Minimally Invasive Glucose Monitoring Using a Highly Porous Gold Microneedles-Based Biosensor: Characterization and Application in Artificial Interstitial Fluid
Abstract
:1. Introduction
2. Results and Discussion
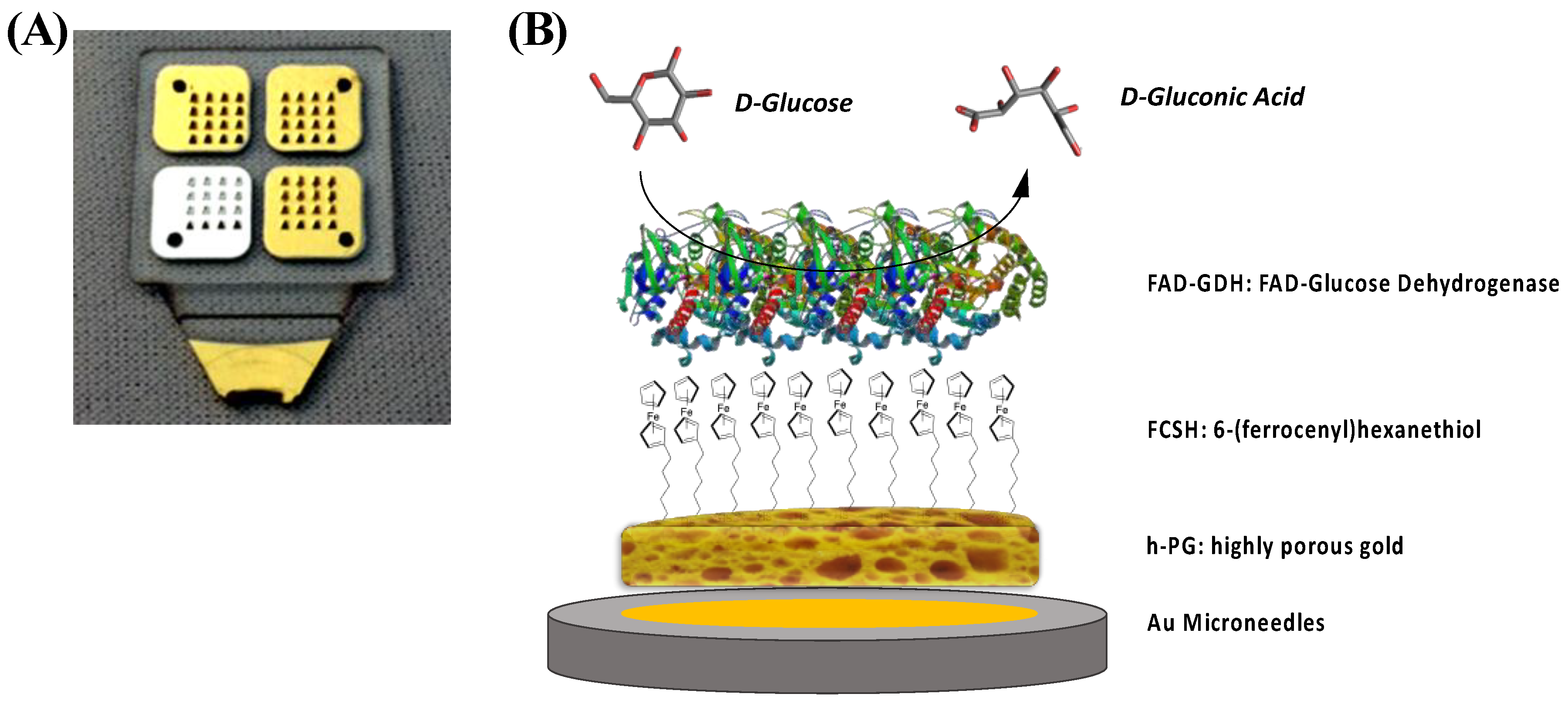
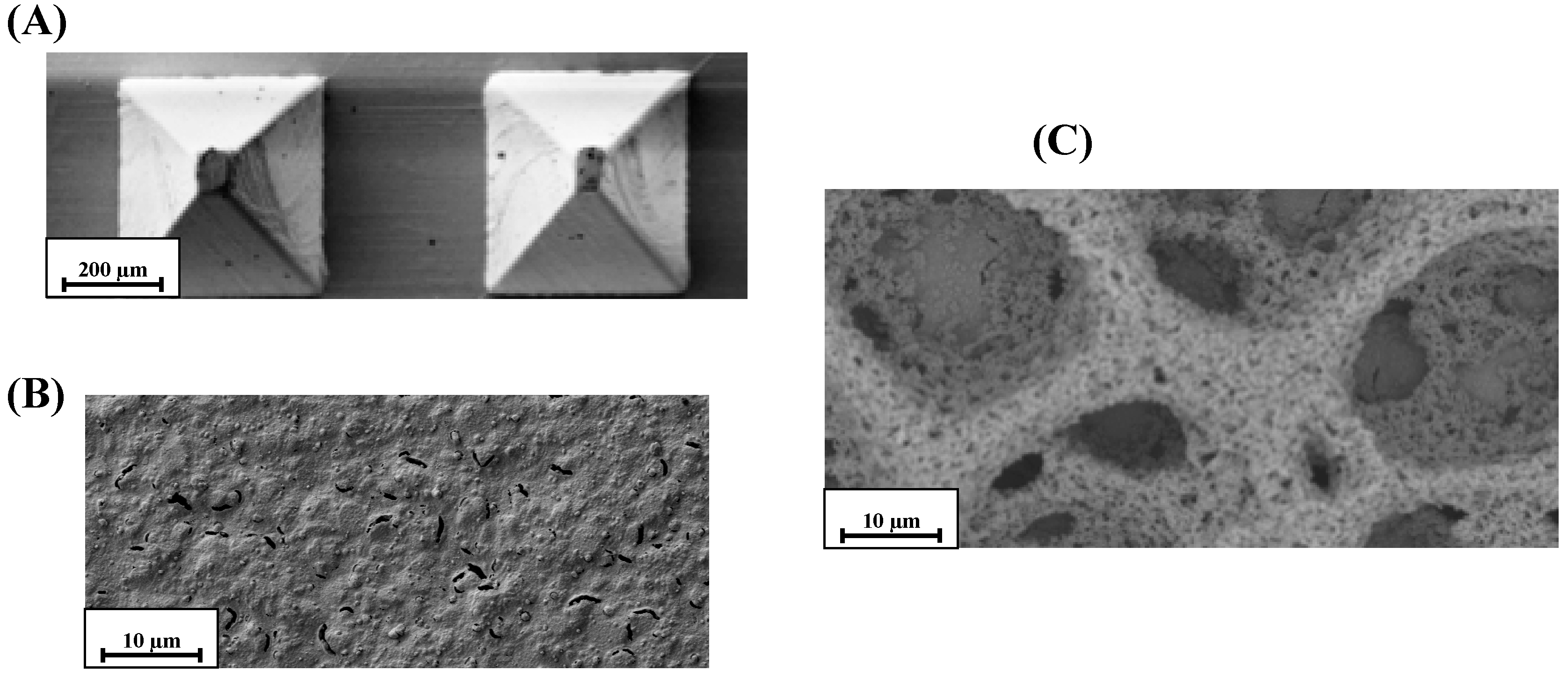
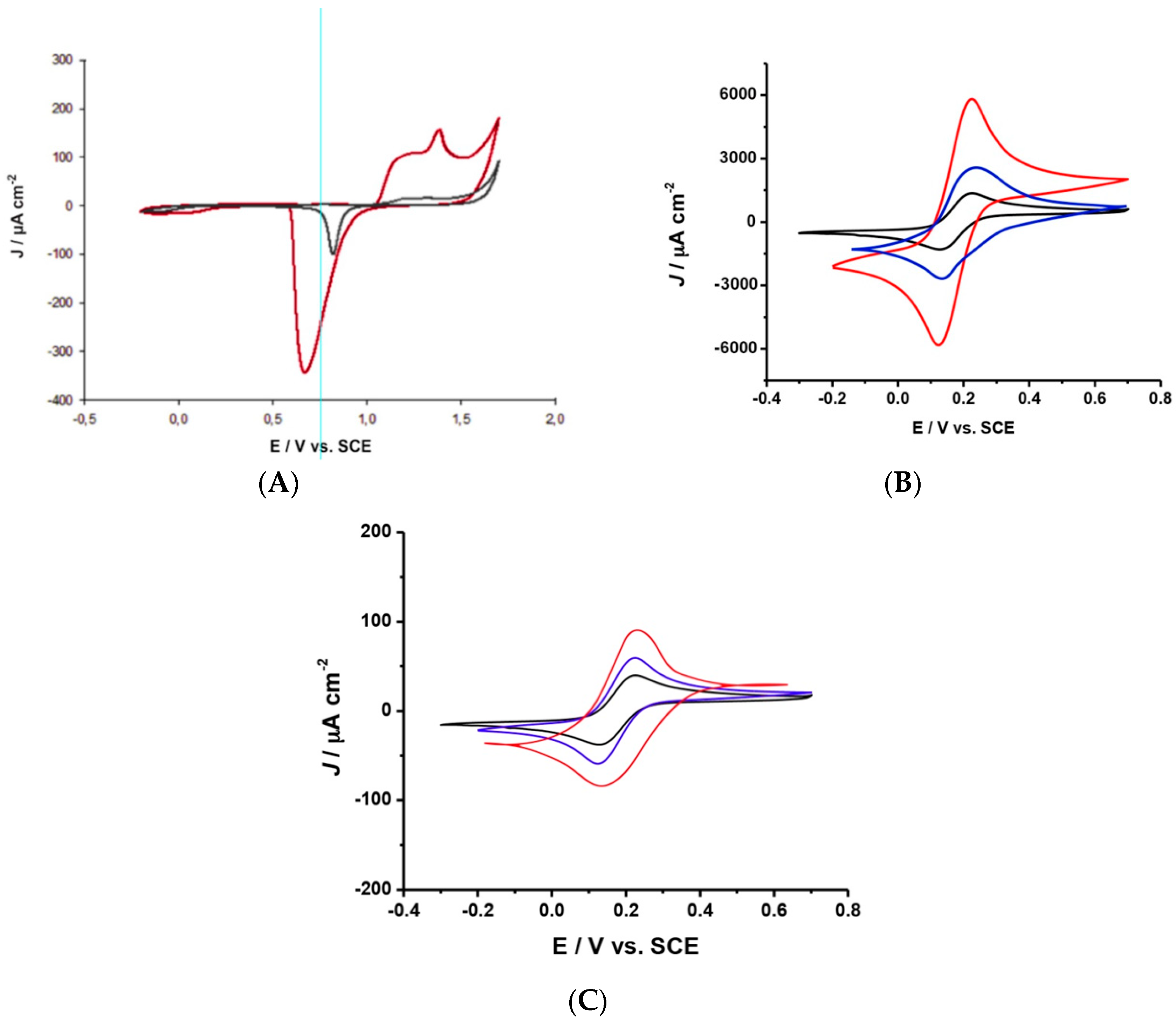
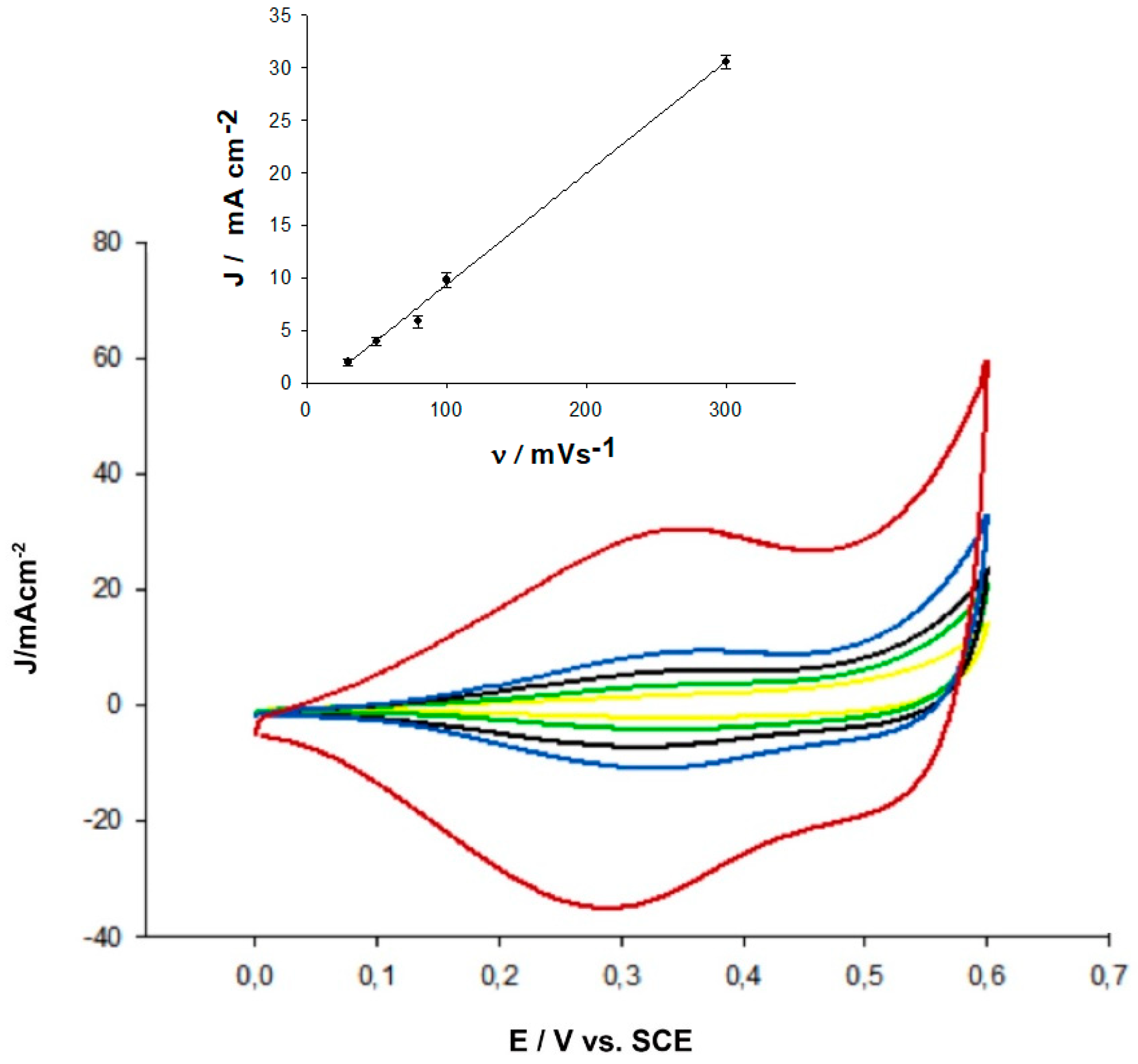
2.1. SEM and Electrochemical Characterization of h-PG/Au Microneedles Electrodes
2.2. Electrochemical Characterization of FcSH/h-PG/Au Microneedles Electrodes
2.3. Application of the h-PG/Au Microneedles-Based Glucose Biosensor in Artificial ISF
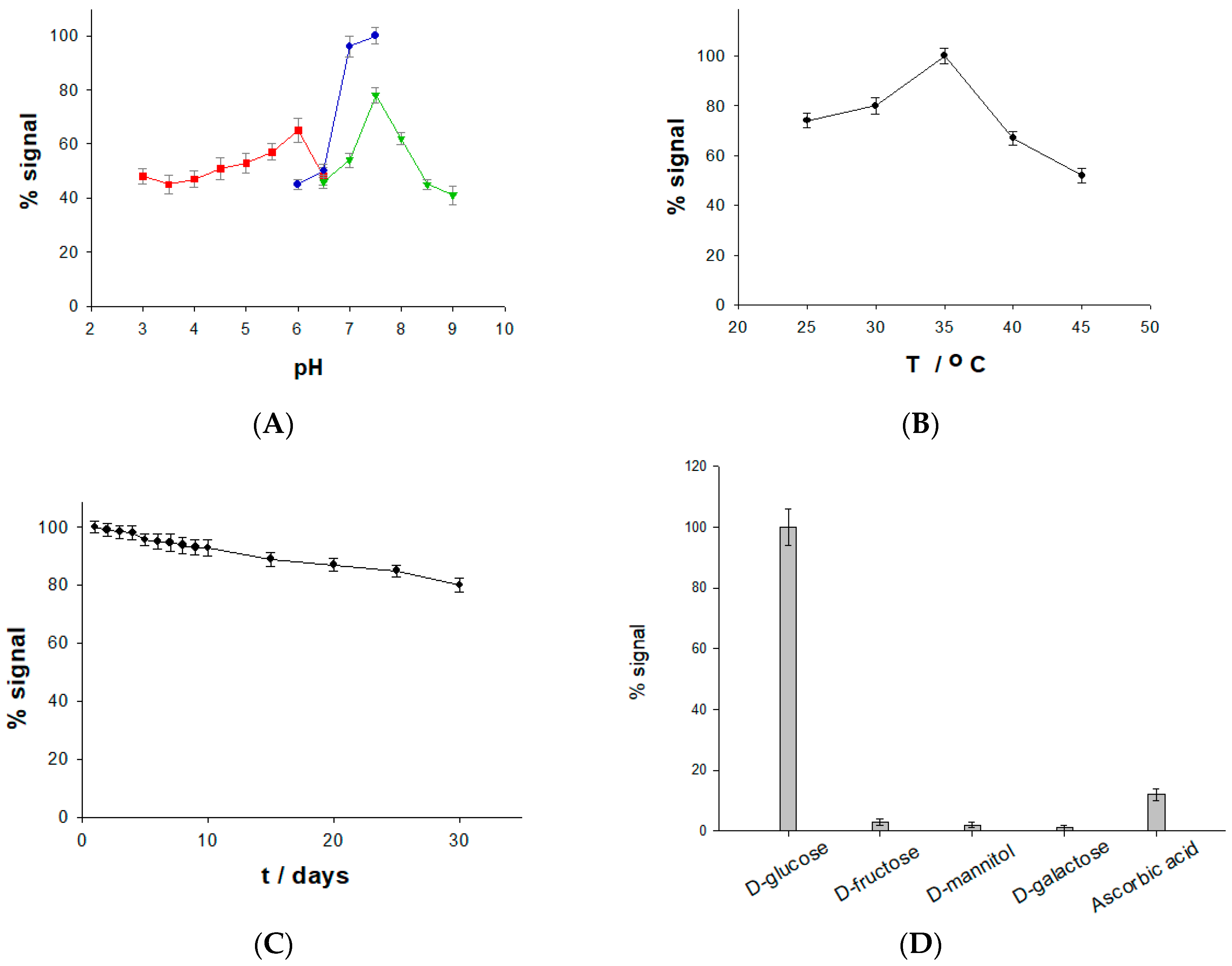
2.4. Effect of pH and Temperature on the Stability and Selectivity of the Glucose Biosensor
2.5. Simulation of Continuous Glucose Monitoring Using a Skin Model
3. Materials and Methods
3.1. Reagents and Apparatus
3.2. Modification of Au Microneedles-Based Electrode
3.3. Modification of Au Planar Electrode
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.E.; Sicree, R.A.; Zimmet, P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 4–14. [Google Scholar] [CrossRef] [PubMed]
- O’Kane, M.J.; Pickup, J. Self-monitoring of blood glucose in diabetes: Is it worth it? Ann. Clin. Biochem. 2009, 46, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Oliver, N.; Toumazou, C.; Cass, A.; Johnston, D. Glucose sensors: A review of current and emerging technology. Diabetic Med. 2009, 26, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Huang, Z.; Rogers, M.; Boutelle, M.; Cass, A.E. Evaluation of a minimally invasive glucose biosensor for continuous tissue monitoring. Anal. Bioanal. Chem. 2016, 408, 8427–8435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pickup, J.C.; Freeman, S.C.; Sutton, A.J. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta-analysis of randomised controlled trials using individual patient data. Br. Med. J. 2011, 343, d3805. [Google Scholar] [CrossRef]
- Chinnadayyala, S.R.; Park, K.D.; Cho, S. Editors’ Choice—Review—In Vivo and In Vitro Microneedle Based Enzymatic and Non-Enzymatic Continuous Glucose Monitoring Biosensors. ECS J. Solid State Sci. Technol. 2018, 7, Q3159–Q3171. [Google Scholar] [CrossRef]
- Paliwal, S.; Hwang, B.H.; Tsai, K.Y.; Mitragotri, S. Diagnostic opportunities based on skin biomarkers. Eur. J. Pharm. Sci. 2013, 50, 546–556. [Google Scholar] [CrossRef]
- Sharma, S.; Saeed, A.; Johnson, C.; Gadegaard, N.; Cass, A.E. Rapid, low cost prototyping of transdermal devices for personal healthcare monitoring. Sens. Biosens. Res. 2017, 13, 104–108. [Google Scholar] [CrossRef]
- Ventrelli, L.; Marsilio Strambini, L.; Barillaro, G. Microneedles for transdermal biosensing: current picture and future direction. Adv. Healthcare Mater. 2015, 4, 2606–2640. [Google Scholar] [CrossRef]
- Miller, P.R.; Narayan, R.J.; Polsky, R.J. Microneedle-based sensors for medical diagnosis. J. Mater. Chem. B. 2016, 4, 1379–1383. [Google Scholar] [CrossRef]
- Windmiller, J.R.; Zhou, N.; Chuang, M.-C.; Valdés-Ramírez, G.; Santhosh, P.; Miller, P.R.; Narayan, R.; Wang, J. Microneedle array-based carbon paste amperometric sensors and biosensors. Analyst 2011, 136, 1846–1851. [Google Scholar] [CrossRef] [PubMed]
- Hwa, K.-Y.; Subramani, B.; Chang, P.-W.; Chien, M.; Huang, J.-T. Transdermal microneedle array-based sensor for real time continuous glucose monitoring. Int. J. Electrochem. Sci 2015, 10, 2455–2466. [Google Scholar]
- El-Laboudi, A.; Oliver, N.S.; Cass, A.; Johnston, D. Use of microneedle array devices for continuous glucose monitoring: A review. Diabetes Technol. Ther. 2013, 15, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Gerstel, M.S.; Place, V.A. Drug delivery device. Google Patents US3964482A, 22 June 1976. [Google Scholar]
- Henry, S.; McAllister, D.V.; Allen, M.G.; Prausnitz, M.R. Microfabricated microneedles: A novel approach to transdermal drug delivery. J. Pharm. Sci. 1998, 87, 922–925. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-C.; Park, J.-H.; Prausnitz, M.R. Microneedles for drug and vaccine delivery. Adv. Drug Delivery Rev. 2012, 64, 1547–1568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valdés-Ramírez, G.; Li, Y.-C.; Kim, J.; Jia, W.; Bandodkar, A.J.; Nuñez-Flores, R.; Miller, P.R.; Wu, S.-Y.; Narayan, R.; Windmiller, J.R. Microneedle-based self-powered glucose sensor. Electrochem. Commun. 2014, 47, 58–62. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Dave, K.; Venuganti, V.V.K. Microneedles in the clinic. J. Controlled Release. 2017, 260, 164–182. [Google Scholar] [CrossRef]
- Strambini, L.; Longo, A.; Scarano, S.; Prescimone, T.; Palchetti, I.; Minunni, M.; Giannessi, D.; Barillaro, G. Self-powered microneedle-based biosensors for pain-free high-accuracy measurement of glycaemia in interstitial fluid. Biosens. Bioelectron. 2015, 66, 162–168. [Google Scholar] [CrossRef]
- Stout, P.J.; Peled, N.; Erickson, B.J.; Hilgers, M.E.; Racchini, J.R.; Hoegh, T.B. Comparison of glucose levels in dermal interstitial fluid and finger capillary blood. Diabetes Technol. Ther. 2011, 3, 81–90. [Google Scholar] [CrossRef]
- Boyne, M.S.; Silver, D.M.; Kaplan, J.; Saudek, C.D. Timing of changes in interstitial and venous blood glucose measured with a continuous subcutaneous glucose sensor. Diabetes 2003, 52, 2790–2794. [Google Scholar] [CrossRef] [PubMed]
- Bollella, P.; Sharma, S.; Cass, A.E.G.; Antiochia, R. Minimally-invasive Microneedle-based Biosensor Array for Simultaneous Lactate and Glucose Monitoring in Artificial Interstitial Fluid. Electroanalysis 2019, 31, 374–382. [Google Scholar] [CrossRef] [Green Version]
- Jina, A.; Tierney, M.J.; Tamada, J.A.; McGill, S.; Desai, S.; Chua, B.; Chang, A.; Christiansen, M.J. Design, development, and evaluation of a novel microneedle array-based continuous glucose monitor. J. Diabetes Sci. Technol. 2014, 8, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Takagi, E.; Cass, T.; Tsugawa, W.; Sode, K. Minimally invasive microneedle array electrodes employing direct electron transfer type glucose dehydrogenase for the development of continuous glucose monitoring sensors. Procedia Technol. 2017, 27, 208–209. [Google Scholar] [CrossRef]
- Caliò, A.; Dardano, P.; Di Palma, V.; Bevilacqua, M.; Di Matteo, A.; Iuele, H.; De Stefano, L. Polymeric microneedles based enzymatic electrodes for electrochemical biosensing of glucose and lactic acid. Sens. Actuators B 2016, 236, 343–349. [Google Scholar] [CrossRef]
- Miller, P.R.; Skoog, S.A.; Edwards, T.L.; Lopez, D.M.; Wheeler, D.R.; Arango, D.C.; Xiao, X.; Brozik, S.M.; Wang, J.; Polsky, R. Multiplexed microneedle-based biosensor array for characterization of metabolic acidosis. Talanta 2012, 88, 739–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bollella, P.; Sharma, S.; Cass, A.E.G.; Antiochia, R. Microneedle-based biosensor for minimally-invasive lactate detection. Biosens. Bioelectron. 2019, 123, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.V.; Windmiller, J.R.; Mishra, R.K.; Wang, J. Continuous minimally-invasive alcohol monitoring using microneedle sensor arrays. Biosens. Bioelectron. 2017, 91, 574–579. [Google Scholar] [CrossRef] [Green Version]
- Rawson, T.M.; Sharma, S.; Georgiou, P.; Holmes, A.; Cass, A.; O’Hare, D. Towards a minimally invasive device for beta-lactam monitoring in humans. Electrochem. Commun. 2017, 82, 1–5. [Google Scholar] [CrossRef]
- Windmiller, J.R.; Valdés-Ramírez, G.; Zhou, N.; Zhou, M.; Miller, P.R.; Jin, C.; Brozik, S.M.; Polsky, R.; Katz, E.; Narayan, R. Bicomponent Microneedle Array Biosensor for Minimally-Invasive Glutamate Monitoring. Electroanalysis 2011, 23, 2302–2309. [Google Scholar] [CrossRef]
- Abrar, M.A.; Dong, Y.; Lee, P.K.; Kim, W.S. Bendable electro-chemical lactate sensor printed with silver nano-particles. Sci. Rep. 2016, 6, 30565. [Google Scholar] [CrossRef] [PubMed]
- Bollella, P.; Gorton, L. Enzyme based amperometric biosensors. Curr. Opin. Electrochem. 2018, 10, 157–173. [Google Scholar] [CrossRef]
- Karadag, M.; Geyik, C.; Demirkol, D.O.; Ertas, F.N.; Timur, S. Modified gold surfaces by 6-(ferrocenyl) hexanethiol/dendrimer/gold nanoparticles as a platform for the mediated biosensing applications. Mater. Sci. Eng. C 2013, 33, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Baldo, T.A.; Seraphim, P.M.; Gomes, H.M.; Teixeira, M.F. Glucose Biosensor Based on the Hexacyanoferrate 11-Mercaptoundecyl-N’, N”, N”’-Trimethylammonium/6-(Ferrocenyl) Hexanethiol. Procedia Eng. 2014, 87, 300–303. [Google Scholar] [CrossRef]
- Antiochia, R.; Lavagnini, I.; Magno, F. Electrocatalytic oxidation of dihydronicotinamide adenine dinucleotide with ferrocene carboxylic acid by diaphorase from Clostridium kluveri. Remarks on the kinetic approaches usually adopted. Electroanalysis 1999, 11, 129–133. [Google Scholar] [CrossRef]
- Zhang, R.; Olin, H. Porous gold films—A short review on recent progress. Materials 2014, 7, 3834–3854. [Google Scholar] [CrossRef] [PubMed]
- Bollella, P.; Hibino, Y.; Kano, K.; Gorton, L.; Antiochia, R. Highly Sensitive Membraneless Fructose Biosensor Based on Fructose Dehydrogenase Immobilized onto Aryl Thiol Modified Highly Porous Gold Electrode: Characterization and Application in Food Samples. Anal. Chem. 2018, 90, 12131–12136. [Google Scholar] [CrossRef]
- Deng, Y.; Huang, W.; Chen, X.; Li, Z. Facile fabrication of nanoporous gold film electrodes. Electrochem. Commun. 2008, 10, 810–813. [Google Scholar] [CrossRef]
- Ben-Ali, S.; Cook, D.A.; Evans, S.A.; Thienpont, A.; Bartlett, P.N.; Kuhn, A. Electrocatalysis with monolayer modified highly organized macroporous electrodes. Electrochem. Commun. 2003, 5, 747–751. [Google Scholar] [CrossRef]
- Plowman, B.J.; Jones, L.A.; Bhargava, S.K. Building with bubbles: The formation of high surface area honeycomb-like films via hydrogen bubble templated electrodeposition. Chem. Comm. 2015, 51, 4331–4346. [Google Scholar] [CrossRef]
- Sanzó, G.; Taurino, L.; Antiochia, R.; Gorton, L.; Favero, G.; Mazzei, F.; De Micheli, G.; Carrara, S. Bubble electrodeposition of gold porous nanocorals for the enzymatic and non-enzymatic detection of glucose. Bioelectrochemistry 2016, 112, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Cass, A.E.; Sharma, S. Microneedle enzyme sensor arrays for continuous in vivo monitoring. Methods Enzymol. 2017, 589, 413–427. [Google Scholar] [PubMed]
- Wannapob, R.; Thavarungkul, P.; Dawan, S.; Numnuam, A.; Limbut, W.; Kanatharana, P. A Simple and Highly Stable Porous Gold-based Electrochemical Sensor for Bisphenol A Detection. Electroanalysis 2017, 29, 472–480. [Google Scholar] [CrossRef]
- Lavagnini, I.; Antiochia, R.; Magno, F. An extended method for the practical evaluation of the standard rate constant from cyclic voltammetric data. Electroanalysis 2004, 16, 505–506. [Google Scholar] [CrossRef]
- Lavagnini, I.; Antiochia, R.; Magno, F. A Calibration-Base Method for the Evaluation of the Detection Limit of an Electrochemical Biosensor. Electroanalysis 2007, 19, 1227–1230. [Google Scholar] [CrossRef]
- Creager, S.E.; Rowe, G.K. Redox properties of ferrocenylalkane thiols coadsorbed with linear n-alkanethiols on polycrystalline bulk gold electrodes. Anal. Chim. Acta 1991, 246, 233–239. [Google Scholar] [CrossRef]
- Belding, S.R.; Campbell, F.W.; Dickinson, E.J.; Compton, R.G. Nanoparticle-modified electrodes. Phys. Chem. Chem. Phys. 2010, 12, 11208–11221. [Google Scholar] [CrossRef]
- Menshykau, D.; Streeter, I.; Compton, R.G. Influence of electrode roughness on cyclic voltammetry. J. Phys. Chem. C 2008, 112, 14428–14438. [Google Scholar] [CrossRef]
- Ferri, S.; Kojima, K.; Sode, K. Review of glucose oxidases and glucose dehydrogenases: a bird’s eye view of glucose sensing enzymes. J. Diabetes Sci. Technol. 2011, 5, 1068–1076. [Google Scholar] [CrossRef]
- Dardano, P.; Caliò, A.; Di Palma, V.; Bevilacqua, M.; Di Matteo, A.; De Stefano, L. Glucose sensing electrode system based on polymeric microneedles. In Proceedings of the IEEE Sensors Appl. Symposium (SAS), Catania, Italy, 20–22 April 2016. [Google Scholar]
- Barrett, C.; Dawson, K.; O’Mahony, C.; O’Riordan, A. Development of low cost rapid fabrication of sharp polymer microneedles for in vivo glucose biosensing applications. ECS J. Solid State Sci. Technol. 2015, 4, S3053–S3058. [Google Scholar] [CrossRef]
- Chen, D.; Wang, C.; Chen, W.; Chen, Y.; Zhang, J.X. PVDF-Nafion nanomembranes coated microneedles for in vivo transcutaneous implantable glucose sensing. Biosens. Bioelectron. 2015, 74, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Hong, Y.J.; Baik, S.; Hyeon, T.; Kim, D.H. Enzyme-based glucose sensor: from invasive to wearable device. Adv. Healthcare Mater. 2018, 7, 17011. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.B.; Lee, W.C.; Cho, C.H.; Park, D.S.; Cho, S.J.; Shim, Y.B. Continuous glucose monitoring using a microneedle array sensor coupled with a wireless signal transmitter. Sens. Actuators B 2019, 281, 14–21. [Google Scholar] [CrossRef]
- Tasca, F.; Tortolini, C.; Bollella, P.; Antiochia, R. Microneedle-based electrochemical devices for transdermal biosensing: A review. Curr. Opin. Electrochem. 2019, 16, 42–49. [Google Scholar] [CrossRef]

| Electrode | k0/10−3 cm s−1 | AEA/cm2 | ρ |
|---|---|---|---|
| Au planar electrode | 1.1 ± 0.1 | 0.08 ± 0.01 | 4.0 ± 0.2 |
| Au planar electrode/h-PG | 3.0 ± 0.8 | 1.12 ± 0.02 | 56.0 ± 0.8 |
| Au planar electrode/Au-MWCNTs | 2.7 ± 0.9 | 1.24 ± 0.02 | 39.5 ± 0.6 |
| Au microneedles | 5.8 ± 0.2 | 2.02 ± 0.18 | 10.1 ± 0.6 |
| Au microneedles/h-PG | 56.2 ± 0.5 | 206.42 ± 0.42 | 1032.1 ± 2.3 |
| Au microneedles/Au-MWCNTs | 16.3 ± 0.4 | 60.36 ± 0.31 | 301.6 ± 1.6 |
| Microneedles Biosensor Platform | Detection Technique | Linear Range (mM) | Biosensor Type | Real ApplicAtion | Ref. |
|---|---|---|---|---|---|
| Au/PEGDA/vFc/GOx | amperometry | 0–0.6 | first generation | - | [51] |
| Au/FcCOOH/GOx | amperometry | 2–13.5 | first generation | - | [52] |
| C/CMC/GOx | amperometry | 0–35 | self-powered | - | [21] |
| AuNPs-PtNPs/PANI/PtNPs/GOx/PVDF-Naf | amperometry | 0–20 | first generation | in vivo | [53] |
| Au/MPA/GOx | Cyclic voltammetry | 2.8–22.2 | first generation | in vitro | [54] |
| Au/PP/GOx | amperometry | - | first generation | in vivo | [5] |
| CP/GOx/TTF-CP/Pt black | polarization curve | 0–25 | self-powered | - | [19] |
| Au/pTCA-GOx | amperometry | 0.05–20 | first generation | - | [55] |
| GDH/pMB/AuMWCNTs/Au | amperometry | 0.05–5 | second generation | - | [23] |
| FAD-GDH/FcSH/h-PG/Au | amperometry | 0.1–10 | second generation | - | this work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bollella, P.; Sharma, S.; Cass, A.E.G.; Tasca, F.; Antiochia, R. Minimally Invasive Glucose Monitoring Using a Highly Porous Gold Microneedles-Based Biosensor: Characterization and Application in Artificial Interstitial Fluid. Catalysts 2019, 9, 580. https://doi.org/10.3390/catal9070580
Bollella P, Sharma S, Cass AEG, Tasca F, Antiochia R. Minimally Invasive Glucose Monitoring Using a Highly Porous Gold Microneedles-Based Biosensor: Characterization and Application in Artificial Interstitial Fluid. Catalysts. 2019; 9(7):580. https://doi.org/10.3390/catal9070580
Chicago/Turabian StyleBollella, Paolo, Sanjiv Sharma, Anthony E. G. Cass, Federico Tasca, and Riccarda Antiochia. 2019. "Minimally Invasive Glucose Monitoring Using a Highly Porous Gold Microneedles-Based Biosensor: Characterization and Application in Artificial Interstitial Fluid" Catalysts 9, no. 7: 580. https://doi.org/10.3390/catal9070580
APA StyleBollella, P., Sharma, S., Cass, A. E. G., Tasca, F., & Antiochia, R. (2019). Minimally Invasive Glucose Monitoring Using a Highly Porous Gold Microneedles-Based Biosensor: Characterization and Application in Artificial Interstitial Fluid. Catalysts, 9(7), 580. https://doi.org/10.3390/catal9070580







