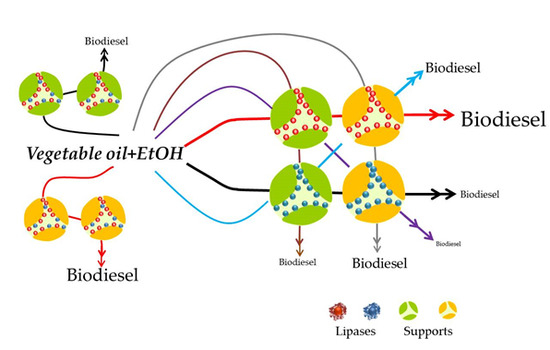
Novel Combi-lipase Systems for Fatty Acid Ethyl Esters Production
Abstract
1. Introduction
2. Results and Discussion
2.1. Immobilization of Lipases on Selected Supports and EE Production Activity
2.2. Effect of Protein Loading in Selected Derivatives
2.3. Composition Effects on EE Production with CL, semiCL and coCL
2.3.1. Semi Combi-lipases (semiCL): TLL-LW/TLL-PU and RML-LW/RML-PU
2.3.2. Combi-lipases Based on Mono-lipasic Derivatives Mixtures (CL)
2.3.3. Co-Immobilized Lipases (coCL)
2.3.4. Application of an Adjusted Kinetic Model
2.4. Reuse of Selected CL
3. Materials and Methods
3.1. Materials
3.2. Esterase Activity and Protein Determination
3.3. One-Step Solvent-Free Ethyl Biodiesel Production (EE)
3.4. Production of Lipase Derivatives
3.5. Production of Co-Immobilized Lipases (coCL)
3.6. Combi-Lipases (CL) and Combi-Catalysts of the Same Enzyme (semiCL)
3.7. Application of an Adjusted Kinetic Model
3.8. Operational Stability of Selected Derivatives in EE Production
3.9. Stability in Ethanol of Selected Biocatalyst
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alves, J.S.; Vieira, N.S.; Cunha, A.S.; Silva, A.M.; Záchia Ayub, M.A.; Fernandez-Lafuente, R.; Rodrigues, R.C. Combi-lipase for heterogeneous substrates: A new approach for hydrolysis of soybean oil using mixtures of biocatalysts. RSC Adv. 2014, 4, 6863. [Google Scholar] [CrossRef]
- Poppe, J.K.; Matte, C.R.; do Carmo Ruaro Peralba, M.; Fernandez-Lafuente, R.; Rodrigues, R.C.; Ayub, M.A.Z. Optimization of ethyl ester production from olive and palm oils using mixtures of immobilized lipases. Appl. Catal. A Gen. 2015, 490, 50–56. [Google Scholar] [CrossRef]
- Poppe, J.K.; Matte, C.R.; Fernandez-Lafuente, R.; Rodrigues, R.C.; Ayub, M.A.Z. Transesterification of Waste Frying Oil and Soybean Oil by Combi-lipases Under Ultrasound-Assisted Reactions. Appl. Biochem. Biotechnol. 2018, 186, 576–589. [Google Scholar] [CrossRef]
- Poppe, J.K.; Matte, C.R.; de Freitas, V.O.; Fernandez-Lafuente, R.; Rodrigues, R.C.; Záchia Ayub, M.A. Enzymatic synthesis of ethyl esters from waste oil using mixtures of lipases in a plug-flow packed-bed continuous reactor. Biotechnol. Prog. 2018, 34, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Zhang, F.; Guan, W.; Zuo, J.; Feng, D. Optimisation of combi-lipases from Aspergillus niger for the synergistic and efficient hydrolysis of soybean oil. Anim. Sci. J. 2017, 88, 772–780. [Google Scholar] [CrossRef]
- Fallavena, L.P.; Antunes, F.H.F.; Alves, J.S.; Paludo, N.; Ayub, M.A.Z.; Fernandez-Lafuente, R.; Rodrigues, R.C. Ultrasound technology and molecular sieves improve the thermodynamically controlled esterification of butyric acid mediated by immobilized lipase from Rhizomucor miehei. RSC Adv. 2014, 4, 8675–8681. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzym. Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, A.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef]
- Guisan, J.M. Immobilization of Enzymes and Cells; Humana Press: New York, NY, USA, 2006; ISBN 978-1-58829-290-2. [Google Scholar]
- Lee, J.H.; Kim, S.B.; Yoo, H.Y.; Lee, J.H.; Han, S.O.; Park, C.; Kim, S.W. Co-immobilization of Candida rugosa and Rhyzopus oryzae lipases and biodiesel production. Korean J. Chem. Eng. 2013, 30, 1335–1338. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, S.B.; Yoo, H.Y.; Lee, J.H.; Park, C.; Han, S.O.; Kim, S.W. Kinetic modeling of biodiesel production by mixed immobilized and co-immobilized lipase systems under two pressure conditions. Korean J. Chem. Eng. 2013, 30, 1272–1276. [Google Scholar] [CrossRef]
- Kazenwadel, F.; Franzreb, M.; Rapp, B.E. Synthetic enzyme supercomplexes: Co-immobilization of enzyme cascades. Anal. Methods 2015, 7, 4030–4037. [Google Scholar] [CrossRef]
- Rocha-Martín, J.; de las Rivas, B.; Muñoz, R.; Guisán, J.M.; López-Gallego, F. Rational Co-Immobilization of Bi-Enzyme Cascades on Porous Supports and their Applications in Bio-Redox Reactions with In Situ Recycling of Soluble Cofactors. ChemCatChem 2012, 4, 1279–1288. [Google Scholar] [CrossRef]
- Fedosov, S.N.; Brask, J.; Pedersen, A.K.; Nordblad, M.; Woodley, J.M.; Xu, X. Kinetic model of biodiesel production using immobilized lipase Candida antarctica lipase B. J. Mol. Catal. B Enzym. 2013, 85–86, 156–168. [Google Scholar] [CrossRef]
- Firdaus, M.Y.; Brask, J.; Nielsen, P.M.; Guo, Z.; Fedosov, S. Kinetic model of biodiesel production catalyzed by free liquid lipase from Thermomyces lanuginosus. J. Mol. Catal. B Enzym. 2016, 133, 55–64. [Google Scholar] [CrossRef]
- Godoy, C.A. New Strategy for the Immobilization of Lipases on Glyoxyl–Agarose Supports: Production of Robust Biocatalysts for Natural Oil Transformation. Int. J. Mol. Sci. 2017, 18, 2130. [Google Scholar] [CrossRef]
- Norjannah, B.; Ong, H.C.; Masjuki, H.H.; Juan, J.C.; Chong, W.T. Enzymatic transesterification for biodiesel production: A comprehensive review. RSC Adv. 2016, 6, 60034–60055. [Google Scholar] [CrossRef]
- Fernandez-Lorente, G.; Cabrera, Z.; Godoy, C.; Fernandez-Lafuente, R.; Palomo, J.M.; Guisan, J.M. Interfacially activated lipases against hydrophobic supports: Effect of the support nature on the biocatalytic properties. Process Biochem. 2008, 43, 1061–1067. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Strategies for the one-step immobilization–purification of enzymes as industrial biocatalysts. Biotechnol. Adv. 2015, 33, 435–456. [Google Scholar] [CrossRef]
- Manoel, E.A.; dos Santos, J.C.S.; Freire, D.M.G.; Rueda, N.; Fernandez-Lafuente, R. Immobilization of lipases on hydrophobic supports involves the open form of the enzyme. Enzym. Microb. Technol. 2015, 71, 53–57. [Google Scholar] [CrossRef]
- Melton, L.; Shahidi, F.; Varelis, P. Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 978-0-12-814045-1. [Google Scholar]
- Rodrigues, R.C.; Fernandez-Lafuente, R. Lipase from Rhizomucor miehei as an industrial biocatalyst in chemical process. J. Mol. Catal. B Enzym. 2010, 64, 1–22. [Google Scholar] [CrossRef]
- Marciello, M.; Filice, M.; Palomo, J.M. Different strategies to enhance the activity of lipase catalysts. Catal. Sci. Technol. 2012, 2, 1531–1543. [Google Scholar] [CrossRef]
- Anandakrishnan, R.; Aguilar, B.; Onufriev, A.V. H++ 3.0: Automating pK prediction and the preparation of biomolecular structures for atomistic molecular modeling and simulations. Nucleic Acids Res. 2012, 40, W537–W541. [Google Scholar] [CrossRef] [PubMed]
- Tacias-Pascacio, V.G.; Virgen-Ortíz, J.J.; Jiménez-Pérez, M.; Yates, M.; Torrestiana-Sanchez, B.; Rosales-Quintero, A.; Fernandez-Lafuente, R. Evaluation of different lipase biocatalysts in the production of biodiesel from used cooking oil: Critical role of the immobilization support. Fuel 2017, 200, 1–10. [Google Scholar] [CrossRef]
- Amini, Z.; Ilham, Z.; Ong, H.C.; Mazaheri, H.; Chen, W.-H. State of the art and prospective of lipase-catalyzed transesterification reaction for biodiesel production. Energy Convers. Manag. 2017, 141, 339–353. [Google Scholar] [CrossRef]
- Mangas-Sánchez, J.; Adlercreutz, P. Highly efficient enzymatic biodiesel production promoted by particle-induced emulsification. Biotechnol. Biofuels 2015, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- AL-Muftah, A.E.; Abu-Reesh, I.M. Effects of internal mass transfer and product inhibition on a simulated immobilized enzyme-catalyzed reactor for lactose hydrolysis. Biochem. Eng. J. 2005, 23, 139–153. [Google Scholar] [CrossRef]
- Abreu Silveira, E.; Moreno-Perez, S.; Basso, A.; Serban, S.; Pestana-Mamede, R.; Tardioli, P.W.; Sanchez-Farinas, C.; Castejon, N.; Fernandez-Lorente, G.; Rocha-Martin, J.; et al. Biocatalyst engineering of Thermomyces lanuginosus lipase adsorbed on hydrophobic supports: Modulation of enzyme properties for ethanolysis of oil in solvent-free systems. J. Biotechnol. 2019, 289, 126–134. [Google Scholar] [CrossRef]
- Utsugi, A.; Kanda, A.; Hara, S. Lipase Specificity in the Transacylation of Triacylglycerin. J. Oleo Sci. 2009, 58, 123–132. [Google Scholar] [CrossRef]
- Caballero, E.; Soto, C.; Olivares, A.; Altamirano, C. Potential Use of Avocado Oil on Structured Lipids MLM-Type Production Catalysed by Commercial Immobilised Lipases. PLoS ONE 2014, 9, e107749. [Google Scholar] [CrossRef]
- Du, W.; Xu, Y.-Y.; Liu, D.-H.; Li, Z.-B. Study on acyl migration in immobilized lipozyme TL-catalyzed transesterification of soybean oil for biodiesel production. J. Mol. Catal. B Enzym. 2005, 37, 68–71. [Google Scholar] [CrossRef]
- Šinkūnienė, D.; Adlercreutz, P. Effects of Regioselectivity and Lipid Class Specificity of Lipases on Transesterification, Exemplified by Biodiesel Production. J. Am. Oil Chem. Soc. 2014, 91, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Mathew, S.; Obbard, J.P. Biodiesel fuel production via transesterification of oils using lipase biocatalyst. GCB Bioenergy 2009, 1, 115–125. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Peirce, S.; Torrestiana-Sanchez, B.; Yates, M.; Rosales-Quintero, A.; Virgen-Ortíz, J.J.; Fernandez-Lafuente, R. Evaluation of different commercial hydrophobic supports for the immobilization of lipases: Tuning their stability, activity and specificity. RSC Adv. 2016, 6, 100281–100294. [Google Scholar] [CrossRef]
- Li, L.; Du, W.; Liu, D.; Wang, L.; Li, Z. Lipase-catalyzed transesterification of rapeseed oils for biodiesel production with a novel organic solvent as the reaction medium. J. Mol. Catal. B Enzym. 2006, 43, 58–62. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, H.; Yan, Y. Optimization of Lipase-Catalyzed Transesterification of Lard for Biodiesel Production Using Response Surface Methodology. Appl. Biochem. Biotechnol. 2008, 160, 504–515. [Google Scholar] [CrossRef]
- Liu, W.-H.; Beppu, T.; Arima, K. Effect of Various Inhibitors on Lipase Action of Thermophilic Fungus Humicola lanuginosa S-38. Agric. Biol. Chem. 1973, 37, 2487–2492. [Google Scholar] [CrossRef]
- Mata, T.M.; Andrade, S.; Correia, D.; Matos, E.; Martins, A.A.; Caetano, N.S. Acidity reduction of mammalian fat by enzymatic esterification. Energy Procedia 2017, 136, 290–295. [Google Scholar] [CrossRef]
- Piyatheerawong, W.; Yamane, T.; Nakano, H.; Iwasaki, Y. Enzymatic preparation of enantiomerically pure sn-2,3-diacylglycerols: A stereoselective ethanolysis approach. J. Am. Oil Chem. Soc. 2006, 83, 603–607. [Google Scholar] [CrossRef]
- Babaki, M.; Yousefi, M.; Habibi, Z.; Mohammadi, M. Process optimization for biodiesel production from waste cooking oil using multi-enzyme systems through response surface methodology. Renew. Energy 2017, 105, 465–472. [Google Scholar] [CrossRef]
- Von der Haar, D.; Stäbler, A.; Wichmann, R.; Schweiggert-Weisz, U. Enzymatic esterification of free fatty acids in vegetable oils utilizing different immobilized lipases. Biotechnol. Lett. 2015, 37, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Yancy-Caballero, D.M.; Guirardello, R. Modeling and parameters fitting of chemical and phase equilibria in reactive systems for biodiesel production. Biomass Bioenergy 2015, 81, 544–555. [Google Scholar] [CrossRef]
- Mendoza, L.D.; Rodriguez, J.A.; Leclaire, J.; Buono, G.; Fotiadu, F.; Carrière, F.; Abousalham, A. An ultraviolet spectrophotometric assay for the screening of sn-2-specific lipases using 1,3-O-dioleoyl-2-O-α-eleostearoyl-sn-glycerol as substrate. J. Lipid Res. 2012, 53, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Salley, S.O.; Ng, K.Y.S. Transesterification of Glycerides Using a Heterogeneous Resin Catalyst Combined with a Homogeneous Catalyst. Energy Fuels 2008, 22, 3594–3599. [Google Scholar] [CrossRef]







| Enzyme | Support | Immobilized Protein (mg/g) | Protein Immobilization Yield (%) a | Immobilized Activity (IU p-NPB/g) | Activity Immobilization Yield (%) a |
|---|---|---|---|---|---|
| Thermomyces lanuginosus lipase (TLL) | Lewatit® VP OC 1600 (LW) | 70.40 ± 9.29 | 77.20 ± 7.66 | 0.814 ± 0.045 | 98.15 ± 4.84 |
| Purolite® ECR 1604 (PU) | 19.40 ± 2.36 | 19.55 ± 2.37 | 0.157 ± 0.008 | 89.34 ± 3.19 | |
| Candida antarctica lipase B (CALB) | LW | 69.12 ± 2.74 | 69.34 ± 0.99 | 0.866 ± 0.026 | 94.42 ± 4.58 |
| PU | 3.76 ± 2.74 | 3.80 ± 2.76 | 0.011 ± 0.026 | 1.20 ± 0.27 | |
| Rhizomucor miehei lipase (RML) | LW | 34.80 ± 1.09 | 34.80 ± 1.10 | 0.257 ± 0.010 | 92.90 ± 3.62 |
| PU | 23.66 ± 1.43 | 23.66 ± 1.43 | 0.317 ± 0.068 | 87.99 ± 2.65 | |
| Novozyme® 435 | LW | 87.22 ± 15.86 | - | - | - |
| Enzyme | Support | Specific Production at 6 h (mmol EE/g Derivative-mg Protein) a | EE After 24 h (%) |
|---|---|---|---|
| TLL | LW | 2.79 ± 0.11 38.0 ± 0.35 b | 92.5 ± 3.32 78.2 ± 3.80 b |
| PU | 9.68 ± 0.07 21.7 ± 0.08 b | 68.1 ± 3.52 70.3 ± 3.41 b | |
| CALB | LW | 2.48 ± 0.72 | 47.4 ± 3.60 |
| RML | LW | 7.79 ± 0.11 9.67 ± 0.61 b | 70.7 ± 4.34 68.6 ± 4.05 b |
| PU | 3.90 ± 0.07 6.42 ± 0.61 b | 69.7 ± 4.34 63.0 ± 3.26 b | |
| Novozyme®435 | LW | 3.62 ± 1.53 | 52.7 ± 3.50 |
| Type | Component C1 | Component C2 | Min. CF a and Composition (%C1) | Max. CF a and Composition (%C1) | Max. EE Yield (%) and Composition (% C1) | |
|---|---|---|---|---|---|---|
| semiCL | TLL-LW | TLL-PU | 1.06 (75) | 1.19 (25) | 86.1 (50) | |
| RML-LW | RML-PU | 0.83 (25) | 0.94 (75) | 70.7 (100) | ||
| CL | TLL-LW | CALB-LW | 1.16 (75) | 1.41 (25) | 81.8 (75) | |
| TLL-LW | RML-LW | 1.01 (75) | 1.15 (25) | 82.0 (25) | ||
| TLL-LW | RML-PU | 1.00 (75) | 1.10 (25) | 78.2 (100) | ||
| TLL-PU | RML-PU | 0.80 (75) | 1.18 (50) | 78.5 (50) | ||
| TLL-PU | RML-LW | 0.95 (75) | 1.07 (25) | 73.7(25) | ||
| TLL-PU | CALB-LW | 1.05 (25) | 1.23 (50) | 81.4 (75) | ||
| RML-LW | CALB-LW | 0.94 (25) | 1.07 (50) | 70.7 (100) | ||
| coCL (co-immobilized) | in LW | TLL | CALB | 1.25 (50) | 1.49 (25) | 89.5 (75) |
| TLL | RML | 1.06 (75) | 1.15 (25) | 80.1 (75) | ||
| in PU | TLL | RML | 1.05 (75) | 1.13 (50) | 75.3 (50) | |
| Type | Component C1 | Component C2 | Max. CF and Composition (%C1) a | Max. %EE Yield and Composition (%C1) b | |||
|---|---|---|---|---|---|---|---|
| Model | Experimental | Model | Experimental | ||||
| semiCL | TLL-LW | TLL-PU | 1.05 (25) | 1.19 (25) | 79.5 (75) | 86.1 (50) | |
| RML-LW | RML-PU | 0.92 (50) | 0.94 (75) | 68.0 (75) | 69.2 (75) | ||
| CL | TLL-LW | CALB-LW | 1.50 (25) | 1.41 (25) | 89.6 (75) | 81.8 (75) | |
| TLL-LW | RML-LW | 1.02 (75) | 1.16 (25) | 76.2 (75) | 82.0 (25) | ||
| TLL-LW | RML-PU | 1.08 50) | 1.10 (25) | 75.0 (75) | 76.2 (50) | ||
| TLL-PU | RML-PU | 1.09 (25) | 1.18 (50) | 69.1 (75) | 78.5 (50) | ||
| TLL-PU | RML-LW | 1.04 (75) | 1.07 (25) | 72.6 (75) | 73.7(25) | ||
| TLL-PU | CALB-LW | 1.23 (50) | 1.19 (75) | 76.9 (75) | 81.4 (75) | ||
| RML-LW | CALB-LW | 1.28 (50) | 1.07 (50) | 74.4 (50) | 67.3(75) | ||
| coCL | in LW | TLL | CALB | 1.50 (25) | 1.49 (25) | 89.6 (75) | 89.5 (75) |
| TLL | RML | 1.02 (75) | 1.15 (25) | 76.2 (75) | 80.1 (75) | ||
| in PU | TLL | RML | 1.09 (25) | 1.13 (50) | 69.1 (75) | 75.3 (50) | |
| RSMD in model value %C1 for CFmax or %EEmax | 29.8 | 26.0 | |||||
| RSMD in model value for CFmax | 0.022 | - | |||||
| RSMD in values for %EEmax | - | 2.28 | |||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toro, E.C.; Rodríguez, D.F.; Morales, N.; García, L.M.; Godoy, C.A. Novel Combi-lipase Systems for Fatty Acid Ethyl Esters Production. Catalysts 2019, 9, 546. https://doi.org/10.3390/catal9060546
Toro EC, Rodríguez DF, Morales N, García LM, Godoy CA. Novel Combi-lipase Systems for Fatty Acid Ethyl Esters Production. Catalysts. 2019; 9(6):546. https://doi.org/10.3390/catal9060546
Chicago/Turabian StyleToro, Esteban C., Diego F. Rodríguez, Nelson Morales, Lina M. García, and César A. Godoy. 2019. "Novel Combi-lipase Systems for Fatty Acid Ethyl Esters Production" Catalysts 9, no. 6: 546. https://doi.org/10.3390/catal9060546
APA StyleToro, E. C., Rodríguez, D. F., Morales, N., García, L. M., & Godoy, C. A. (2019). Novel Combi-lipase Systems for Fatty Acid Ethyl Esters Production. Catalysts, 9(6), 546. https://doi.org/10.3390/catal9060546