Synthesis of Hollow Flower-Like Fe3O4/MnO2/Mn3O4 Magnetically Separable Microspheres with Valence Heterostructure for Dye Degradation
Abstract
:1. Introduction
2. Results and Discussion
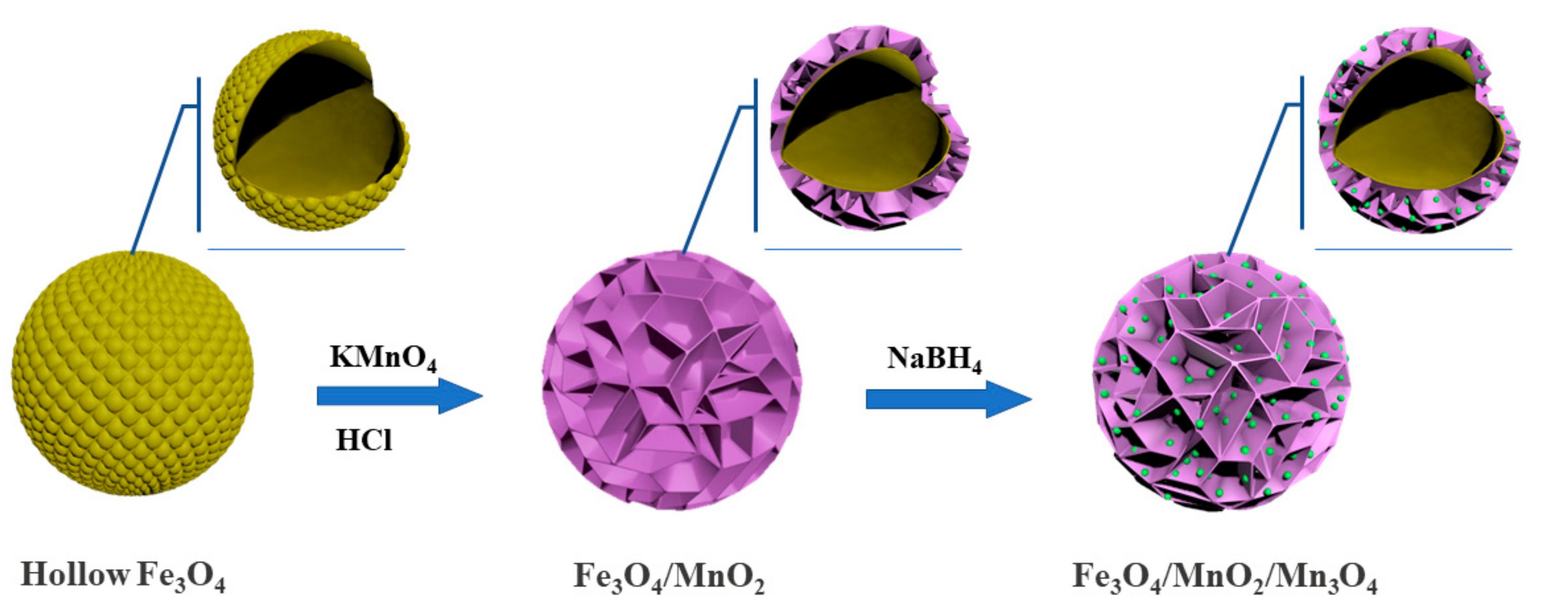
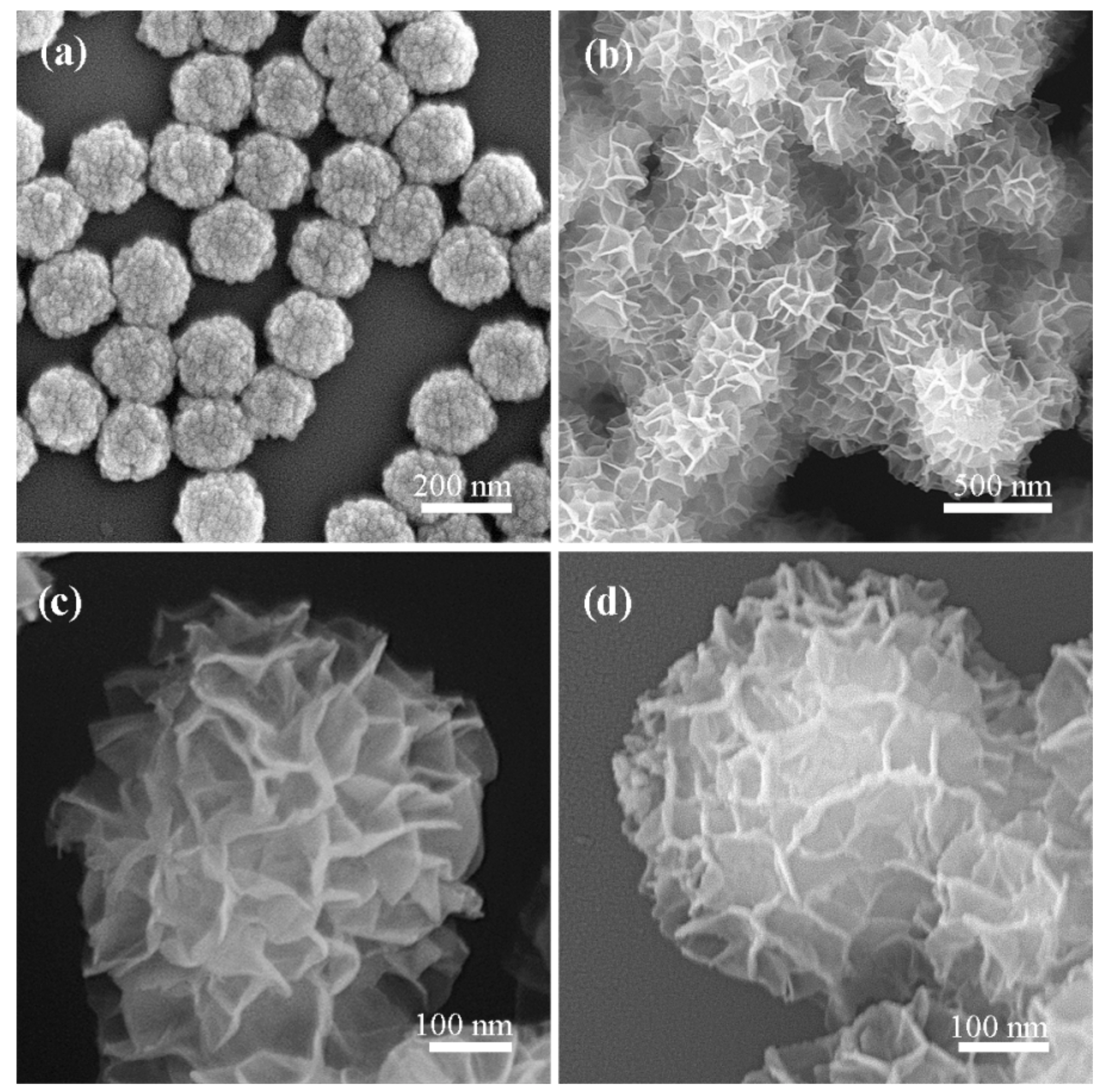
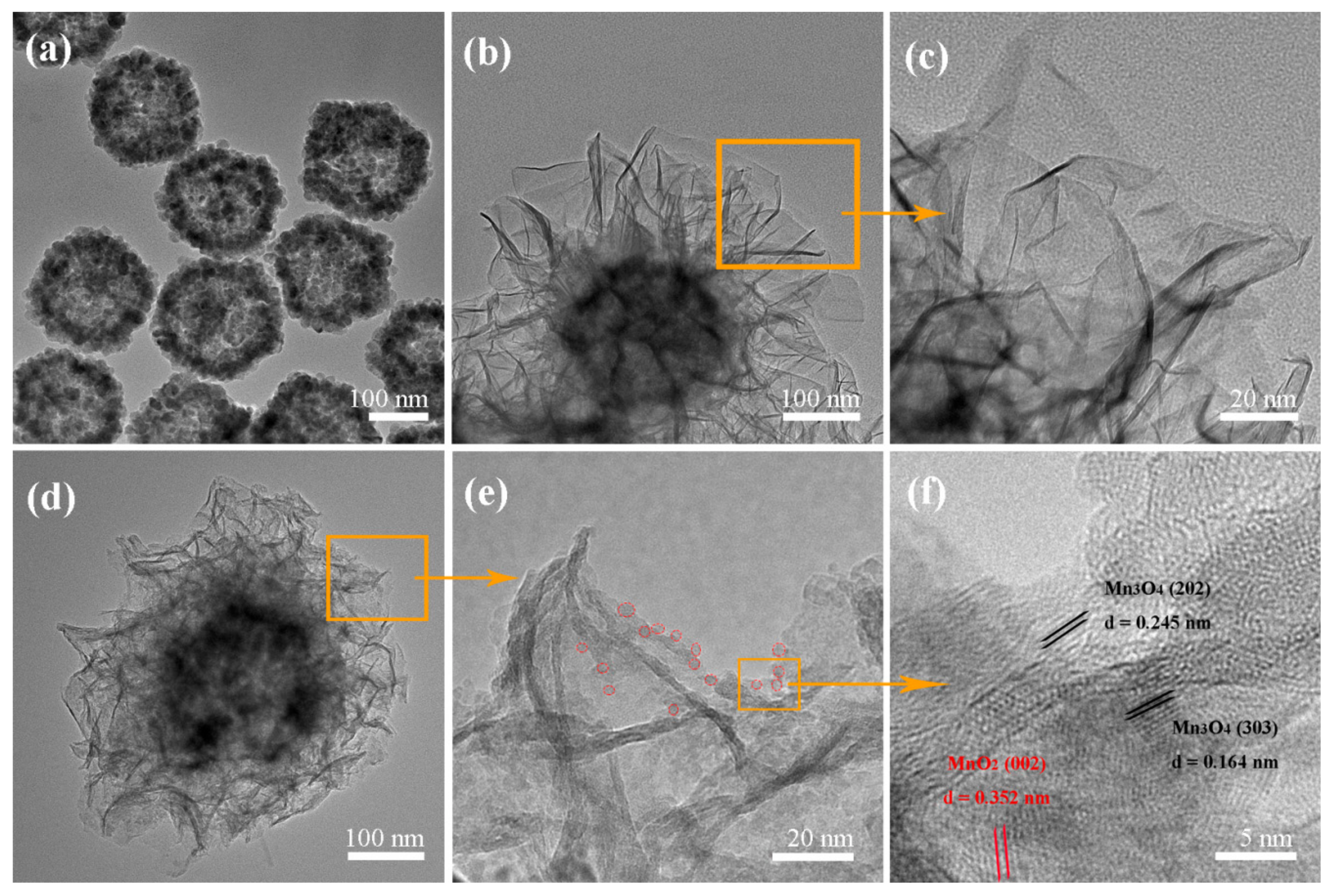
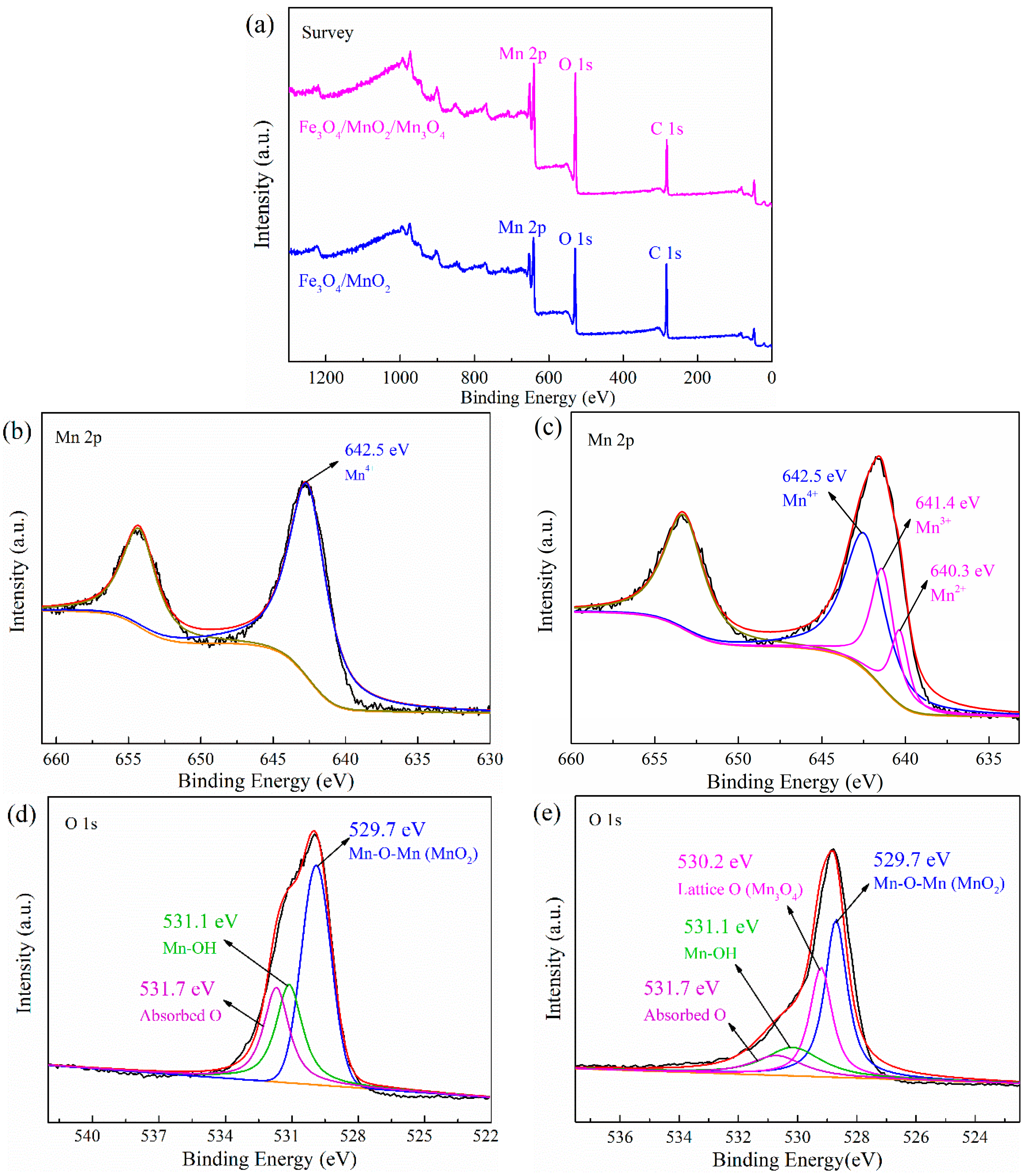
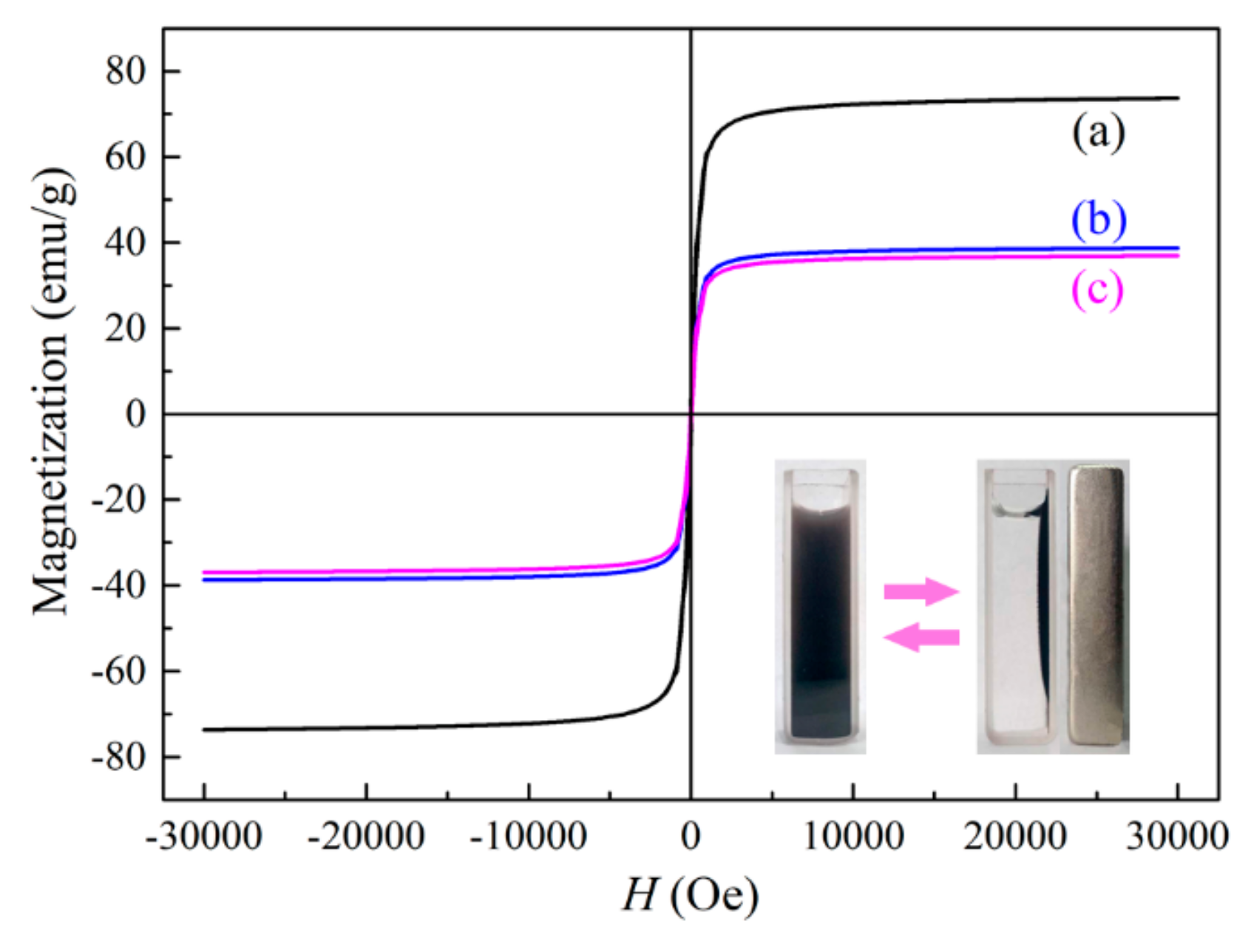
2.1. Characterization of the Photocatalyst
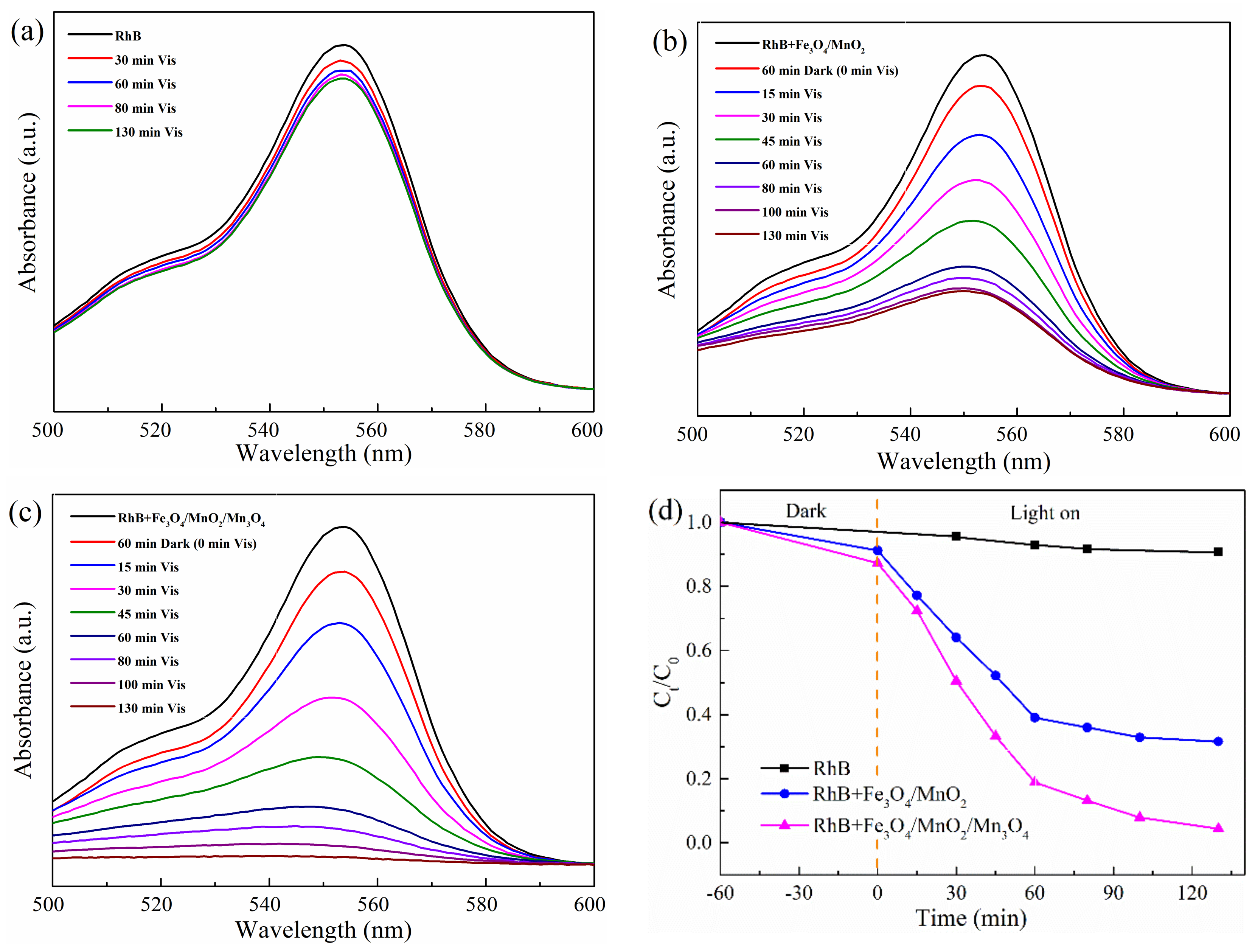
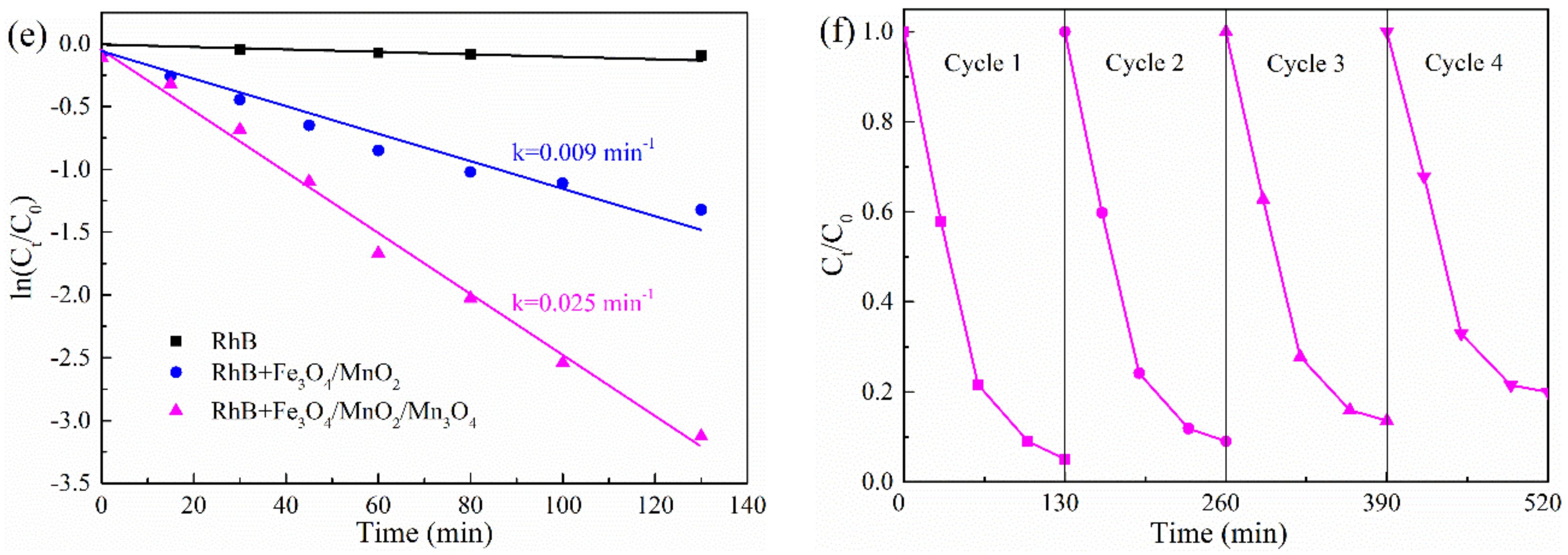
2.2. Photocatalytic Tests
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Flower-Like Fe3O4/MnO2 Microspheres
3.3. Synthesis of Flower-Like Fe3O4/MnO2/Mn3O4 Microspheres
3.4. Photocatalytic Tests
3.5. Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Guo, R.; Jiao, T.; Li, R.; Chen, Y.; Guo, W.; Zhang, L.; Zhou, J.; Zhang, Q.; Peng, Q. Sandwiched Fe3O4/carboxylate graphene oxide nanostructures constructed by layer-by-layer assembly for highly efficient and magnetically recyclable dye removal. ACS Sustain. Chem. Eng. 2018, 6, 1279–1288. [Google Scholar] [CrossRef]
- Kaviyarasu, K.; Kotsedi, L.; Simo, A.; Fuku, X.; Mola, G.T.; Kennedy, J.; Maaza, M. Photocatalytic activity of ZrO 2 doped lead dioxide nanocomposites: Investigation of structural and optical microscopy of RhB organic dye. Appl. Surf. Sci. 2017, 421, 234–239. [Google Scholar] [CrossRef]
- Dong, W.-H.; Wu, D.-D.; Luo, J.-M.; Xing, Q.-J.; Liu, H.; Zou, J.-P.; Luo, X.-B.; Min, X.-B.; Liu, H.-L.; Luo, S.-L.; et al. Coupling of photodegradation of RhB with photoreduction of CO2 over rGO/SrTi0.95Fe0.05O3-delta catalyst: A strategy for one-pot conversion of organic pollutants to methanol and ethanol. J. Catal. 2017, 349, 218–225. [Google Scholar] [CrossRef]
- Chitiphon, C.; Radheshyam, P.; Keiko, S. Dye-sensitized photocatalyst of sepiolite for organic dye degradation. Catalysts 2019, 3, 235. [Google Scholar]
- Oveisi, M.; Asli, M.A.; Mahmoodi, N.M. MIL-Ti metal-organic frameworks (MOFs) nanomaterials as superior adsorbents: Synthesis and ultrasound-aided dye adsorption from multicomponent wastewater systems. J. Hazard. Mater. 2018, 347, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Chen, J.; Wang, J.; Huyan, Y.; Zhang, H.; Zhang, Q. Flowerlike BSA/Zn3(PO4)2/Fe3O4 magnetic hybrid particles: preparation and application to adsorption of copperions. J. Chem. Eng. Data 2018, 63, 3913–3922. [Google Scholar] [CrossRef]
- Zhang, B.; Huyan, Y.; Wang, J.; Chen, X.; Zhang, H.; Zhang, Q. Fe 3 O 4 @SiO 2 @CCS porous magnetic microspheres as adsorbent for removal of organic dyes in aqueous phase. J. Alloy. Compd. 2018, 735, 1986–1996. [Google Scholar] [CrossRef]
- Zhang, J.; Yi, J.; Jiao, Y. Preparation and application of water-soluble TiO2-ionic liquids hybrid nanomaterials. J. Inorg. Mater. 2018, 33, 577–581. [Google Scholar]
- Cheng, L.; Xiang, Q.; Liao, Y.; Zhang, H. CdS-based photocatalysts. Energy Environ. Sci. 2018, 11, 1362–1391. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Jaroniec, M. Hierarchical photocatalysts. Chem. Soc. Rev. 2016, 45, 2603–2636. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Li, Q.; Lv, K.; Li, M. Heterojunction construction between TiO2 hollowsphere and ZnIn2S4 flower for photocatalysis application. Appl. Surf. Sci. 2017, 398, 81–88. [Google Scholar] [CrossRef]
- Samuel Osei-Bonsu, O.; Francis, O.; Govender, P.P. Tuning the electronic and structural properties of Gd-TiO2-GO nanocomposites for enhancing photodegradation of IC dye: The role of Gd3+ ion. Appl. Catal. B: Environ. 2019, 243, 106–120. [Google Scholar]
- Xu, Y.; Li, A.; Yao, T.; Ma, C.; Zhang, X.; Shah, J.H.; Han, H. Strategies for Efficient Charge Separation and Transfer in Artificial Photosynthesis of Solar Fuels. ChemSusChem 2017, 10, 4277–4305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Hou, C.; Zhang, H.; Qiao, M.; Chen, Y.; Zhang, H.; Zhang, Q.; Guo, Z. Morphology-dependent electrochemical supercapacitors in multi-dimensional polyaniline nanostructures. J. Mater. Chem. A 2017, 5, 14041–14052. [Google Scholar] [CrossRef]
- Xiong, X.; Ji, Y.; Xie, M.; You, C.; Yang, L.; Liu, Z.; Asiri, A.M.; Sun, X. MnO2-CoP3 nanowires array: An efficient electrocatalyst for alkaline oxygen evolution reaction with enhanced activity. Electrochem. Commun. 2018, 86, 161–165. [Google Scholar] [CrossRef]
- Miao, L.; Wang, J.; Zhang, P. Review on manganese dioxide for catalytic oxidation of airborne formaldehyde. Appl. Surf. Sci. 2019, 466, 441–453. [Google Scholar] [CrossRef]
- Gao, F.; Tang, X.; Yi, H.; Chu, C.; Li, N.; Li, J.; Zhao, S. In-situ DRIFTS for the mechanistic studies of NO oxidation over alpha-MnO2, beta-MnO2 and gamma-MnO2 catalysts. Chem. Eng. J. 2017, 322, 525–537. [Google Scholar] [CrossRef]
- Jiang, C.; Ge, Y.; Chen, W.; Hua, L.; Li, H.; Zhang, Y.; Cao, S. Hierarchically-structured TiO2/MnO2 hollow spheres exhibiting the complete mineralization of phenol. Catalysts 2019, 9, 13. [Google Scholar] [CrossRef]
- Zhu, G.; Zhu, J.; Jiang, W.; Zhang, W.J.; Zhu, Y.; Zhang, Q. Surface oxygen vacancy induced alpha-MnO2 nanofiber for highly efficient ozone elimination. Appl. Catal. B Environ. 2017, 209, 729–737. [Google Scholar] [CrossRef]
- Tan, X.; Wan, Y.; Huang, Y.; He, C.; Zhang, Z.; He, Z.; Hu, L.; Zeng, J.; Shu, D. Three-dimensional MnO2 porous hollow microspheres for enhanced activity as ozonation catalysts in degradation of bisphenol A. J. Hazard. Mater. 2017, 321, 162–172. [Google Scholar] [CrossRef]
- Zhu, S.; Li, X.; Kang, J.; Duan, X.; Wang, S. Persulfate activation on crystallographic manganese oxides: Mechanism of singlet oxygen evolution for nonradical selective degradation of aqueous contaminants. Environ. Sci. Technol. 2019, 53, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Meng, X.; Tang, X.; Ji, P. Core-Shell MnO2-SiO2 Nanorods for Catalyzing the Removal of Dyes from Water. Catalysts 2017, 7, 19. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction photocatalysts. Adv. Mater. 2017, 29, 20. [Google Scholar] [CrossRef] [PubMed]
- Marschall, R. Semiconductor composites: strategies for enhancing charge carrier separation to improve photocatalytic activity. Adv. Funct. Mater. 2014, 24, 2421–2440. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zheng, Y.; Chen, C.; Zhan, Y.; Lin, X.; Zheng, Q.; Wei, K.; Zhu, J. Network Structured SnO2/ZnO Heterojunction Nanocatalyst with High Photocatalytic Activity. Inorg. Chem. 2009, 48, 1819–1825. [Google Scholar] [CrossRef] [PubMed]
- Chiu, M.-H.; Zhang, C.; Shiu, H.-W.; Chuu, C.-P.; Chen, C.-H.; Chang, C.-Y.S.; Chen, C.-H.; Chou, M.-Y.; Shih, C.-K.; Li, L.-J. Determination of band alignment in the single-layer MoS2/WSe2 heterojunction. Nat. Commun. 2015, 6, 7666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, F.; Zhao, Z.; Xiong, T.; Ni, Z.; Zhang, W.; Sun, Y.; Ho, W.-K. In Situ Construction of g-C 3 N 4 /g-C 3 N 4 Metal-Free Heterojunction for Enhanced Visible-Light Photocatalysis. ACS Appl. Mater. Interfaces 2013, 5, 11392–11401. [Google Scholar] [CrossRef]
- He, Z.; Shi, Y.; Gao, C.; Wen, L.; Chen, J.; Song, S. BiOCl/BiVO4 p-n heterojunction with enhanced photocatalytic activity under visible-light irradiation. J. Phys. Chem. C 2014, 118, 389–398. [Google Scholar] [CrossRef]
- Ma, Y.; Hou, C.; Zhang, H.; Zhang, Q.; Liu, H.; Wu, S.; Guo, Z. Three-dimensional core-shell Fe3O4/Polyaniline coaxial heterogeneous nanonets: Preparation and high performance supercapacitor electrodes. Electrochimica Acta 2019, 315, 114–123. [Google Scholar] [CrossRef]
- Ma, Y.M.; Yang, R.; Feng, L.; Jia, G.; Chen, W.; Li, P.L. Preparation and characterization of magnetic hollow Fe3O4/P(GMA-EGDMA)-SO3H/Au-PPy recyclable catalyst for catalytic reduction of 4-nitrophenol. Appl. Organomet. Chem. 2018, 32, e4534. [Google Scholar]
- Ma, M.; Yang, Y.; Li, W.; Feng, R.; Li, Z.; Lyu, P.; Ma, Y. Gold nanoparticles supported by amino groups on the surface of magnetite microspheres for the catalytic reduction of 4-nitrophenol. J. Mater. Sci. 2019, 54, 323–334. [Google Scholar] [CrossRef]
- Ma, M.; Yang, Y.; Liao, D.; Lyu, P.; Zhang, J.; Liang, J.; Zhang, L. Synthesis, characterization and catalytic performance of core-shell structure magnetic Fe3O4/P(GMA-EGDMA)-NH2/HPG-COOH-Pd catalyst. Appl. Organometall. Chem. 2019, 33, e4708. [Google Scholar] [CrossRef]
- Ma, M.; Yang, Y.; Liu, Y.; Li, W.; Chen, G.; Ma, Y.; Lyu, P.; Li, S.; Wang, Y.; Wu, G. Preparation of magnetic Fe 3 O 4 /P (GMA-DVB)-PEI/Pd highly efficient catalyst with core-shell structure. Appl. Organomet. Chem. 2019, 33, e4850. [Google Scholar] [CrossRef]
- Shokouhimehr, M.; Hong, K.; Lee, T.H.; Moon, C.W.; Hong, S.-P.; Zhang, K.; Suh, J.M.; Choi, K.S.; Varma, R.S.; Jang, H.W. Magnetically retrievable nanocomposite adorned with Pd nanocatalysts: Efficient reduction of nitroaromatics in aqueous media. Green Chem. 2018, 20, 3809–3817. [Google Scholar] [CrossRef]
- Ahadi, A.; Rostamnia, S.; Panahi, P.; Wilson, L.D.; Kong, Q.; An, Z.; Shokouhimehr, M. Palladium Comprising Dicationic Bipyridinium Supported Periodic Mesoporous Organosilica (PMO): Pd@Bipy–PMO as an Efficient Hybrid Catalyst for Suzuki–Miyaura Cross-Coupling Reaction in Water. Catalysts 2019, 9, 140. [Google Scholar] [CrossRef]
- Ma, M.; Zhang, Q.; Dou, J.; Zhang, H.; Geng, W.; Yin, D.; Chen, S. Fabrication of 1D Fe3O4/P(NIPAM-MBA) thermosensitive nanochains by magnetic-field-induced precipitation polymerization. Colloid Polym. Sci. 2012, 290, 1207–1213. [Google Scholar] [CrossRef]
- Qiao, M.; Lei, X.; Ma, Y.; Tian, L.; Wang, W.; Su, K.; Zhang, Q. Facile synthesis and enhanced electromagnetic microwave absorption performance for porous core-shell Fe3O4@MnO2 composite microspheres with lightweight feature. J. Alloy. Compd. 2017, 693, 432–439. [Google Scholar] [CrossRef]
- Geng, Z.; Wang, Y.; Liu, J.; Li, G.; Li, L.; Huang, K.; Yuan, L.; Feng, S. Delta-MnO2-Mn3O4 nanocomposite for photochemical water oxidation: Active structure stabilized in the interface. ACS Appl. Mater. Interfaces 2016, 8, 27825–27831. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, Z.; Li, N.; Nan, J.; Yu, R.; Du, J. Visible-light-driven photocatalytic degradation of ciprofloxacin by a ternary Mn2O3/Mn3O4/MnO2 valence state heterojunction. Chem. Eng. J. 2018, 353, 805–813. [Google Scholar] [CrossRef]
- Xiong, M.; Chen, L.; Yuan, Q.; He, J.; Luo, S.-L.; Au, C.-T.; Yin, S.-F. Controlled synthesis of graphitic carbon nitride/beta bismuth oxide composite and its high visible-light photocatalytic activity. Carbon 2015, 86, 217–224. [Google Scholar] [CrossRef]
- Ramírez, A.; Hillebrand, P.; Stellmach, D.; May, M.; Bogdanoff, P.; Fiechter, S. Evaluation of MnOx, Mn2O3, and Mn3O4 electrodeposited films for the oxygen evolution reaction of water. J. Phys. Chem. C 2014, 118, 14073–14081. [Google Scholar] [CrossRef]
- Zhao, J.; Nan, J.; Zhao, Z.; Li, N.; Liu, J.; Cui, F. Energy-efficient fabrication of a novel multivalence Mn3O4-MnO2 heterojunction for dye degradation under visible light irradiation. Appl. Catal. B: Environ. 2017, 202, 509–517. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, M.; Yin, X.; Shao, Q.; Lu, N.; Feng, Y.; Lu, Y.; Wujcik, E.K.; Mai, X.; Wang, C.; et al. Tuning polyaniline nanostructures via end group substitutions and their morphology dependent electrochemical performances. Polym. 2018, 156, 128–135. [Google Scholar] [CrossRef]
- Sun, H.; He, Q.; She, P.; Zeng, S.; Xu, K.; Li, J.; Liang, S.; Liu, Z. One-pot synthesis of Au@TiO 2 yolk-shell nanoparticles with enhanced photocatalytic activity under visible light. J. Colloid Interface Sci. 2017, 505, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Zhu, B.; Cheng, B.; Yu, J.; Xu, J. 2D/2D g-C3N4/MnO2 nanocomposite as a direct Z-scheme photocatalyst for enhanced photocatalytic activity. ACS Sustain. Chem. Eng. 2018, 6, 965–973. [Google Scholar] [CrossRef]
- Zheng, X.Z.; Han, W.; Yang, F.; Qu, B.; Liu, X.W. 3D Co3O4@MnO2 heterostructures grown on a flexible substrate and their applications in super-capacitor electrodes and photocatalysts. Dalton Trans. 2016, 45, 16850–16858. [Google Scholar]
- Zhou, J.; Wang, Y.; Ma, Y.; Zhang, B.; Zhang, Q. Surface molecularly imprinted thermo-sensitive polymers based on light-weight hollow magnetic microspheres for specific recognition of BSA. Appl. Surf. Sci. 2019, 486, 265–273. [Google Scholar] [CrossRef]
- Zhang, L.; Lian, J.; Wu, L.; Duan, Z.; Jiang, J.; Zhao, L. Synthesis of a Thin-Layer MnO2 Nanosheet-Coated Fe3O4 Nanocomposite as a Magnetically Separable Photocatalyst. Langmuir 2014, 30, 7006–7013. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Zhang, H.; Fan, X.; Liu, Y.; Zhang, Q. One-pot hydrothermal synthesis of highly monodisperse water-dispersible hollow magnetic microspheres and construction of photonic crystals. Chem. Eng. J. 2015, 259, 779–786. [Google Scholar] [CrossRef]

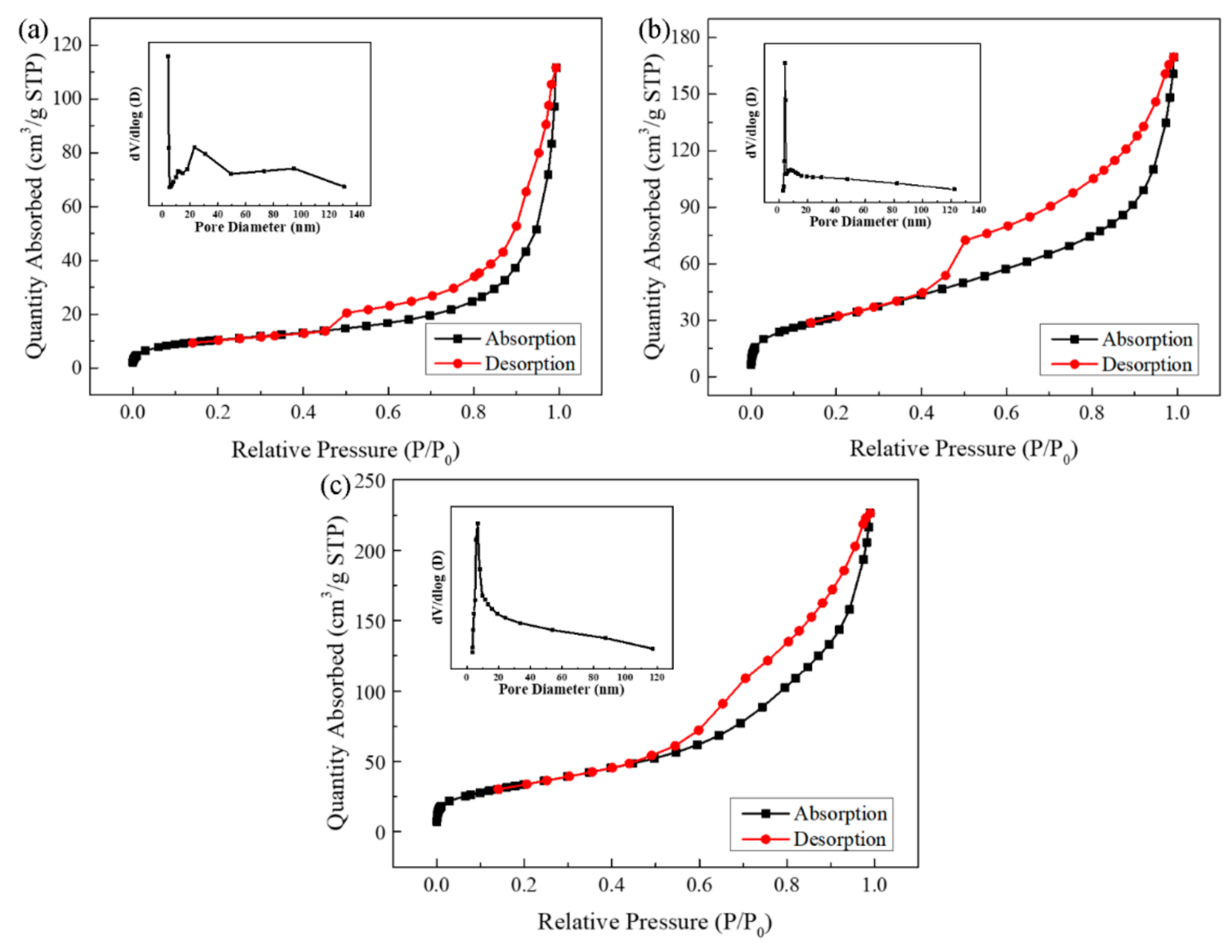


| Sample | Surface Area (m2/g) | Pore Volume (cm3/g) |
|---|---|---|
| Fe3O4 | 38.47 | 0.17 |
| Fe3O4/MnO2 | 117.66 | 0.27 |
| Fe3O4/MnO2/Mn3O4 | 143.03 | 0.34 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, M.; Yang, Y.; Chen, Y.; Wu, F.; Li, W.; Lyu, P.; Ma, Y.; Tan, W.; Huang, W. Synthesis of Hollow Flower-Like Fe3O4/MnO2/Mn3O4 Magnetically Separable Microspheres with Valence Heterostructure for Dye Degradation. Catalysts 2019, 9, 589. https://doi.org/10.3390/catal9070589
Ma M, Yang Y, Chen Y, Wu F, Li W, Lyu P, Ma Y, Tan W, Huang W. Synthesis of Hollow Flower-Like Fe3O4/MnO2/Mn3O4 Magnetically Separable Microspheres with Valence Heterostructure for Dye Degradation. Catalysts. 2019; 9(7):589. https://doi.org/10.3390/catal9070589
Chicago/Turabian StyleMa, Mingliang, Yuying Yang, Yan Chen, Fei Wu, Wenting Li, Ping Lyu, Yong Ma, Weiqiang Tan, and Weibo Huang. 2019. "Synthesis of Hollow Flower-Like Fe3O4/MnO2/Mn3O4 Magnetically Separable Microspheres with Valence Heterostructure for Dye Degradation" Catalysts 9, no. 7: 589. https://doi.org/10.3390/catal9070589
APA StyleMa, M., Yang, Y., Chen, Y., Wu, F., Li, W., Lyu, P., Ma, Y., Tan, W., & Huang, W. (2019). Synthesis of Hollow Flower-Like Fe3O4/MnO2/Mn3O4 Magnetically Separable Microspheres with Valence Heterostructure for Dye Degradation. Catalysts, 9(7), 589. https://doi.org/10.3390/catal9070589




