Degradation of Sulfamethoxazole Using Iron-Doped Titania and Simulated Solar Radiation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural, Optical and Morphological Properties of Iron-Doped Materials
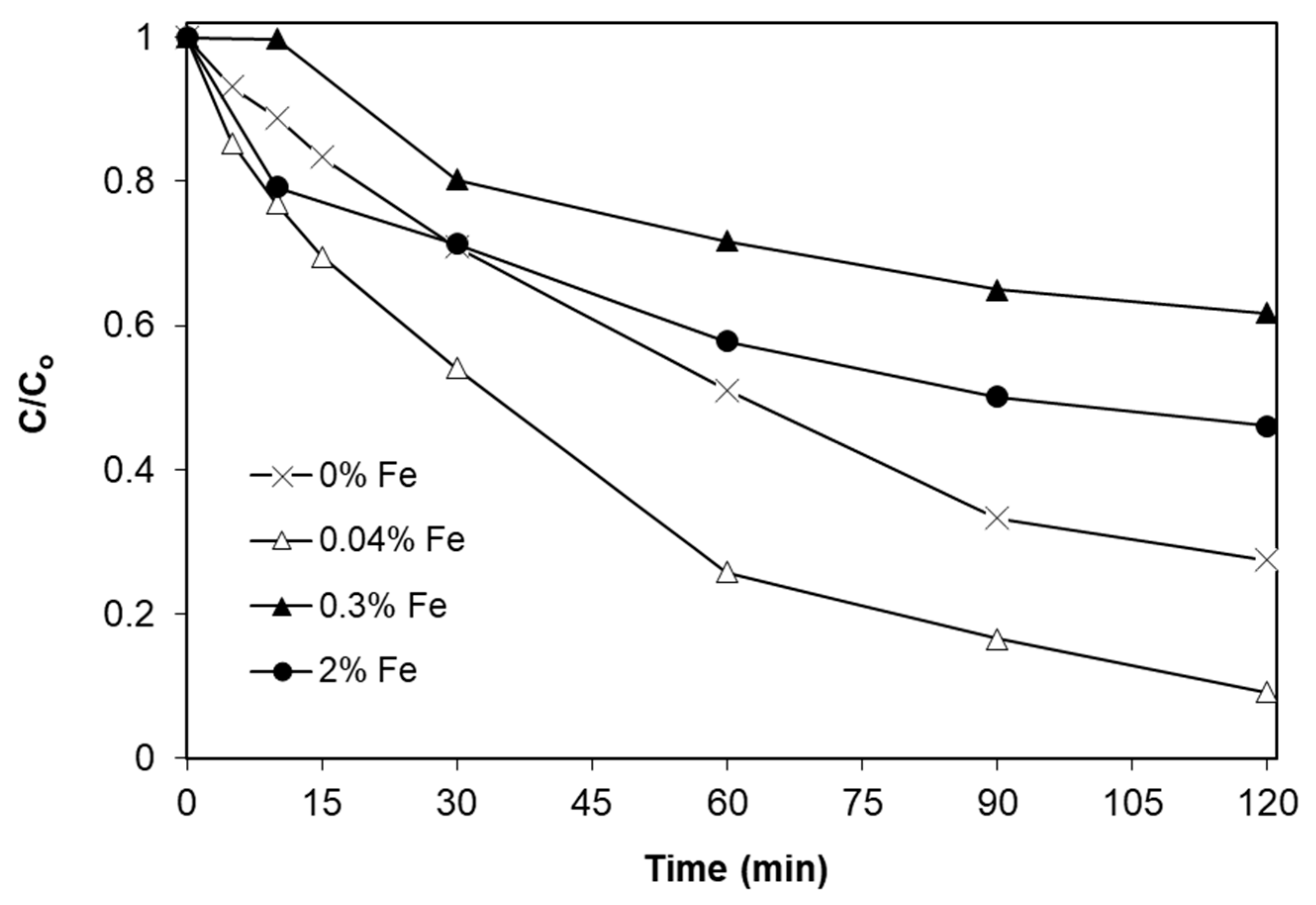
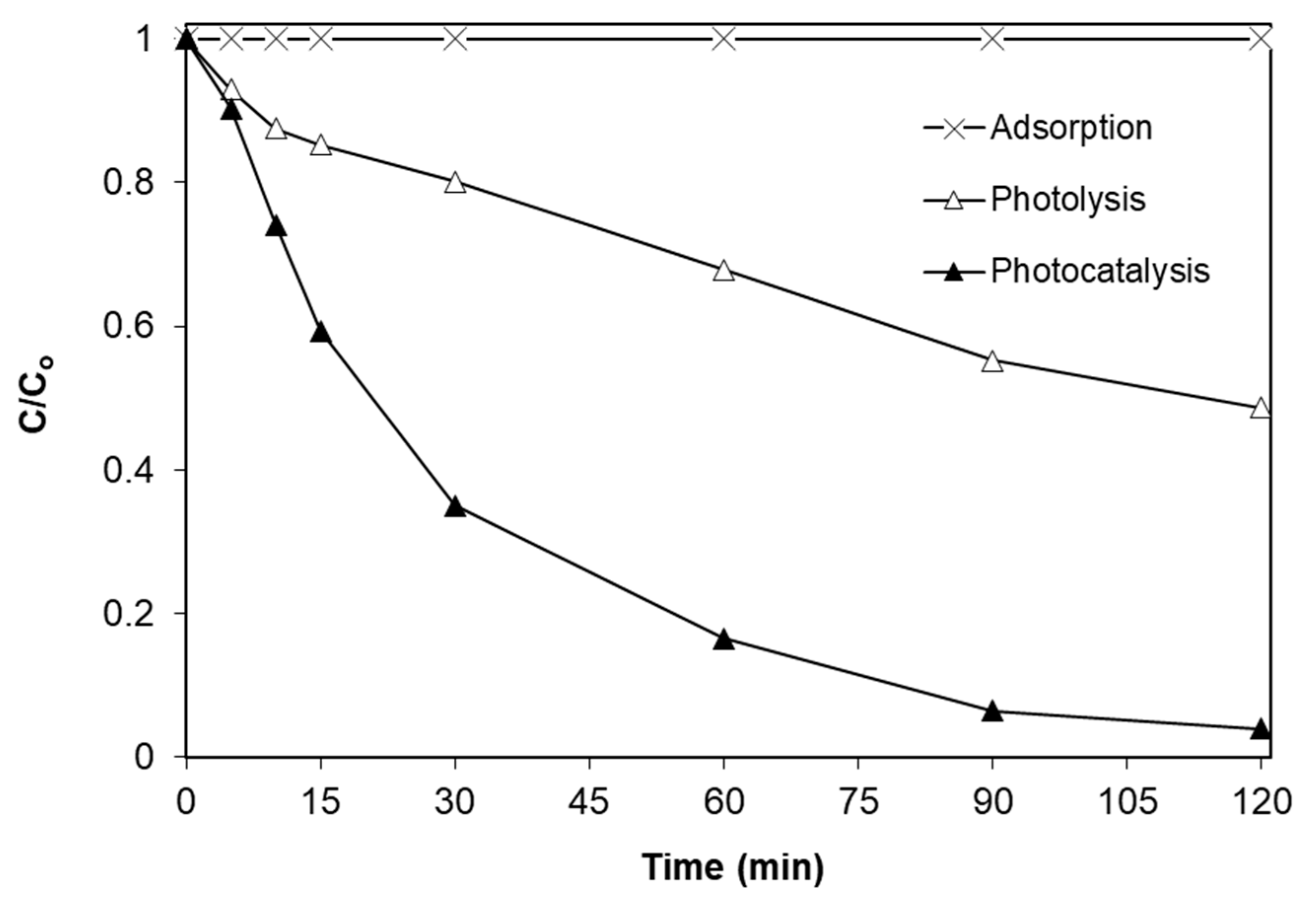
2.2. Effect of Iron Doping Level
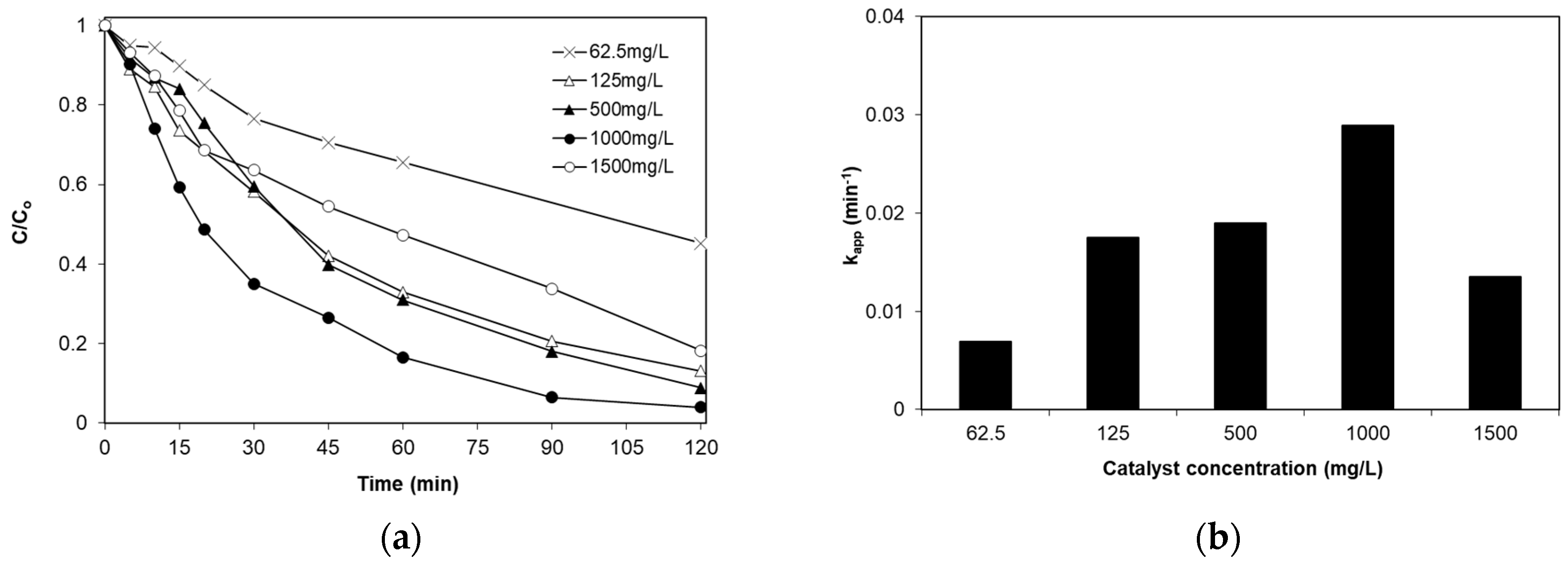
2.3. Effect of Catalyst Loading
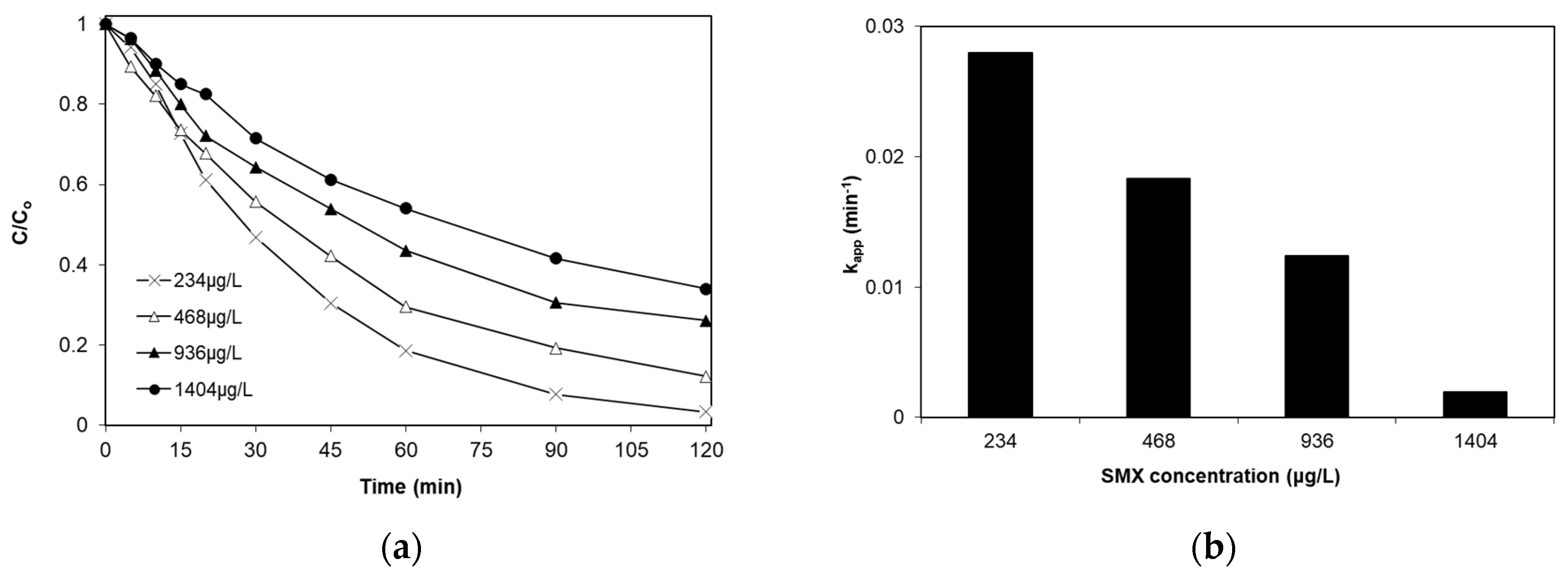
2.4. Effect of Initial SMX Concentration
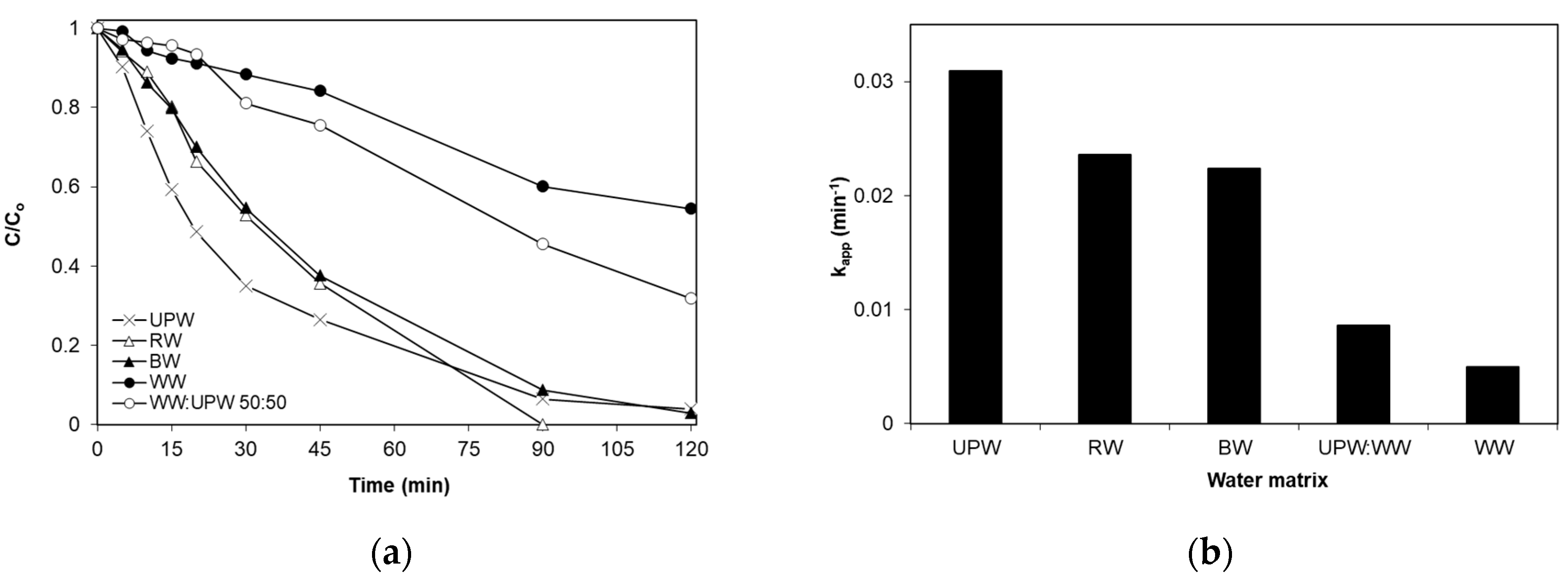
2.5. Effect of pH and the Water Matrix
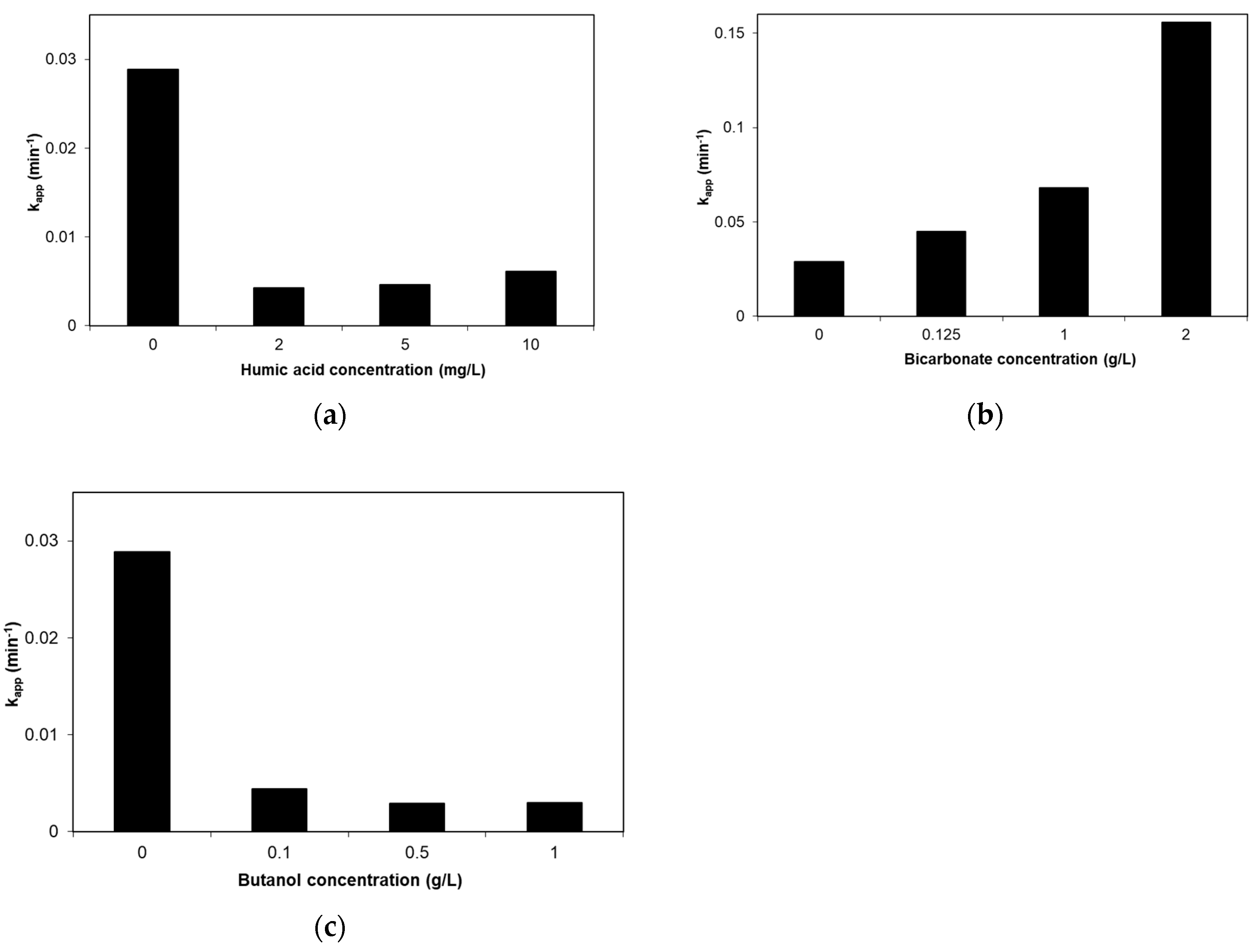
2.6. Effect of Humic Acid, Bicarbonate and Tert-Butanol
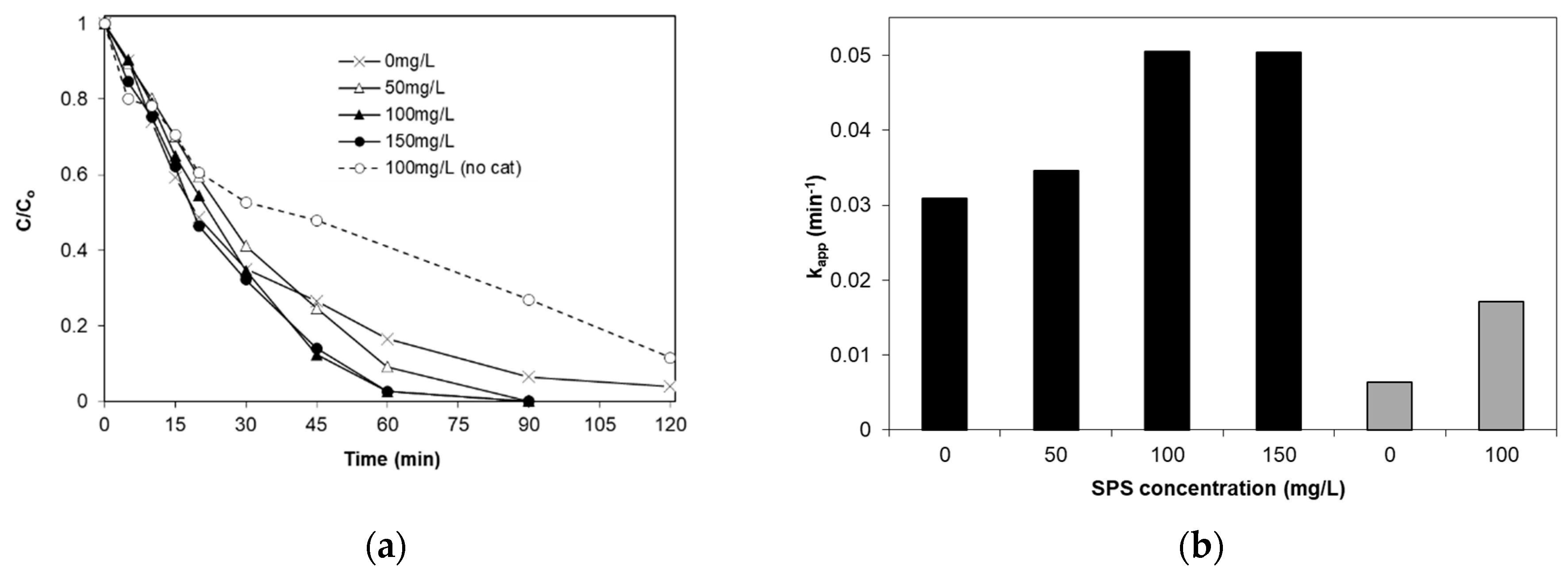
2.7. Effect of Sodium Persulfate
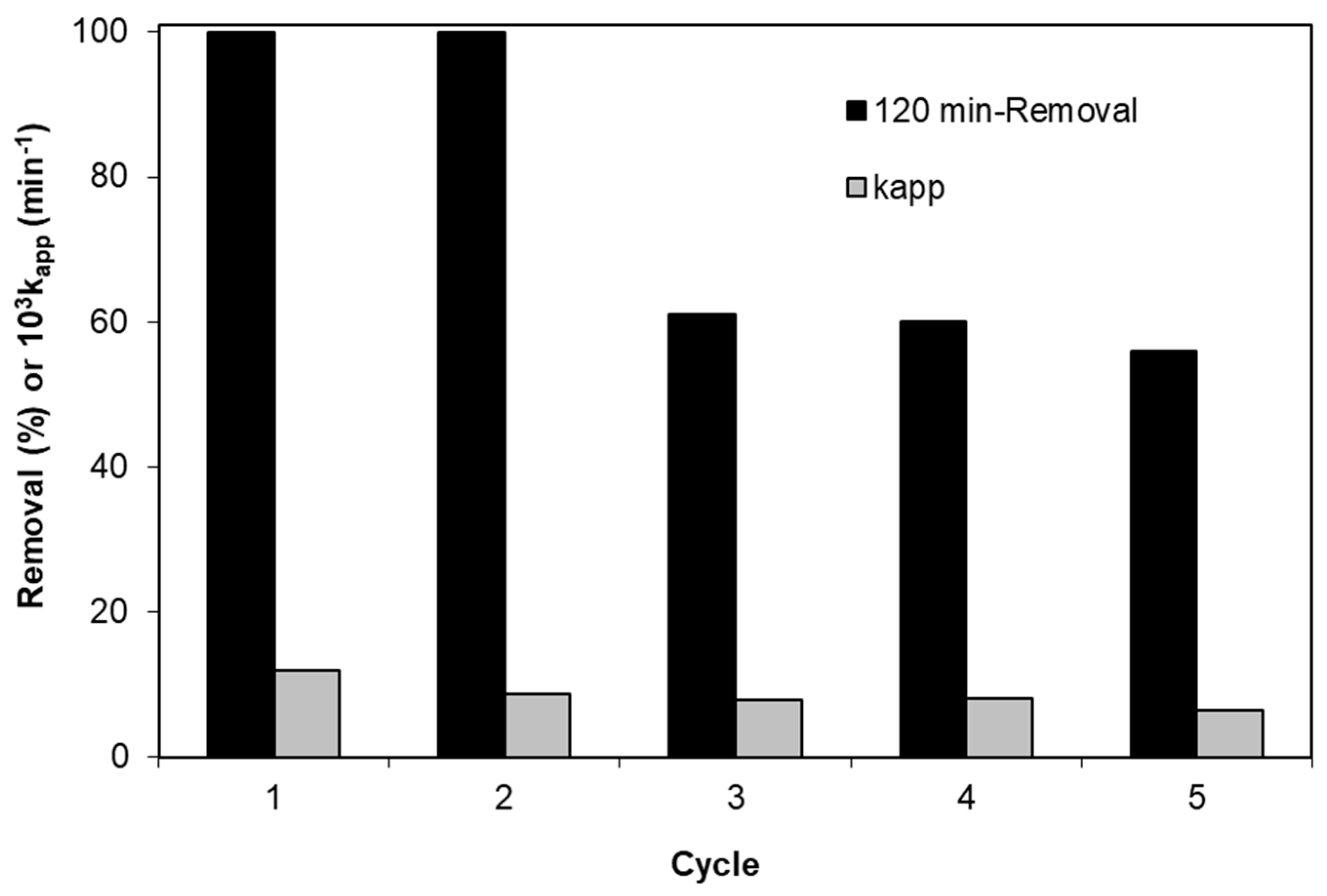
2.8. Catalyst Reuse
3. Materials and Methods
3.1. Catalyst Synthesis and Characterization
3.2. Chemicals
3.3. Photocatalytic Experiments
3.4. High Performance Liquid Chromatography
3.5. Water Matrices
4. Conclusions
- The degradation of SMX follows pseudo-first-order kinetics with the apparent kinetic constant decreasing with increasing initial SMX concentration. The reaction is favored at low levels of iron doping (0.04%), increased catalyst concentrations (up to 1 g/L) and at the solution’s inherent pH.
- Complex water matrices such as secondary treated wastewaters considerably retard SMX degradation, and this may partly be associated with the presence of species, such as humic acid. Conversely, bicarbonate seems to have a positive effect.
- The presence of an electron acceptor (sodium persulfate) enhances SMX removal. The contribution of persulfate activation to the photocatalytic SMX degradation is additive rather than synergistic.
- Partial photocatalyst deactivation occurs after five successive experiments with the same catalyst sample.
- The same family of iron-doped titania samples was employed in our previous work [29] for the effective photocatalytic elimination of Staphylococcus aureus; the implications for water/wastewater treatment are encouraging since modified titania can exploit solar light for the combined decontamination and disinfection of polluted waters.
Author Contributions
Funding
Conflicts of Interest
References
- Aminov, R.I. A Brief History of the Antibiotic Era: Lessons Learned and Challenges for the Future. Front. Microbiol. 2010, 1, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischbach, M.A.; Walsh, C.T. Antibiotics for emerging pathogens. Science 2009, 325, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Jaimes, J.A.; Postigo, C.; Melgoza-Alemán, R.M.; Aceña, J.; Barceló, D.; López de Alda, M. Study of pharmaceuticals in surface and wastewater from Cuernavaca, Morelos, Mexico: Occurrence and environmental risk assessment. Sci. Total Environ. 2018, 613–614, 1263–1274. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U.; Mohan, D. Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quesada, H.B.; Baptista, A.T.A.; Cusioli, L.F.; Seibert, D.; Bezerra, C.D.O.; Bergamasco, R. Surface water pollution by pharmaceuticals and an alternative of removal by low-cost adsorbents: A review. Chemosphere 2019, 222, 766–780. [Google Scholar] [CrossRef] [PubMed]
- White, D.; Lapworth, D.J.; Civil, W.; Williams, P. Tracking changes in the occurrence and source of pharmaceuticals within the River Thames, UK; from source to sea. Environ. Pollut. 2019, 249, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Deng, H.; Benskin, J.P.; Radke, M. Biodegradation of sulfamethoxazole photo-transformation products in a water/sediment test. Chemosphere 2016, 148, 518–525. [Google Scholar] [CrossRef]
- Barnes, K.K.; Kolpin, D.W.; Furlong, E.T.; Zaugg, S.D.; Meyer, M.T.; Barber, L.B. A national reconnaissance of pharmaceuticals and other organic wastewater contaminants in the United States—I) Groundwater. Sci. Total Environ. 2008, 402, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Batt, A.L.; Kim, S.; Aga, D.S. Comparison of the occurrence of antibiotics in four full-scale wastewater treatment plants with varying designs and operations. Chemosphere 2007, 68, 428–435. [Google Scholar] [CrossRef]
- Rizzo, L.; Manaia, C.; Merlin, C.; Schwartz, T.; Dagot, C.; Ploy, M.; Michael, I.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: A review. Sci. Total Environ. 2013, 447, 345–360. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhuang, Y.; Geng, J.; Ren, H.; Xu, K.; Ding, L. Reduction of antibiotic resistance genes in municipal wastewater effluent by advanced oxidation processes. Sci. Total Environ. 2016, 550, 184–191. [Google Scholar] [CrossRef]
- Dantas, R.F.; Contreras, S.; Sans, C.; Esplugas, S. Sulfamethoxazole abatement by means of ozonation. J. Hazard. Mater. 2008, 150, 790–794. [Google Scholar] [CrossRef]
- Boudreau, J.; Bejan, D.; Li, S.; Bunce, N.J. Competition between Electrochemical Advanced Oxidation and Electrochemical Hypochlorination of Sulfamethoxazole at a Boron-Doped Diamond Anode. Ind. Eng. Chem. Res. 2010, 49, 2537–2542. [Google Scholar] [CrossRef]
- Ribeiro, R.S.; Frontistis, Z.; Mantzavinos, D.; Venieri, D.; Antonopoulou, M.; Konstantinou, I.; Silva, A.M.; Faria, J.L.; Gomes, H.T. Magnetic carbon xerogels for the catalytic wet peroxide oxidation of sulfamethoxazole in environmentally relevant water matrices. Appl. Catal. B Environ. 2016, 199, 170–186. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J. Comparative study on sulfamethoxazole degradation by Fenton and Fe(ii)-activated persulfate process. RSC Adv. 2017, 7, 48670–48677. [Google Scholar] [CrossRef] [Green Version]
- Kemmou, L.; Frontistis, Z.; Vakros, J.; Manariotis, I.D.; Mantzavinos, D. Degradation of antibiotic sulfamethoxazole by biochar-activated persulfate: Factors affecting the activation and degradation processes. Catal. Today 2018, 313, 128–133. [Google Scholar] [CrossRef]
- Al-Hamadani, Y.A.; Chu, K.H.; Flora, J.R.; Kim, D.-H.; Jang, M.; Sohn, J.; Joo, W.; Yoon, Y. Sonocatalytical degradation enhancement for ibuprofen and sulfamethoxazole in the presence of glass beads and single-walled carbon nanotubes. Ultrason. Sonochem. 2016, 32, 440–448. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, X.; Jiang, J.; Ma, J.; Liu, G.; Cao, Y.; Liu, W.; Li, J.; Pang, S.; Kong, X.; et al. Degradation of sulfamethoxazole by UV, UV/H2O2 and UV/persulfate (PDS): Formation of oxidation products and effect of bicarbonate. Water Res. 2017, 118, 196–207. [Google Scholar] [CrossRef]
- Mirzaei, A.; Yerushalmi, L.; Chen, Z.; Haghighat, F.; Guo, J. Enhanced photocatalytic degradation of sulfamethoxazole by zinc oxide photocatalyst in the presence of fluoride ions: Optimization of parameters and toxicological evaluation. Water Res. 2018, 132, 241–251. [Google Scholar] [CrossRef]
- Grilla, E.; Petala, A.; Frontistis, Z.; Konstantinou, I.K.; Kondarides, D.I.; Mantzavinos, D. Solar photocatalytic abatement of sulfamethoxazole over Ag3PO4/WO3 composites. Appl. Catal. B Environ. 2018, 231, 73–81. [Google Scholar] [CrossRef]
- Ioannidou, E.; Frontistis, Z.; Antonopoulou, M.; Venieri, D.; Konstantinou, I.; Kondarides, D.I.; Mantzavinos, D. Solar photocatalytic degradation of sulfamethoxazole over tungsten—Modified TiO2. Chem. Eng. J. 2017, 318, 143–152. [Google Scholar] [CrossRef]
- Długosz, M.; Żmudzki, P.; Kwiecień, A.; Szczubiałka, K.; Krzek, J.; Nowakowska, M. Photocatalytic degradation of sulfamethoxazole in aqueous solution using a floating TiO2-expanded perlite photocatalyst. J. Hazard. Mater. 2015, 298, 146–153. [Google Scholar]
- Bhatkhande, D.S.; Pangarkar, V.G.; Beenackers, A.A. Photocatalytic degradation for environmental applications—A review. J. Chem. Technol. Biotechnol. 2002, 77, 102–116. [Google Scholar] [CrossRef]
- Braham, R.J.; Harris, A.T. Review of Major Design and Scale-up Considerations for Solar Photocatalytic Reactors. Ind. Eng. Chem. Res. 2009, 48, 8890–8905. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.; Hamilton, J.W.; Byrne, J.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Fang, Y.; Li, Z.; Xu, S. Visible-Light Nanostructured Photocatalysts—A Review. J. Nanosci. Nanotechnol. 2015, 15, 889–920. [Google Scholar] [CrossRef]
- Petala, A.; Noe, A.; Frontistis, Z.; Drivas, C.; Kennou, S.; Mantzavinos, D.; Kondarides, D.I. Synthesis and characterization of CoOx/BiVO4 photocatalysts for the degradation of propyl paraben. J. Hazard. Mater. 2019, 372, 52–60. [Google Scholar] [CrossRef]
- Repousi, V.; Petala, A.; Frontistis, Z.; Antonopoulou, M.; Konstantinou, I.; Kondarides, D.I.; Mantzavinos, D. Photocatalytic degradation of bisphenol A over Rh/TiO2 suspensions in different water matrices. Catal. Today 2017, 284, 59–66. [Google Scholar] [CrossRef]
- Venieri, D.; Tournas, F.; Gounaki, I.; Binas, V.; Zachopoulos, A.; Kiriakidis, G.; Mantzavinos, D. Inactivation of Staphylococcus aureus in water by means of solar photocatalysis using metal doped TiO2 semiconductors. J. Chem. Technol. Biotechnol. 2017, 92, 43–51. [Google Scholar] [CrossRef]
- Majeed Khan, M.A.; Siwach, R.; Kumar, S.; Alhazaa, A.N. Role of Fe doping in tuning photocatalytic and photoelectrochemical properties of TiO2 for photodegradation of methylene blue. Opt. Laser Technol. 2019, 118, 170–178. [Google Scholar] [CrossRef]
- Moradi, V.; Ahmed, F.; Jun, M.B.G.; Blackburn, A.; Herring, R.A. Acid-treated Fe-doped TiO2 as a high performance photocatalyst used for degradation of phenol under visible light irradiation. J. Environ. Sci. 2019, 83, 183–194. [Google Scholar] [CrossRef]
- Crişan, M.; Mardare, D.; Ianculescu, A.; DrĂgan, N.; Nitoi, I.; Crişan, D.; Voicescu, M.; Todan, L.; Oancea, P.; Adomnitei, C.; et al. Iron doped TiO2 films and their photoactivity in nitrobenzene removal from water. Appl. Surf. Sci. 2018, 455, 201–215. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, Y.; Liu, X.; Jin, H.; Lv, H.; He, S.; Hao, H.; Li, C. A Mild in-Situ Method to Construct Fe-Doped Cauliflower-Like Rutile TiO2 Photocatalysts for Degradation of Organic Dye in Wastewater. Catalysts 2019, 9, 426. [Google Scholar] [CrossRef]
- Vargas, X.; Tauchert, E.; Marin, J.-M.; Restrepo, G.; Dillert, R.; Bahnemann, D. Fe-doped titanium dioxide synthesized: Photocatalytic activity and mineralization study for azo dye. J. Photochem. Photobiol. A Chem. 2012, 243, 17–22. [Google Scholar] [CrossRef]
- Li, X.; Yue, P.-L.; Kutal, C. Synthesis and photocatalytic oxidation properties of iron doped titanium dioxide nanosemiconductor particles. New J. Chem. 2003, 27, 1264. [Google Scholar] [CrossRef]
- Daskalaki, V.M.; Kondarides, D.I. Efficient production of hydrogen by photo-induced reforming of glycerol at ambient conditions. Catal. Today 2009, 144, 75–80. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Dimitrakopoulou, D.; Rethemiotaki, I.; Frontistis, Z.; Xekoukoulotakis, N.P.; Venieri, D.; Mantzavinos, D. Degradation, mineralization and antibiotic inactivation of amoxicillin by UV-A/TiO2 photocatalysis. J. Environ. Manag. 2012, 98, 168–174. [Google Scholar] [CrossRef]
- Dirany, A.; Sirés, I.; Oturan, N.; Oturan, M.A. Electrochemical abatement of the antibiotic sulfamethoxazole from water. Chemosphere 2010, 81, 594–602. [Google Scholar] [CrossRef]
- Safari, G.H.; Nasseri, S.; Mahvi, A.H.; Yaghmaeian, K.; Nabizadeh, R.; Alimohammadi, M. Optimization of sonochemical degradation of tetracycline in aqueous solution using sono-activated persulfate process. J. Environ. Health Sci. Eng. 2015, 13, 24. [Google Scholar] [CrossRef]
- Xekoukoulotakis, N.P.; Drosou, C.; Brebou, C.; Chatzisymeon, E.; Hapeshi, E.; Fatta-Kassinos, D.; Mantzavinos, D. Kinetics of UV-A/TiO2 photocatalytic degradation and mineralization of the antibiotic sulfamethoxazole in aqueous matrices. Catal. Today 2011, 161, 163–168. [Google Scholar] [CrossRef]
- Choi, J.; Lee, H.; Choi, Y.; Kim, S.; Lee, S.; Lee, S.; Choi, W.; Lee, J. Heterogeneous photocatalytic treatment of pharmaceutical micropollutants: Effects of wastewater effluent matrix and catalyst modifications. Appl. Catal. B Environ. 2014, 147, 8–16. [Google Scholar] [CrossRef]
- Xu, H.; Cooper, W.J.; Jung, J.; Song, W. Photosensitized degradation of amoxicillin in natural organic matter isolate solutions. Water Res. 2011, 45, 632–638. [Google Scholar] [CrossRef]
- Stefan, M.I. Advanced Oxidation Processes for Water Treatment: Fundamentals and Applications; IWA Publishing: London, UK, 2017. [Google Scholar]
- Chen, S.-N.; Hoffman, M.Z.; Parsons, G.H. Reactivity of the carbonate radical toward aromatic compounds in aqueous solution. J. Phys. Chem. 1975, 79, 1911–1912. [Google Scholar] [CrossRef]
- Metheniti, M.E.; Frontistis, Z.; Ribeiro, R.S.; Silva, A.M.T.; Faria, J.L.; Gomes, H.T.; Mantzavinos, D. Degradation of propyl paraben by activated persulfate using iron-containing magnetic carbon xerogels: Investigation of water matrix and process synergy effects. Environ. Sci. Pollut. Res. 2018, 25, 34801–34810. [Google Scholar] [CrossRef]
- Ioannidi, A.; Frontistis, Z.; Mantzavinos, D. Destruction of propyl paraben by persulfate activated with UV-A light emitting diodes. J. Environ. Chem. Eng. 2018, 6, 2992–2997. [Google Scholar] [CrossRef]
- Wang, W.; Wang, H.; Li, G.; An, T.; Zhao, H.; Wong, P.K. Catalyst-free activation of persulfate by visible light for water disinfection: Efficiency and mechanisms. Water Res. 2019, 157, 106–118. [Google Scholar] [CrossRef]
- Frontistis, Z.; Mantzavinos, D. Chapter 11: Advanced oxidation processes for wastewater treatment. In Wastewater and Biosolids Management, 1st ed.; Kalavrouziotis, I., Ed.; IWA Publishing: London, UK, 2017. [Google Scholar]
- Özkal, C.B.; Frontistis, Z.; Antonopoulou, M.; Konstantinou, I.; Mantzavinos, D.; Meriç, S. Removal of antibiotics in a parallel-plate thin-film-photocatalytic reactor: Process modeling and evolution of transformation by-products and toxicity. J. Environ. Sci. 2017, 60, 114–122. [Google Scholar] [CrossRef]

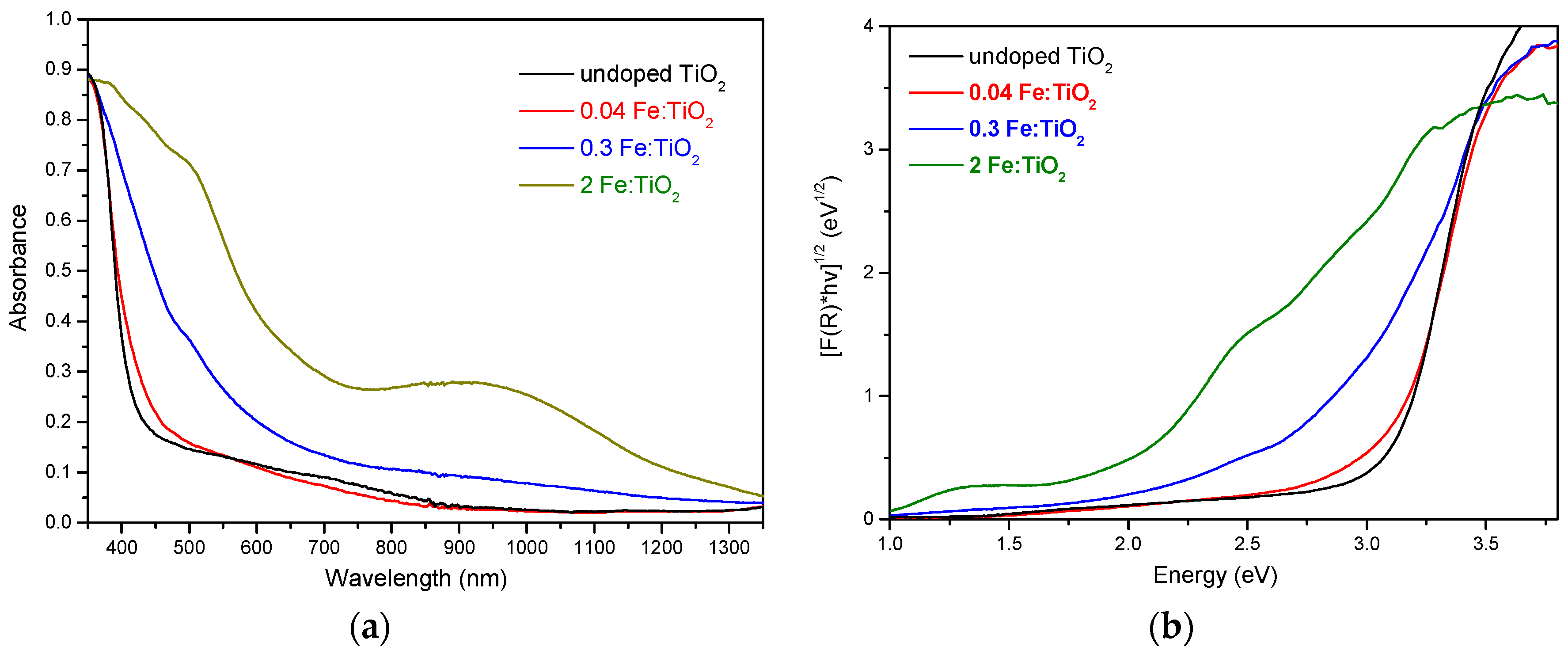
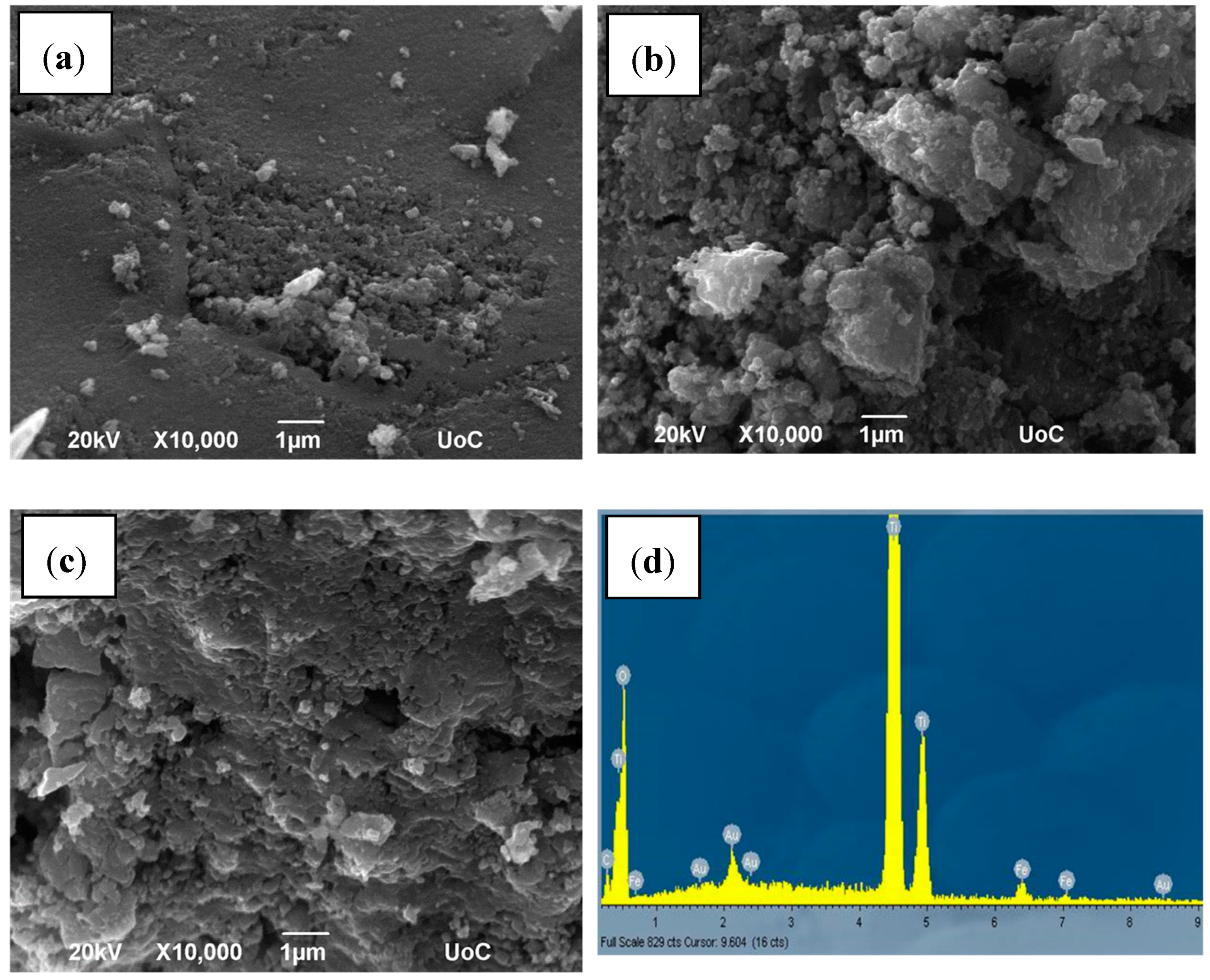

| Fe Doping (%) | Particle Size (nm) | Lattice Parameters (Ǻ) | Cell Volume (Ǻ3) | fanatase | Band Gap (eV) | |
|---|---|---|---|---|---|---|
| a = b | c | |||||
| 0 | 40 | 3.786 | 9.518 | 136.416 | 100 | 3.2 |
| 0.04 | 27 | 3.810 | 9.541 | 138.498 | 100 | 3 |
| 0.3 | 33 | 3.796 | 9.547 | 137.569 | 92 | 2.85 |
| 2 | 30 | - | - | - | 10 | 2.4–1.85 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsiampalis, A.; Frontistis, Z.; Binas, V.; Kiriakidis, G.; Mantzavinos, D. Degradation of Sulfamethoxazole Using Iron-Doped Titania and Simulated Solar Radiation. Catalysts 2019, 9, 612. https://doi.org/10.3390/catal9070612
Tsiampalis A, Frontistis Z, Binas V, Kiriakidis G, Mantzavinos D. Degradation of Sulfamethoxazole Using Iron-Doped Titania and Simulated Solar Radiation. Catalysts. 2019; 9(7):612. https://doi.org/10.3390/catal9070612
Chicago/Turabian StyleTsiampalis, Athanasios, Zacharias Frontistis, Vassilios Binas, George Kiriakidis, and Dionissios Mantzavinos. 2019. "Degradation of Sulfamethoxazole Using Iron-Doped Titania and Simulated Solar Radiation" Catalysts 9, no. 7: 612. https://doi.org/10.3390/catal9070612
APA StyleTsiampalis, A., Frontistis, Z., Binas, V., Kiriakidis, G., & Mantzavinos, D. (2019). Degradation of Sulfamethoxazole Using Iron-Doped Titania and Simulated Solar Radiation. Catalysts, 9(7), 612. https://doi.org/10.3390/catal9070612








