Facile Synthesis of a Polycatenane Compound Based on Ag-triazole Complexes and Phosphomolybdic Acid for the Catalytic Epoxidation of Olefins with Molecular Oxygen
Abstract
:1. Introduction
2. Results and Discussions
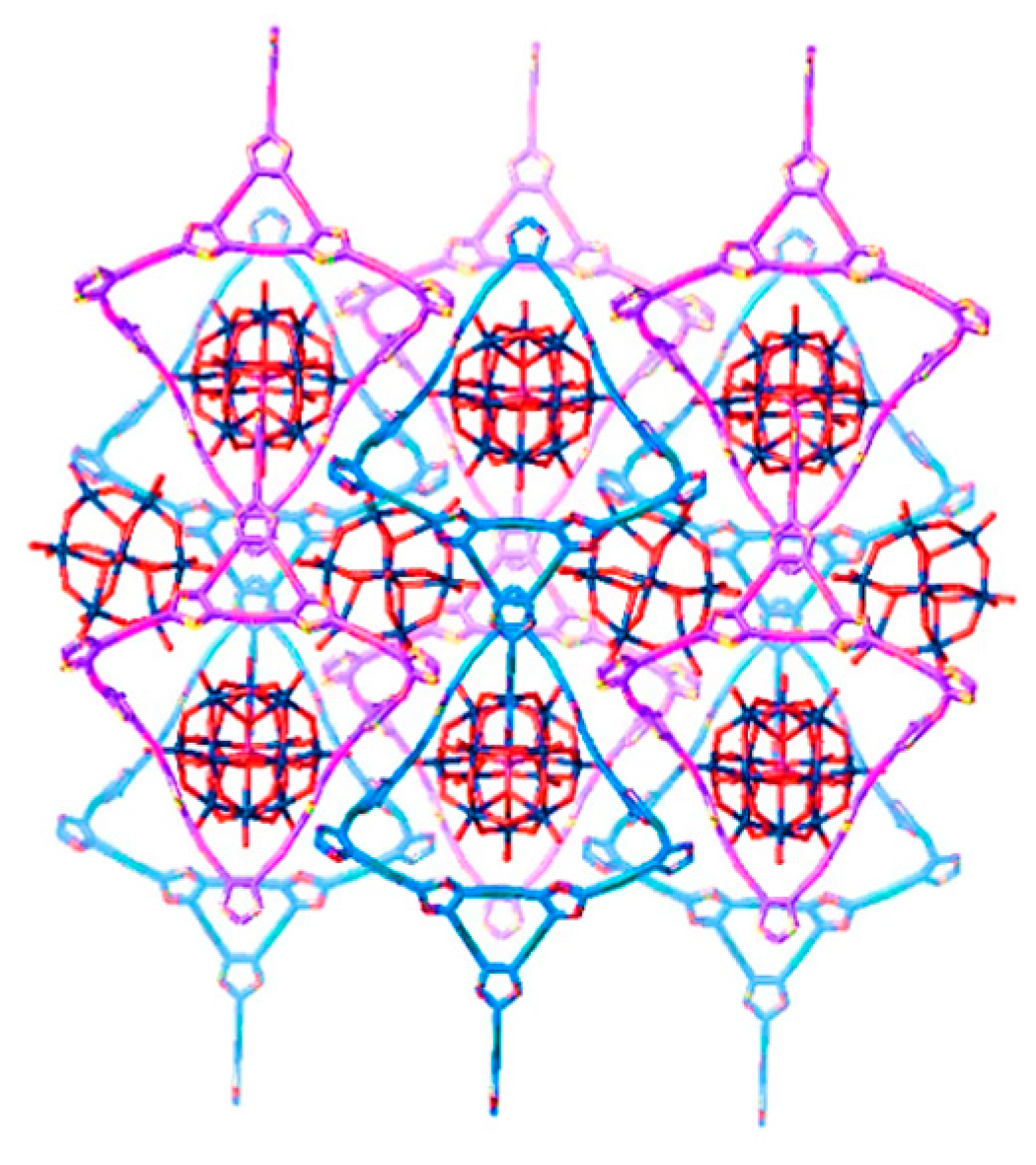
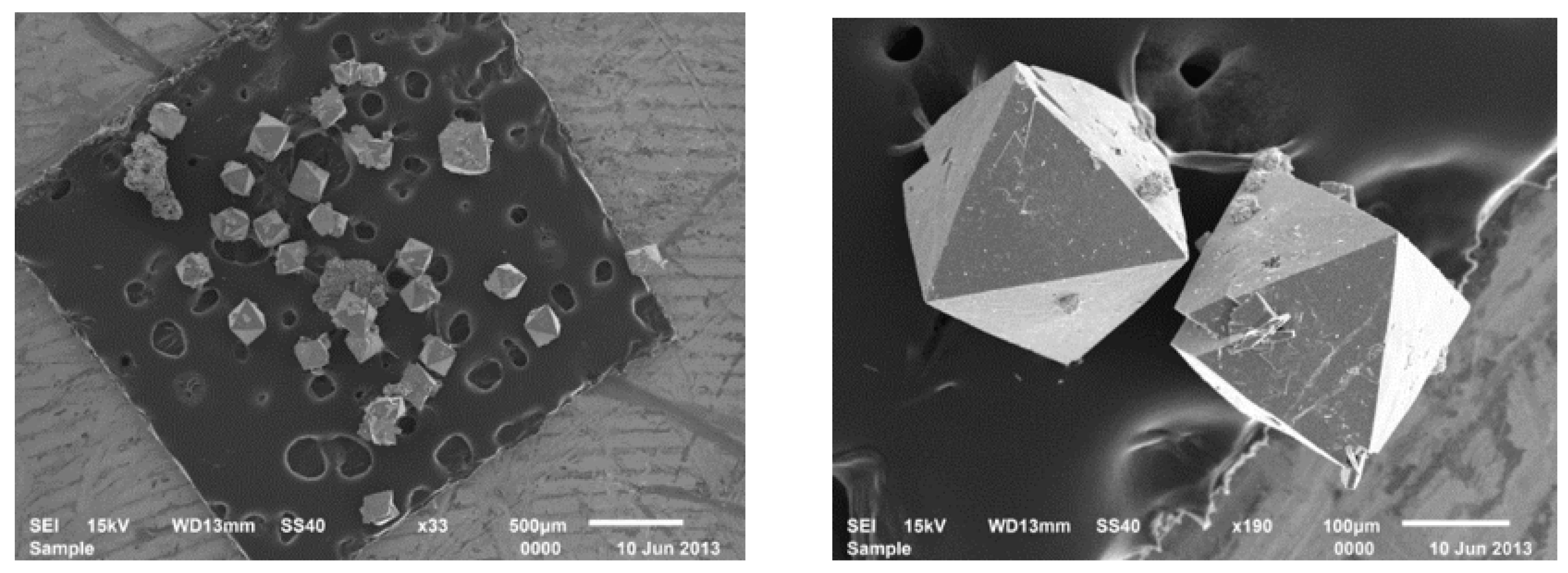
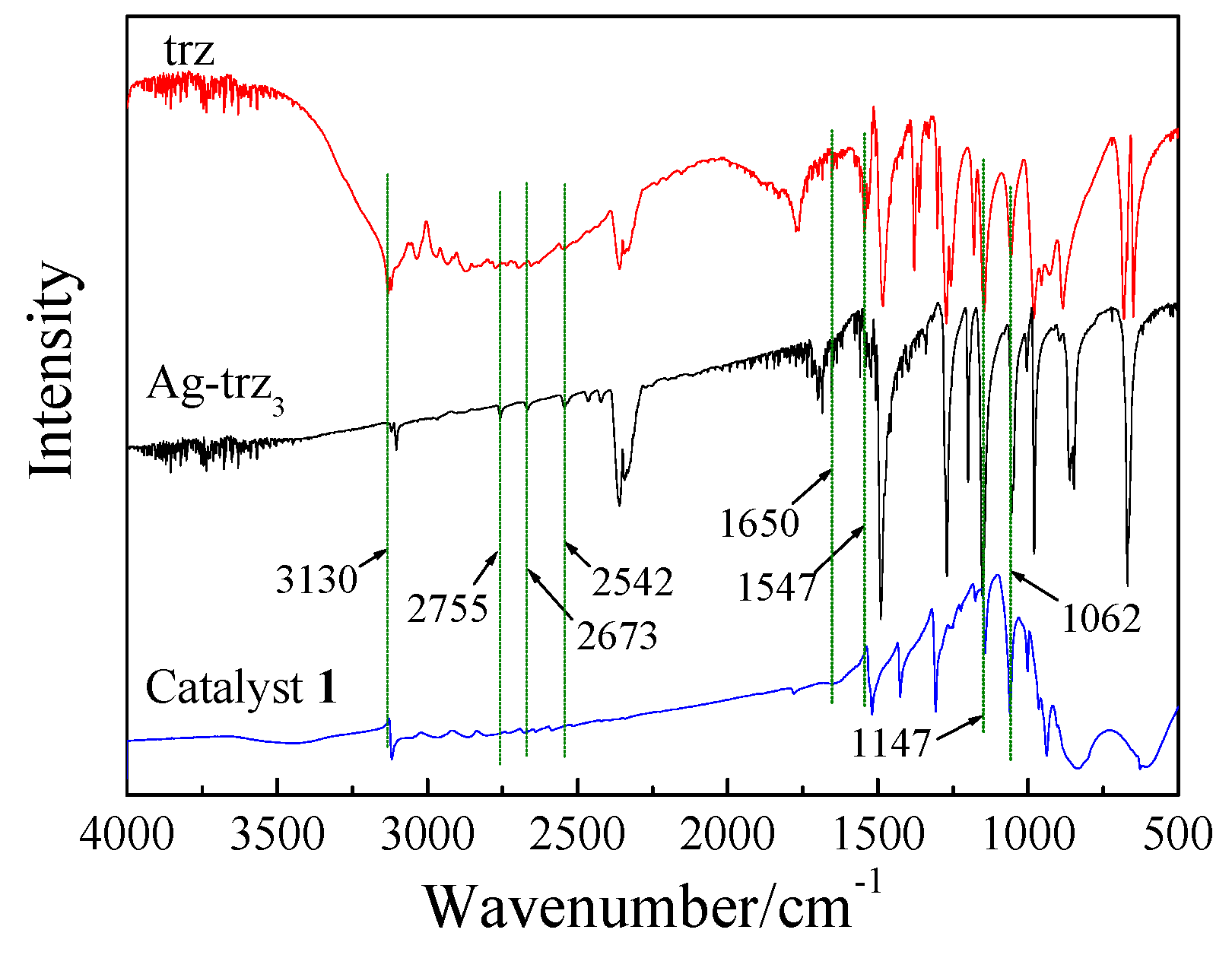
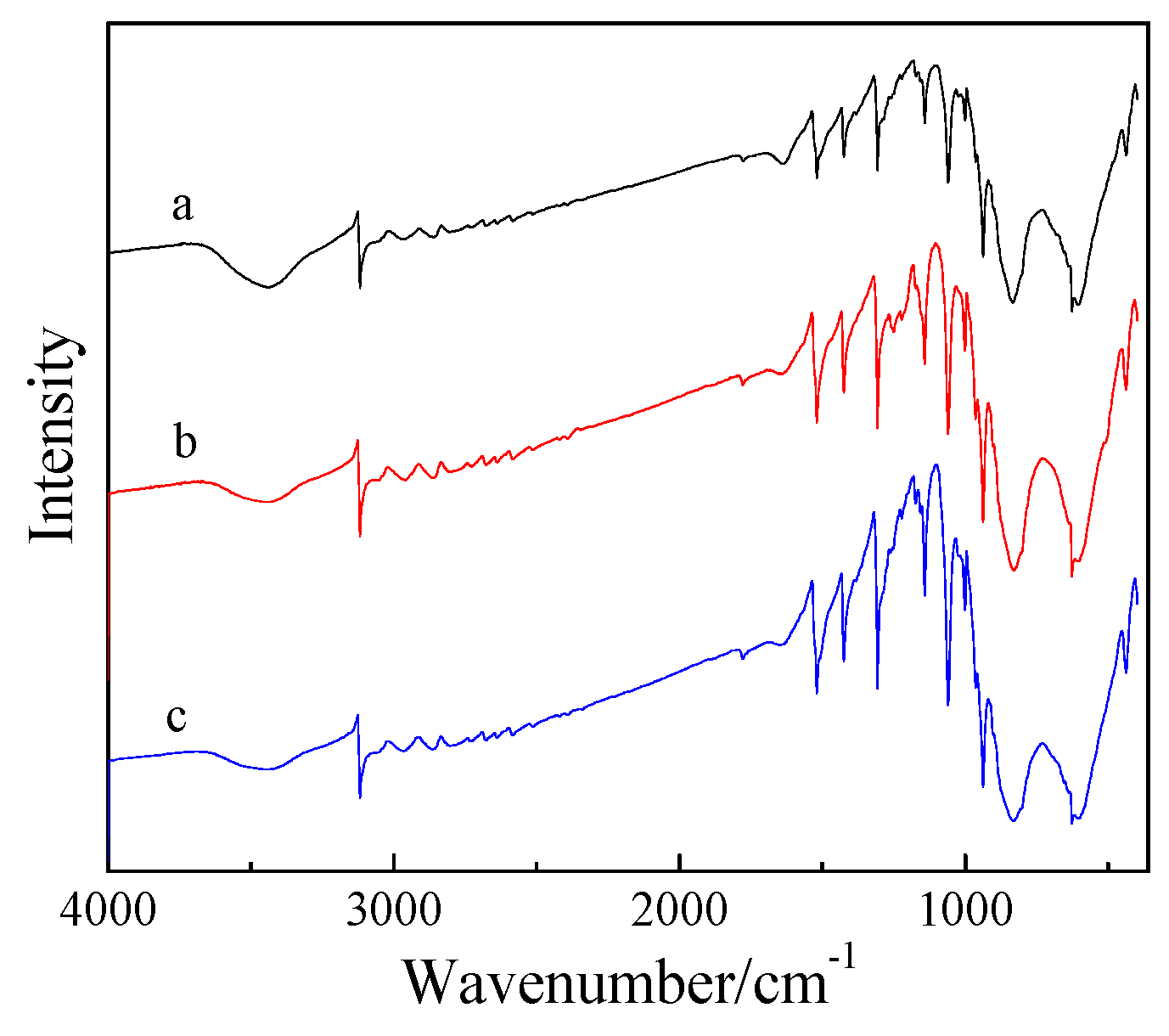
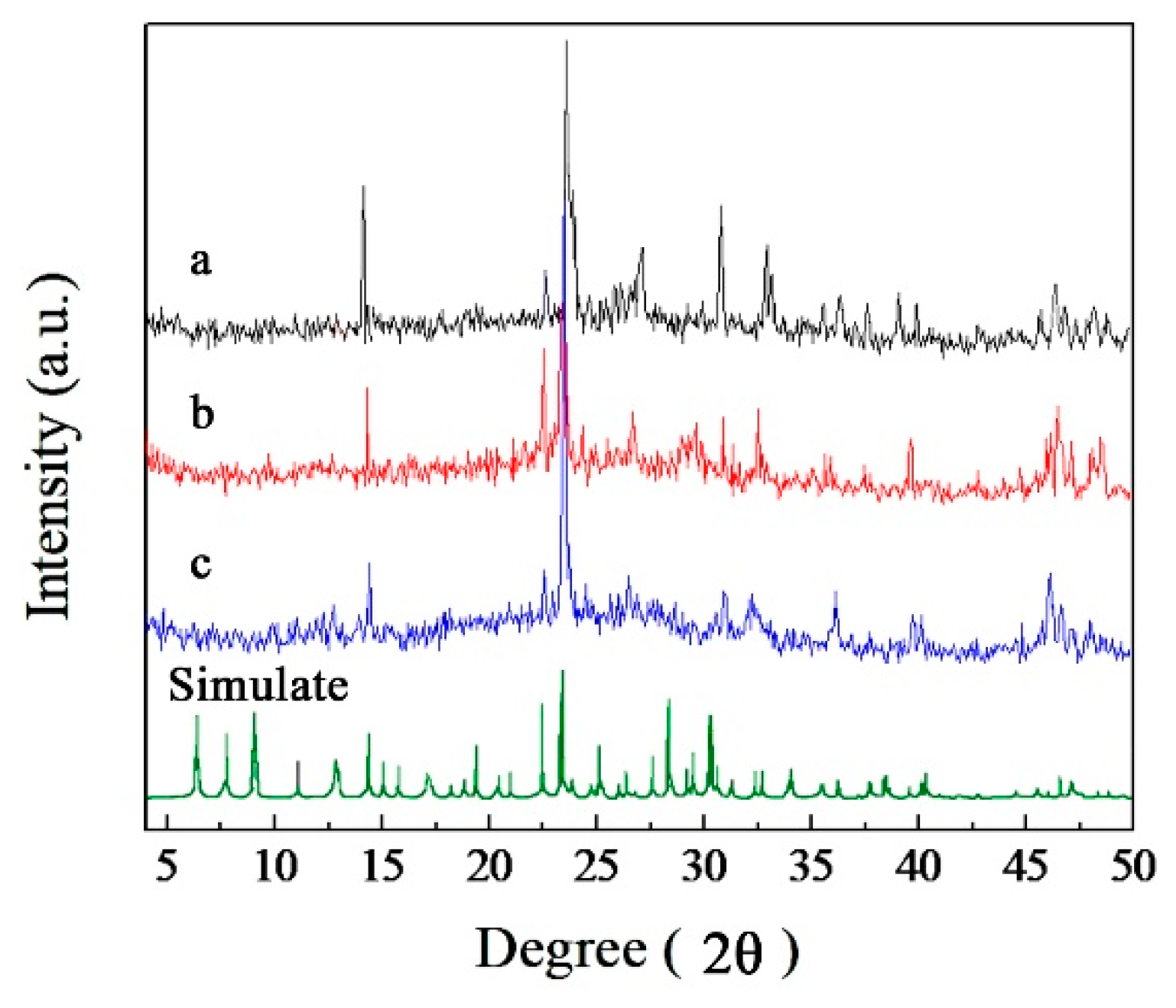
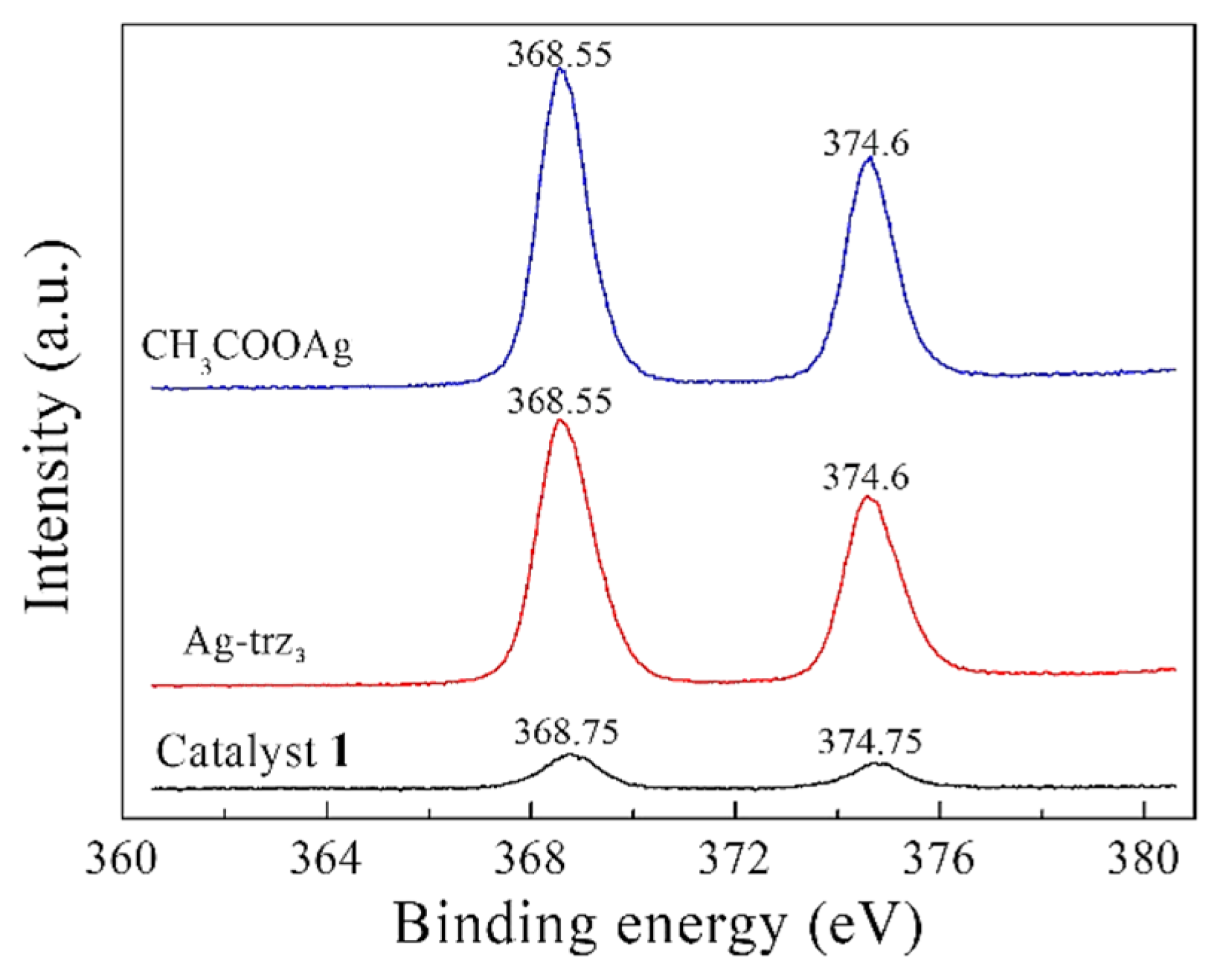
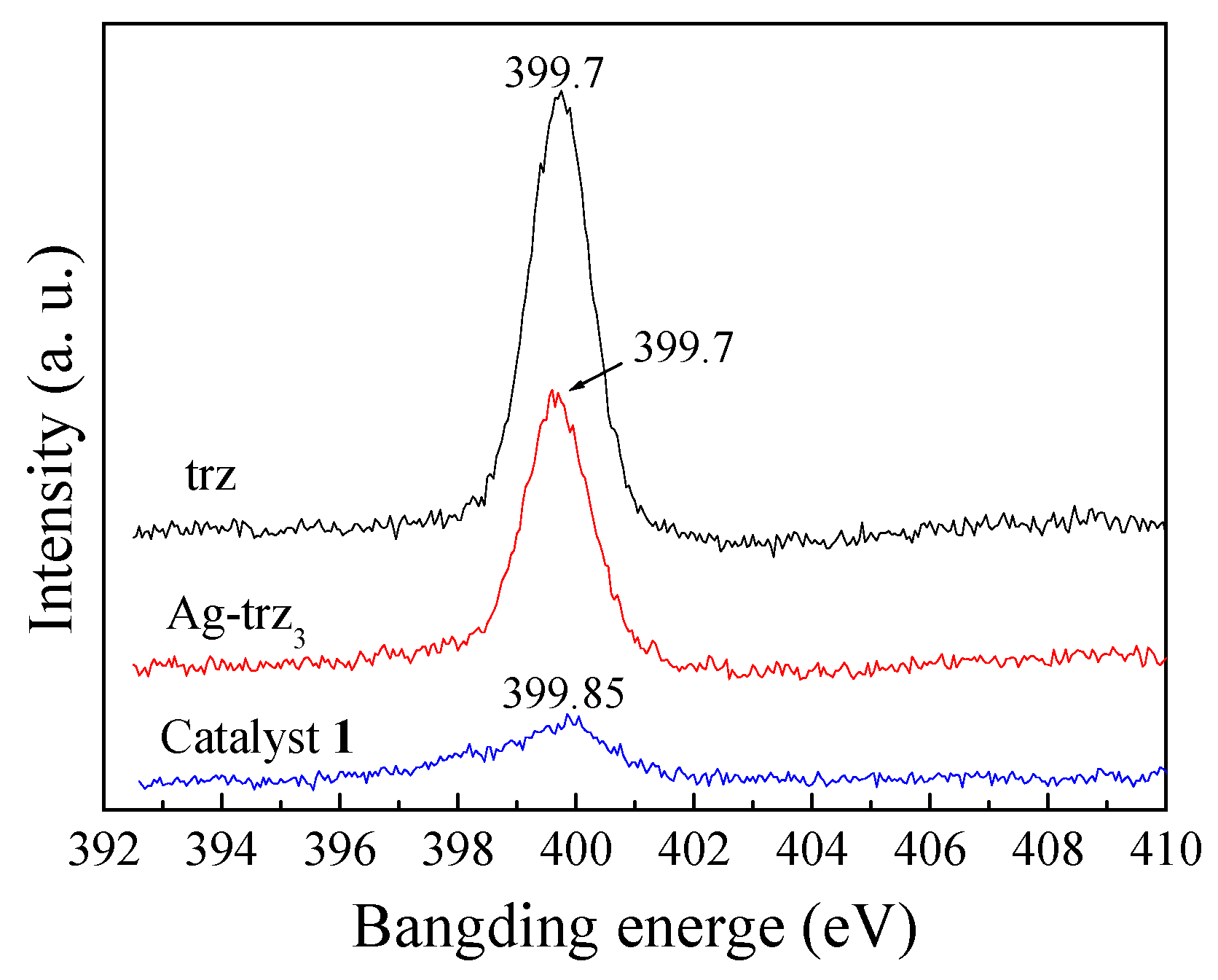
2.1. Synthesis and Structure of the Catalysts
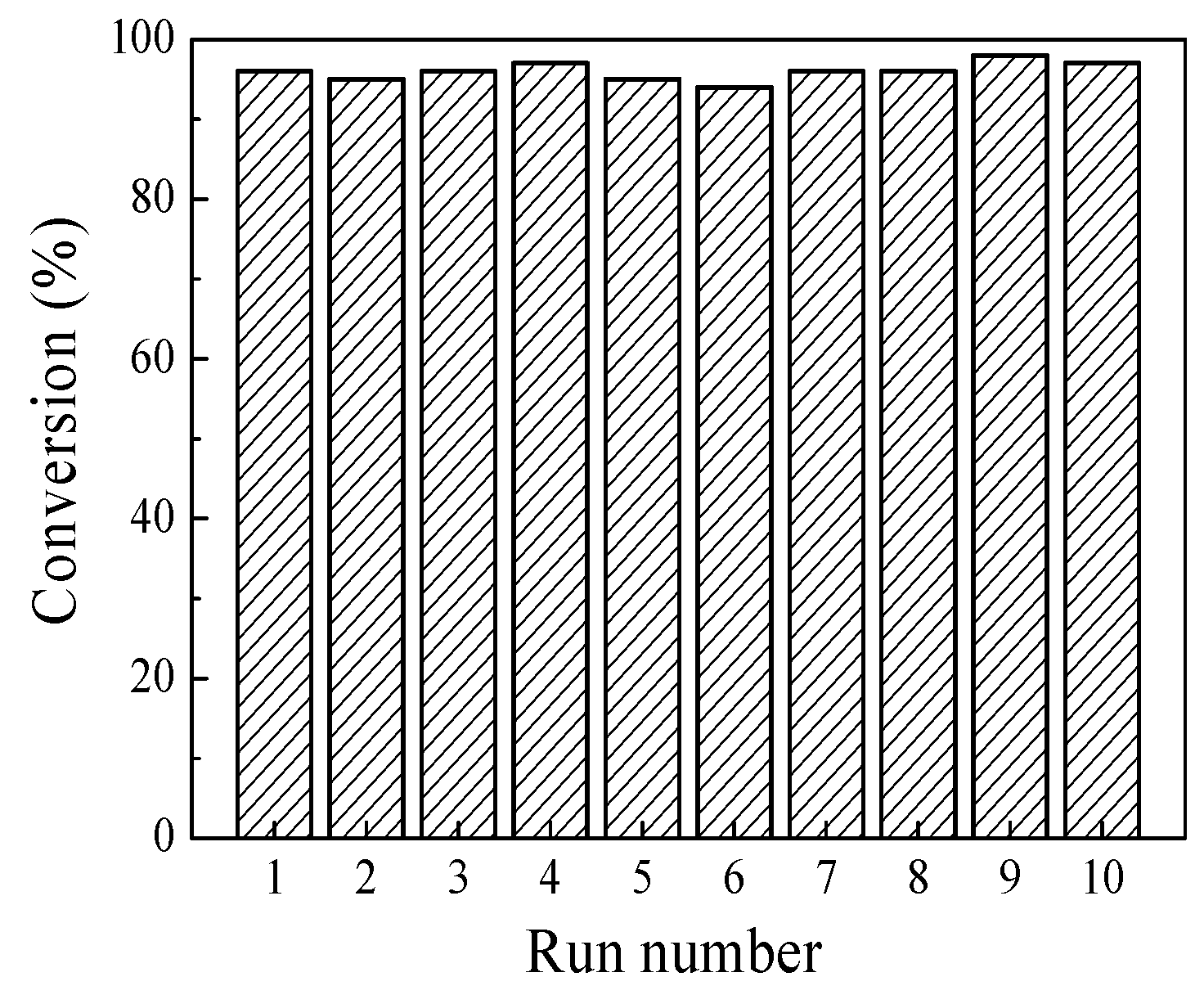
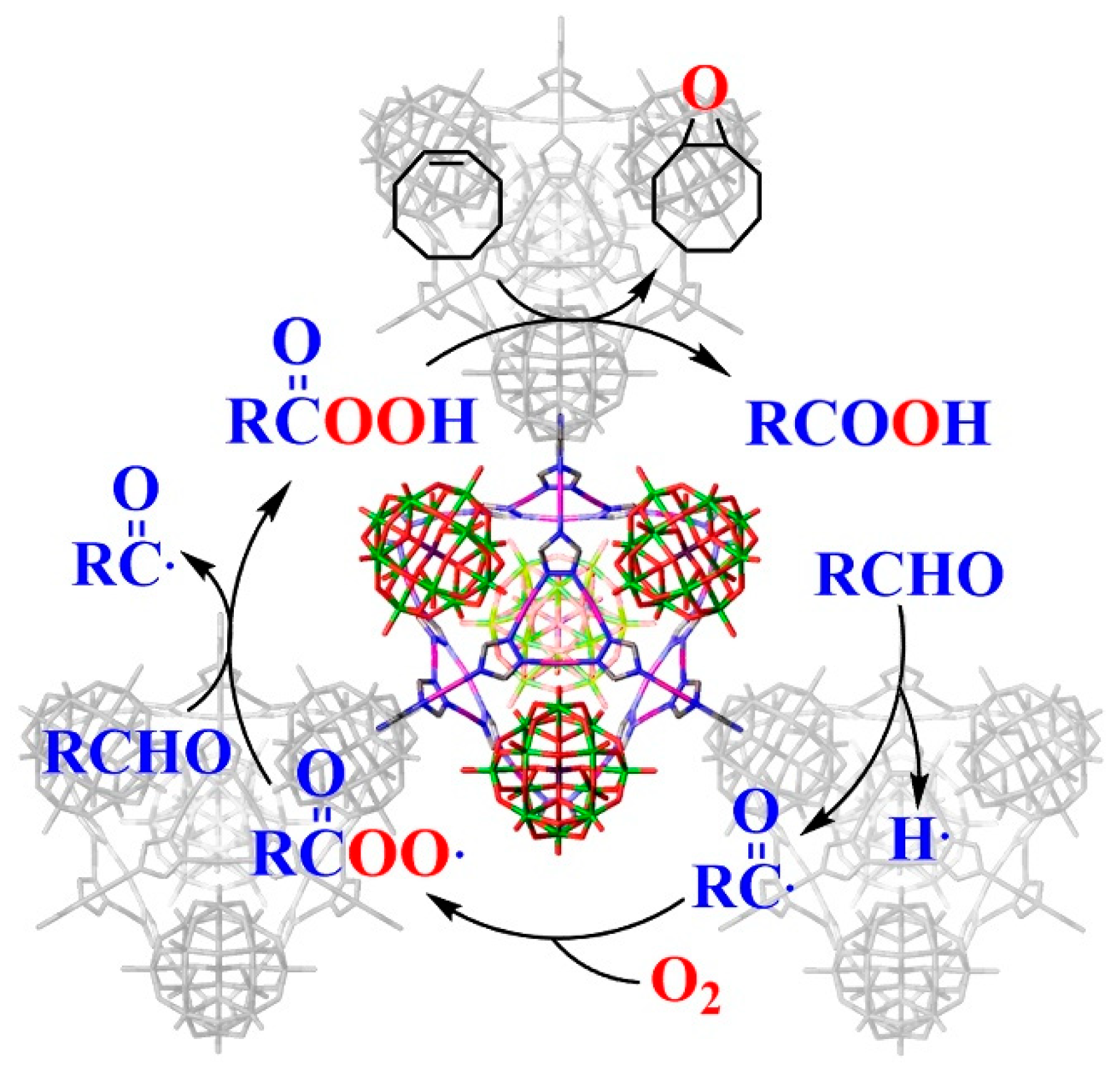
2.2. Catalytic Properties
3. Materials and Methods
3.1. Synthesis of the Catalysts
3.1.1. Synthesis of {[Ag2(trz)2] [Ag24(trz)18]}[PMo12O40]2 (1)
3.1.2. Reference Sample Ag-trz3
3.2. Materials and Methods
3.3. Catalyst Test
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Roy, S.; Vemuri, V.; Maiti, S.; Manoj, K.S.; Subbarao, U.; Peter, S.C. Two Keggin-Based Isostructural POMOF Hybrids: Synthesis, Crystal Structure, and Catalytic Properties. Inorg. Chem. 2018, 57, 12078–12092. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, K.F.; Tong, Z.B.; Yang, J.B.; Sheng, N.; Li, J.S.; Sha, J.Q. Keggin polyoxometalates based hybrid compounds containing helix/nanocages for colorimetric biosensing. J. Solid State Chem. 2018, 265, 372–380. [Google Scholar] [CrossRef]
- Jiang, W.; Chen, L.; Cheng, W.W.; Dong, Y.Y.; Gong, J.J.; Shen, H.C.; Xu, Y. Three hybrid tungstosilicates: Confinement of SiW12O404- anions in rigid concave surfaces with excellent nonlinear optical performance and interesting magnetic properties. Polyhedron 2018, 151, 185–191. [Google Scholar] [CrossRef]
- Tian, P.; He, X.; Li, W.X.; Zhao, L.; Fang, W.; Chen, H.; Zhang, F.Q.; Zhang, W.Q.; Wang, W. Zr-MOFs based on Keggin-type polyoxometalates for photocatalytic hydrogen production. J. Mater. Sci. 2018, 53, 12016–12029. [Google Scholar] [CrossRef]
- Kaczmarek, A.M. Eu3+/Tb3+ and Dy3+ POM@MOFs and 2D coordination polymers based on pyridine-2, 6-dicarboxylic acid for ratiometric optical temperature sensing. J. Mater. Chem. C 2018, 6, 5916–5925. [Google Scholar] [CrossRef]
- Sha, J.Q.; Li, X.; Li, J.S.; Yang, X.Y.; Zhang, H.F.; Yue, M.B.; Zhou, K.F. Acidity Considerations in the Self-Assembly of POM/Ag/trz-Based Compounds with Efficient Electrochemical Activities in LIBs. Cryst. Growth Des. 2018, 18, 2289–2296. [Google Scholar] [CrossRef]
- Wang, X.L.; Zhao, D.; Tian, A.X.; Ying, J. Three 3D silver-bis(triazole) metal–organic frameworks stabilized by high-connected Wells–Dawson polyoxometallates. Dalton Trans. 2014, 43, 5211–5220. [Google Scholar] [CrossRef]
- Ying, J.; Chen, Y.G.; Wang, X.Y. A series of 0D to 3D Anderson-type polyoxometalate-based compounds obtained under ambient and hydrothermal conditions. CrystEngComm 2019, 21, 1168–1179. [Google Scholar] [CrossRef]
- Somayeh, T.; Masoud, M.; Hossein, E.H.; Antonio, F. Tuning the topology of hybrid inorganic–organic materials based on the study of flexible ligands and negative charge of polyoxometalates: A crystal engineering perspective. Coord. Chem. Rev. 2016, 309, 84–106. [Google Scholar]
- Hao, X.L.; Jia, S.F.; Ma, Y.Y.; Wang, H.Y.; Li, Y.G. Two new Keggin-type polyoxometalate-based entangled coordination networks constructed from metal-organic chains with dangling arms. Inorg. Chem. Commun. 2016, 72, 132–137. [Google Scholar] [CrossRef]
- Bai, X.; Lin, H.Y.; Sun, J.J.; Liu, G.C.; Wang, X.; Wang, X.L. Two Anderson-type polyoxometalate-induced various Co-complexes based on a rigid pyrazine-bis(triazole) ligand. Inorg. Chem. Commun. 2018, 92, 151–156. [Google Scholar] [CrossRef]
- Song, X.J.; Yan, Y.; Wang, Y.N.; Hu, D.W.; Xiao, L.N.; Yu, J.H.; Zhang, W.X.; Jia, M.J. Hybrid compounds assembled from copper-triazole complexes and phosphomolybdic acid as advanced catalysts for the oxidation of olefins with oxygen. Dalton Trans. 2017, 46, 16655–16662. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yang, J.; Yang, G.C.; Ma, J.F. Four New Three-Dimensional Polyoxometalate-Based Metal–Organic Frameworks Constructed From [Mo6O18(O3AsPh)2]4– Polyoxoanions and Copper(I)-Organic Fragments: Syntheses, Structures, Electrochemistry, and Photocatalysis Properties. Inorg. Chem. 2013, 52, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.Y.; Gu, X.J.; Peng, J.; Yu, X.; Wang, E.B. From Molecular Double-Ladders to an Unprecedented Polycatenation: A Parallel Catenated 3D Network Containing Bicapped Keggin Polyoxometalate Clusters. Eur. J. Inorg. Chem. 2006, 2, 385–388. [Google Scholar] [CrossRef]
- Wang, X.L.; Lin, H.Y.; Bi, Y.F.; Chen, B.K.; Liu, G.C. An unprecedented extended architecture constructed from a 2-D interpenetrating cationic coordination framework templated by SiW12O404− anion. J. Solid State Chem. 2008, 181, 556–561. [Google Scholar] [CrossRef]
- Du, X.D.; Li, C.H.; Zhang, Y.; Liu, S.; Ma, Y.; You, X.Z. Coordination polymers based on the octamolybdate and flexible bis(triazole) ligands with different spacer lengths. CrystEngComm 2011, 13, 2350–2357. [Google Scholar] [CrossRef]
- Kuang, X.F.; Wu, X.Y.; Yu, R.M.; Donahue, J.P.; Huang, J.S.; Lu, C.Z. Assembly of a metal-organic framework by sextuple intercatenation of discrete adamantine-like cages. Nat. Chem. 2010, 2, 461–465. [Google Scholar] [CrossRef]
- Sha, J.Q.; Li, M.T.; Yang, X.Y.; Sheng, N.; Li, J.S.; Zhu, M.L.; Liu, G.D.; Jiang, J.Z. New Route toward POM[6]Catenane Members for Lithium-Ion Batteries. Cryst. Growth Des. 2017, 17, 3775–3782. [Google Scholar] [CrossRef]
- Sahoo, D.P.; Patnaik, S.; Rath, D.; Parida, K.M. Synergistic effects of plasmon induced Ag@Ag3VO4/ZnCr LDH ternary heterostructures towards visible light responsive O2 evolution and phenol oxidation reactions. Inorg. Chem. Front. 2018, 5, 879–896. [Google Scholar] [CrossRef]
- Cao, L.J.; Tao, P.P.; Li, M.C.; Lyu, F.C.; Wang, Z.Y.; Wu, S.S.; Wang, W.X.; Huo, Y.F.; Huang, L.; Lu, Z.G. Synergistic Effects of C/α-MoC and Ag for Efficient Oxygen Reduction Reaction. J. Phys. Chem. Lett. 2018, 9, 779–784. [Google Scholar] [CrossRef]
- Laura, M.; Jiménez, D.; Luis, A.P. Molecular oxygen adsorption and dissociation on Au12M clusters with M=Cu, Ag or Ir. Eur. Phys. J. D 2018, 72, 51–60. [Google Scholar]
- Luo, W.W.; Liu, G.; Wang, X.; Lei, X.L.; Ouyang, C.Y.; Liu, S.Q. The adsorption and dissociation of oxygen on Ag (111) supported χ3 borophene. Phys. B Condens. Matter 2018, 537, 1–6. [Google Scholar] [CrossRef]
- Wu, Y.S.; Yuan, S.S.; Feng, R.; Ma, Z.C.; Gao, Y.Z.; Xing, S.T. Comparative study for low-temperature catalytic oxidation of o-xylene over doped OMS-2 catalysts: Role of Ag and Cu. Mol. Catal. 2017, 442, 164–172. [Google Scholar] [CrossRef]
- Li, Y.F.; Liu, F.F.; Fan, Y.; Cheng, G.; Song, W.; Zhou, J.L. Silver palladium bimetallic core-shell structure catalyst supported on TiO2 for toluene oxidation. Appl. Surf. Sci. 2018, 462, 207–212. [Google Scholar] [CrossRef]
- Gümüs, M.K.; Kansız, S.; Aydemir, E.; Gorobets, N.Y.; Dege, N. Structural features of 7-methoxy-5-methyl-2-(pyridin-3-yl)-11,12-dihydro-5,11-methano[1,2,4] triazolo[1,5-c][1,3,5]benzoxadiazocine: Experimental and theoretical (HF and DFT) studies, surface properties (MEP, Hirshfeld). J. Mol. Struct. 2018, 1168, 280–290. [Google Scholar] [CrossRef]
- Jin, R.Y.; Zeng, C.Y.; Liang, X.H.; Sun, X.H.; Liu, Y.F.; Wang, Y.Y.; Zhou, S. Design, synthesis, biological activities and DFT calculation of novel 1,2,4-triazole Schiff base derivatives. Bioorganic Chem. 2018, 80, 253–260. [Google Scholar] [CrossRef]
- Calu, L.; Badea, M.; Korošin, N.Č.; Chifiriuc, M.C.; Bleotu, C.; Stanică, N.; Silvestro, L.; Maurer, M.; Olar, R. Spectral, thermal and biological characterization of complexes with a Schiff base bearing triazole moiety as potential antimicrobial species. J. Therm. Anal. Calorim. 2018, 134, 1839–1850. [Google Scholar] [CrossRef]
- Aouad, M.R.; Messali, M.; Rezki, N.; Zaqri, N.A.; Warad, I. Single proton intramigration in novel 4-phenyl-3-((4-phenyl-1H-1,2,3-triazol-1-yl)methyl)-1H-1,2,4-triazole-5(4H)-thione: XRD-crystal interactions, physicochemical, thermal, Hirshfeld surface, DFT realization of thiol/thione tautomerism. J. Mol. Liq. 2018, 264, 621–630. [Google Scholar] [CrossRef]
- Vinita; Tiwari, M.; Prakash, R. Colorimetric detection of picric acid using silver nanoparticles modified with 4-amino-3-hydrazino-5-mercapto-1, 2, 4-triazole. Appl. Surf. Sci. 2018, 449, 174–180. [Google Scholar] [CrossRef]
- Dudley, H.W.; Lan, F. Spectroscopic Methods in Organic Chemistry; Mcgraw-Hill: London, UK, 1966; Volume 222, price 38s. [Google Scholar]
- Wang, S.; Liu, Y.W.; Zhang, Z.; Li, X.H.; Tian, H.R.; Yan, T.T.; Zhang, X.; Liu, S.; Sun, X.W.; Xu, L.; et al. One-Step Template-Free Fabrication of Ultrathin Mixed-Valence Polyoxovanadate-Incorporated Metal-Organic Framework Nanosheets for Highly Efficient Selective Oxidation Catalysis in Air. ACS Appl. Mater. Interfaces 2019. [Google Scholar] [CrossRef]
- Wang, J.; Zou, Y.C.; Sun, Y.; Hemgesberg, M.; Schaffner, D.; Gao, H.C.; Song, X.J.; Zhang, W.X.; Jia, M.J.; Thiel, W.R. Electrostatic immobilization of phosphomolybdic acid on imidazolium-based mesoporous organosilicas for catalytic olefin epoxidation. Chin. J. Catal. 2014, 35, 532–539. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, X.Y.; Zhu, G.X.; Gao, Y.J.; Cheng, Q.F.; Cheng, X.W. Synthesis of silver phosphate/sillenite bismuth ferrite/graphene oxide nanocomposite and its enhanced visible light photocatalytic mechanism. Sep. Purif. Technol. 2019, 215, 490–499. [Google Scholar] [CrossRef]
- Feng, Z.Z.; Yu, J.J.; Sun, D.P.; Wang, T.H. Visible-light-driven photocatalysts Ag/AgCl dispersed on mesoporous Al2O3 with enhanced photocatalytic performance. J. Colloid Interface Sci. 2016, 480, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Nagaraju, P.; Hafedh, D.; Mohammed, R.A.R.; Abdulrahim, A.A.Z.; Muhammad, A.D.; Arshid, M.A.; Sharif, F.Z.; Lachezar, A.P. Methanol Synthesis Using CO2 and H2 on Nano Silver-Ceria Zirconia Catalysts: Influence of Preparation Method. J. Nanosci. Nanotechnol. 2019, 19, 3197–3204. [Google Scholar]
- Gambardella, A.; Berni, M.; Graziani, G.; Kovtun, A.; Liscio, A.; Russo, A.; Visani, A.; Bianchi, M. Nanostructured Ag thin films deposited by pulsed electron ablation. Appl. Surf. Sci. 2019, 475, 917–925. [Google Scholar] [CrossRef]
- Ji, X.B.; Chen, Y.X.; Paul, B.; Vadivel, S. Photocatalytic oxidation of aromatic alcohols over silver supported on cobalt oxide nanostructured catalyst. J. Alloy. Compd. 2019, 783, 583–592. [Google Scholar] [CrossRef]
- Raghunath, D.; Venkata, S.S.; Hugues, K.P.; Madhumita, B.; Vinesh, M.; Arjun, M. Silver decorated magnetic nanocomposite (Fe3O4@PPy-MAA/Ag) as highly active catalyst towards reduction of 4-nitrophenol and toxic organic dyes. Appl. Catal. B Environ. 2019, 244, 546–558. [Google Scholar]
- Matjaž, F. EQCM and XPS analysis of 1, 2, 4-triazole and 3-amino-1, 2, 4-triazole as copper corrosion inhibitors in chloride solution. Corros. Sci. 2013, 77, 350–359. [Google Scholar]
- Yang, L.Y.; Xin, L.L.; Gu, W.; Tian, J.L.; Liao, S.Y.; Du, P.Y.; Tong, Y.Z.; Zhang, Y.P.; Lv, R.; Wang, J.Y.; et al. A new carboxyl-copper-organic framework and its excellent selective absorbability for proteins. J. Solid State Chem. 2014, 218, 64–70. [Google Scholar] [CrossRef]
- Bentiss, F.; Traisnel, M.; Gengembre, L.; Lagrenée, M. A new triazole derivative as inhibitor of the acid corrosion of mild steel: Electrochemical studies, weight loss determination, SEM and XPS. Appl. Surf. Sci. 1999, 152, 237–249. [Google Scholar] [CrossRef]
- Gao, W.X.; Sun, X.Y.; Niu, H.L.; Song, X.J.; Li, K.G.; Gao, H.C.; Zhang, W.X.; Yu, J.H.; Jia, M.J. Phosphomolybdic acid functionalized covalent organic frameworks: Structure characterization and catalytic properties in olefin epoxidation. Microporous Mesoporous Mater. 2015, 213, 59–67. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Miao, A.J.; Jiang, M. Fabrication, characterization and electrochemistry of organice inorganic multilayer films containing polyoxometalate and polyviologen via layer-by-layer self-assembly. Mater. Chem. Phys. 2013, 141, 482–487. [Google Scholar] [CrossRef]
- Song, X.J.; Hu, D.W.; Yang, X.T.; Zhang, H.; Zhang, W.X.; Li, J.Y.; Jia, M.J.; Yu, J.H. Polyoxomolybdic Cobalt Encapsulated within Zr-Based Metal-Organic Frameworks as Efficient Heterogeneous Catalysts for Olefins Epoxidation. ACS Sustain. Chem. Eng. 2019, 7, 3624–3631. [Google Scholar] [CrossRef]
- Du, J.; Yu, J.H.; Tang, J.Y.; Wang, J.; Zhang, W.X.; Thiel, W.R.; Jia, M.J. Supramolecular Assemblies Directed by Hydrogen Bonds and π–π Interactions and Based on N-Heterocyclic-Ligand-Modified β-Octamolybdate-Structure and Catalytic Application in Olefin Epoxidation. Eur. J. Inorg. Chem. 2011, 15, 2361–2365. [Google Scholar] [CrossRef]
- Charles, F.H.; Hendrik, C.A.; Van, B.; Pieter, M.H. Reactions of Some Peracids and Hydroperoxides with Cobalt(II) and Cobalt(III) Acetate in Acetic Acid Solution. Ind. Eng. Chem. Prod. Res. Dev. 1979, 18, 38–43. [Google Scholar]
- Wang, Q.B.; Cheng, Y.W.; Wang, L.J.; Li, X. Semicontinuous Studies on the Reaction Mechanism and Kinetics for the Liquid-Phase Oxidation of p-Xylene to Terephthalic Acid. Ind. Eng. Chem. Res. 2007, 46, 8980–8992. [Google Scholar] [CrossRef]
- Denisov, E.T. Redox Reactions of Atoms and Radicals with Ions in Solution. Russ. Chem. Rev. 1971, 40, 24–32. [Google Scholar] [CrossRef]
- Kamiya, Y.; Ingold, K.U. The Metal-Catalyzed Autoxidation of Tetralin. Can. Jocrnal Chem. 1964, 42, 1027–1043. [Google Scholar] [CrossRef]
- Navarro, M.; Escobar, A.; Landaeta, V.R.; Visbal, G.; Linares, F.L.; Luis, M.L.; Fuentes, A. Catalytic Oxidation of Tetralin by Biologically Active Copper and Palladium Complexes. Appl. Catal. A Gen. 2009, 363, 27–31. [Google Scholar] [CrossRef]
- Chung, Y.M.; Ahn, W.S.; Lim, P.K. Organic-Water Interfacial Synthesis of α-Tetralone Using Nickel-Tetraethylenepentamine Complex Catalysts. J. Catal. 1998, 173, 210–218. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS; Siemens Analytical X-ray Instrument Division: Madison, WI, USA, 1995. [Google Scholar]
- Sheldrick, G.M. SHELXL-97: A Program for Crystal Structure Determination; University of Gottingen: Gottingen, Germany, 1997. [Google Scholar]



| Catalyst | Substrate | Product | Time (h) | Conversion (%) | Related work |
|---|---|---|---|---|---|
| 1 |  |  | 3 | 96 | this work |
| Ag-trz3 | 3 | 23 | this work | ||
| CH3COOAg | 3 | 51 | this work | ||
| PMA | 3 | 5 | this work | ||
| 1 b | 2.5 | 94 | this work | ||
| POM@MOF c | 4 | 95 d | Ref-31 | ||
| 1 |  |  | 4 | 90 | this work |
| Ag-trz3 | 4 | 15 | this work | ||
| CH3COOAg | 4 | 37 | this work | ||
| PMA | 4 | 11 | this work | ||
| POM@MOFc | 7 | 73 d | Ref-31 |
| Entry | Solvent | Temperature (°C) | Conversion (%) |
|---|---|---|---|
| 1 | EtOH | 60 | 64 |
| 2 | CHCl3 | 60 | 62 |
| 3 | CH3CN | 25 | 81 |
| 4 | CH3CN | 35 | 89 |
| 5 | CH3CN | 40 | 96 |
| 6 | CH3CN | 60 | 98 |
| Entry | Substrate | Temperature (°C) | Time (h) | Conversion (%) | Selectivity (%) | |
|---|---|---|---|---|---|---|
| Epoxidation | Others | |||||
| 1 |  | 35 | 3 | 96 | 84 | 16 |
| 2 |  | 61 | 8 | 74 | 75 | 25 |
| 3 |  | 61 | 6 | 82 | >99 | -- |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, J.; Liu, X.; Zhang, J.; Liu, Y.; Zhu, E.; Che, G.; Jia, M. Facile Synthesis of a Polycatenane Compound Based on Ag-triazole Complexes and Phosphomolybdic Acid for the Catalytic Epoxidation of Olefins with Molecular Oxygen. Catalysts 2019, 9, 568. https://doi.org/10.3390/catal9070568
Du J, Liu X, Zhang J, Liu Y, Zhu E, Che G, Jia M. Facile Synthesis of a Polycatenane Compound Based on Ag-triazole Complexes and Phosphomolybdic Acid for the Catalytic Epoxidation of Olefins with Molecular Oxygen. Catalysts. 2019; 9(7):568. https://doi.org/10.3390/catal9070568
Chicago/Turabian StyleDu, Juan, Xinyu Liu, Jiaxin Zhang, Yucun Liu, Enwei Zhu, Guangbo Che, and Mingjun Jia. 2019. "Facile Synthesis of a Polycatenane Compound Based on Ag-triazole Complexes and Phosphomolybdic Acid for the Catalytic Epoxidation of Olefins with Molecular Oxygen" Catalysts 9, no. 7: 568. https://doi.org/10.3390/catal9070568





