal. Chem. 2003, 554, 15–23. [Google Scholar] [CrossRef]
- Yang, J.; Sebastian, P.; Duca, M.; Hoogenboom, T.; Koper, M.T.M. pH dependence of the electroreduction of nitrate on Rh and Pt polycrystalline electrodes. Chem. Commun. 2014, 50, 2148–2151. [Google Scholar] [CrossRef] [PubMed]
- Reyter, D.; Odziemkowski, M.; Bélanger, D.; Roué, L. Electrochemically activated copper electrodes: Surface characterization, electrochemical behavior, and properties for the electroreduction of nitrate. J. Electrochem. Soc. 2007, 154, K36–K44. [Google Scholar] [CrossRef]
- Pérez-Gallent, E.; Figueiredo, M.C.; Katsounaros, I.; Koper, M.T.M. Electrocatalytic reduction of nitrate on copper single crystals in acidic and alkaline solutions. Electrochim. Acta 2017, 227, 77–84. [Google Scholar] [CrossRef]
- Reyter, D.; Chamoulaud, G.; Bélanger, D.; Roué, L. Electrocatalytic reduction of nitrate on copper electrodes prepared by high-energy ball milling. J. Electroanal. Chem. 2006, 596, 13–24. [Google Scholar] [CrossRef]
- Öznülüer, T.; Özdurak, B.; Öztürk Doğan, H. Electrochemical reduction of nitrate on graphene modified copper electrodes in alkaline media. J. Electroanal. Chem. 2013, 699, 1–5. [Google Scholar] [CrossRef]
- Katsounaros, I.; Figueiredo, M.; Calle-Vallejo, F.; Li, H.J.; Gewirth, A.; Markovic, N.; Koper, M.T.M. On the mechanism of the electrochemical conversion of ammonia to dinitrogen on Pt(1 0 0) in alkaline environment. J. Catal. 2018, 359, 82–91. [Google Scholar] [CrossRef] [Green Version]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Li, Y.; Li, L.; Zhao, Y.; Shi, S.; Jiang, R.; Ma, H. Cu Modified Pt Nanoflowers with Preferential (100) Surfaces for Selective Electroreduction of Nitrate. Catalysts 2019, 9, 536. https://doi.org/10.3390/catal9060536
Chen T, Li Y, Li L, Zhao Y, Shi S, Jiang R, Ma H. Cu Modified Pt Nanoflowers with Preferential (100) Surfaces for Selective Electroreduction of Nitrate. Catalysts. 2019; 9(6):536. https://doi.org/10.3390/catal9060536
Chicago/Turabian StyleChen, Ting, Yuxuan Li, Luyan Li, Yanjie Zhao, Shuhua Shi, Rongyan Jiang, and Houyi Ma. 2019. "Cu Modified Pt Nanoflowers with Preferential (100) Surfaces for Selective Electroreduction of Nitrate" Catalysts 9, no. 6: 536. https://doi.org/10.3390/catal9060536
APA StyleChen, T., Li, Y., Li, L., Zhao, Y., Shi, S., Jiang, R., & Ma, H. (2019). Cu Modified Pt Nanoflowers with Preferential (100) Surfaces for Selective Electroreduction of Nitrate. Catalysts, 9(6), 536. https://doi.org/10.3390/catal9060536