Optimization of the Washcoat Slurry for Hydrotalcite-Based LNT Catalyst
Abstract
:1. Introduction
2. Results and Discussion
2.1. Factors Affecting Slurry Viscosity
2.1.1. pH
2.1.2. Solid Content
2.1.3. Binder Mass Fraction
2.1.4. Additive Content
2.2. Particle Size Distribution
2.3. Characterization of Catalyst Washcoat
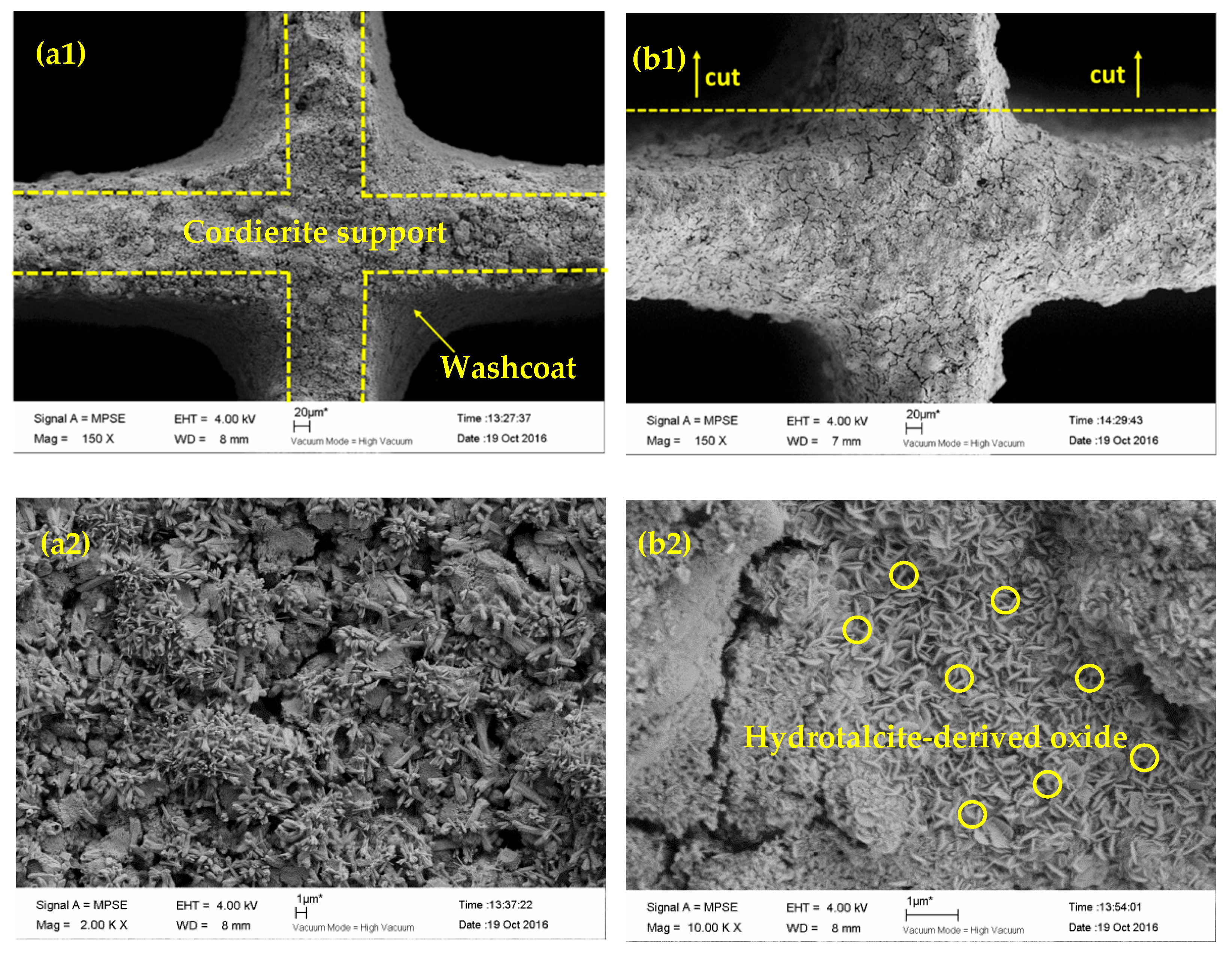
2.3.1. SEM
2.3.2. XRD Analysis
2.3.3. BET Measurement
2.4. Performance of the Monolithic Catalyst
2.4.1. SEM
2.4.2. NOx Storage Capacity
2.4.3. NOx-Temperature Programmed Desorption (TPD)
2.4.4. NOx Reduction
3. Experimental
3.1. Catalyst Preparation and Coating
3.2. Characterization of Slurry and Washcoat
3.3. Characterization of Pt,Ba-Containing Monolithic Catalyst
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shinjoh, H.; Takahashi, N.; Yokota, K.; Sugiura, M. Effect of periodic operation over Pt catalysts in simulated oxidizing exhaust gas. Appl. Catal. B 1998, 15, 189–201. [Google Scholar] [CrossRef]
- Liu, Z.M.; Woo, S.I. Recent advances in catalytic DeNOx science and technology. Catal. Rev. Sci. Eng. 2006, 48, 43–89. [Google Scholar] [CrossRef]
- Epling, W.S.; Campbell, L.E.; Yezerets, A.; Currier, N.W.; Parks, J.E. Overview of the Fundamental reactions and degradation Mechanisms of NOx storage/reduction catalysts. Catal. Rev. Sci. Eng. 2004, 46, 163–265. [Google Scholar] [CrossRef]
- Matsumoto, S. Recent advances in automobile exhaust catalysts. Catal. Today 2004, 90, 183–190. [Google Scholar] [CrossRef]
- Roy, S.; Baiker, A. NOx storage-reduction catalysis: From mechanism and materials properties to storage-reduction performance. Chem. Rev. 2009, 109, 4054–4091. [Google Scholar] [CrossRef]
- Fornasaria, G.; Glfcklerb, R.; Livia, M.; Vaccari, A. Role of the Mg/Al atomic ratio in hydrotalcite-based catalysts for NOx storage/reduction. Appl. Clay Sci. 2005, 29, 258–266. [Google Scholar] [CrossRef]
- Centi, G.; Fornasari, G.; Gobbi, C.; Livi, M.; Trifiro, F.; Vaccari, A. NOx storage-reduction catalysts based on hydrotalcite. Effect of Cu in promoting resistance to deactivation. Catal. Today 2002, 73, 287–296. [Google Scholar] [CrossRef]
- Braterman, P.S.; Xu, Z.P.; Yarberry, F. Handbook of Layered Materials; Auerbach, S.M., Carrado, K.A., Dutta, P.K., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2004; p. 373. [Google Scholar]
- Liu, L.; Li, S.L.; An, Y.L.; Sun, X.C.; Wu, H.L.; Li, J.Z.; Chen, X.; Li, H.D. Hybridization of Nanodiamond and CuFe-LDH as Heterogeneous Photoactivator for Visible-Light Driven Photo-Fenton Reaction: Photocatalytic Activity and Mechanism. Catalysts 2019, 9, 118. [Google Scholar] [CrossRef]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Li, B.S.; Yuan, S.L. Synthesis, characterization, and evaluation of TiMgAlCu mixed oxides as novel SOx removal catalysts. Ceram. Int. 2014, 40, 11559–11566. [Google Scholar] [CrossRef]
- Wang, Z.P.; Li, Q.; Wang, L.G.; Shangguan, W.F. Simultaneous catalytic removal of NOx and soot particulates over CuMgAl hydrotalcites derived mixed metal oxides. Appl. Clay Sci. 2012, 55, 125–130. [Google Scholar] [CrossRef]
- Li, Q.; Meng, M.; Tsubaki, N.; Li, X.G.; Li, Z.Q.; Xie, Y.N.; Hu, T.D.; Zhang, J. Performance of K-promoted hydrotalcite-derived CoMgAlO catalysts used for soot combustion, NOx storage and simultaneous soot-NOx removal. Appl. Catal. B Environ. 2009, 91, 406–415. [Google Scholar] [CrossRef]
- Li, Q.; Meng, M.; Dai, F.F.; Zha, Y.Q.; Xie, Y.N.; Hu, T.D.; Zhang, J. Multifunctional hydrotalcite-derived K/MnMgAlO catalysts used for soot combustion, NOx storage and simultaneous soot–NOx removal. Chem. Eng. J. 2012, 184, 106–112. [Google Scholar] [CrossRef]
- Sikander, U.; Sufian, S.; Salam, M.A. Synthesis and Structural Analysis of Double Layered Ni-Mg-Al Hydrotalcite Like Catalyst. Procedia Eng. 2016, 148, 261–267. [Google Scholar] [CrossRef] [Green Version]
- Martin, H.; Jaroslav, K.; Karel, F.; Aleš, V. Mg-Fe mixed oxides and their rehydrated mixed oxides as catalysts for transesterification. J. Clean. Prod. 2017, 161, 1423–1431. [Google Scholar]
- Tahir, N.; Abdelssadek, Z.; Halliche, D.; Saadi, A.; Cherifi, O.; Bachari, K. Study of the benzylation of aromatics over Mg-Cr-hydrotalcite catalysts. Stud. Surf. Sci. Catal. 2008, 174, 1287–1290. [Google Scholar]
- Jabłonska, M.; Palomares, A.E.; Chmielarz, L. NOx storage/reduction catalysts based on Mg/Zn/Al/Fe hydrotalcite-like materials. Chem. Eng. J. 2013, 231, 273–280. [Google Scholar] [CrossRef]
- Wang, R.N.; Wu, X.; Zou, C.L.; Li, X.J.; Du, Y.L. NOx Removal by Selective Catalytic Reduction with Ammonia over a Hydrotalcite-Derived NiFe Mixed Oxide. Catalysts 2018, 8, 384. [Google Scholar] [CrossRef]
- Dai, F.F.; Zhang, Y.X.; Meng, M.; Zhang, J.; Zheng, L.R.; Hu, T.D. Enhanced soot combustion over partially substituted hydrotalcite-drived mixed oxide catalysts CoMgAlLaO. J. Mol. Catal. A Chem. 2014, 393, 68–74. [Google Scholar] [CrossRef]
- Tomasic, V.; Jovic, F. State-of-the-art in the monolithic catalysts/reactors. Appl. Catal. A 2006, 311, 112–121. [Google Scholar] [CrossRef]
- Nijhuis, T.A.; Beers, A.E.W.; Vergunst, T.; Hoek, I.; Kapteijn, F.; Moulijn, J.A. Preparation of monolithic catalysts. Catal. Rev. Sci. Eng. 2001, 43, 345–380. [Google Scholar] [CrossRef]
- Blachou, V.; Goula, D.; Philippopoulos, C. Wet milling of alumina and preparation of slurries for monolithic structures impregnation. Ind. Eng. Chem. Res. 1992, 31, 364–369. [Google Scholar] [CrossRef]
- Shimrock, T.; Taylor, R.D.; Collins, J. Method of impregnating ceramic monolithic structures with predetermined amounts of catalyst. Europe Patent 0157651, 9 October 1985. [Google Scholar]
- Gao, J.L.; Liu, J.X.; Zhang, D.Q.; Li, D.F.; Zhang, J.R. Influence of Additive on Al2O3 washcoat of Porous Ceramic Substrate. J. Chem. Eng. Chin. Univ. 2006, 20, 142–146. [Google Scholar]
- Li, B. Study on the NOx Reduction for Diesel Engines Using the Modified-Hydrotalcite Derived LNT catalyst. Ph.D. Thesis, Tianjin University, Tianjin, China, 2018. [Google Scholar]
- Agrafiotis, C.; Tsetsekou, A. Deposition of meso-porous g-alumina coatings on ceramic honeycombs by sol-gel methods. J. Eur. Ceram. Soc. 2002, 22, 423–434. [Google Scholar] [CrossRef]
- Jiang, P.P.; Lu, G.Z.; Guo, Y.; Guo, Y.L.; Zhang, S.H.; Wang, X.Y. Preparation and properties of a γ-Al2O3 washcoat deposited on a ceramic honeycomb. Surf. Coat. Technol. 2005, 190, 314–320. [Google Scholar] [CrossRef]
- Pereda-Ayo, B.; López-Fonseca, R.; González-Velasco, J.R. Influence of the preparation procedure of NSR monolithic catalysts on the Pt-Ba dispersion and distribution. Appl. Catal. A Gen. 2009, 363, 73–80. [Google Scholar] [CrossRef]
- Pereda-Ayo, B.; González-Velasco, J.R. NOx Storage and Reduction for Diesel Engine Exhaust Aftertreatment. In Diesel Engine—Combustion, Emissions and Condition Monitoring, 1st ed.; Bari, S., Ed.; Intech: Rijeka, Croatia, 2013; pp. 161–196. [Google Scholar]
- Agrafiotis, C.; Tsetsekou, A. The effect of processing parameters on the properties of γ-alumina washcoats deposited on ceramic honeycombs. J. Mater. Sci. 2000, 35, 951–960. [Google Scholar] [CrossRef]
- Adamowska, M.; Costa, P.D. Structured Pd/γ-Al2O3 Prepared by Washcoated Deposition on a Ceramic Honeycomb for Compressed Natural Gas Applications. J. Nanopart. 2015, 9. [Google Scholar] [CrossRef]
- Tian, J.Y.; Lu, J.S.; Wu, H. Effect of Additive Polyethylene Glycol on γ-Al2O3 Washcoat Properties of Cordierite Ceramic Honeycomb. J. Chem. Eng. Chin. Univ. 2010, 24, 167–170. [Google Scholar]
- Zheng, J.; Tian, X.; Yu, K.; Wang, L.; Yang, C.; He, M. Hydrothermal Synthesis and Characterization of Regular and Homogeneous Nanocrystalline Hydrotalcite. Acta Chim. Sin. 2006, 64, 2231–2234. [Google Scholar]
- Tascón, A. Influence of particle size distribution skewness on dust explosibility. Powder Technol. 2018, 338, 438–445. [Google Scholar] [CrossRef]
- Yi, H.H.; Xie, X.Z.; Tang, X.L.; Zhao, S.Z.; Yang, K.; Huang, Y.H.; Yang, Z.Y. Demonstration of low-temperature toluene degradation mechanism on hydrotalcite-derived oxides with ultrasonic intervention. Chem. Eng. J. 2019, 374, 370–380. [Google Scholar] [CrossRef]
- Jabłońska, M.; Nothdurft, K.; Nocuń, M.; Girmanc, V.; Palkovits, R. Redox-performance correlations in Ag–Cu–Mg–Al, Ce–Cu–Mg–Al, and Ga–Cu–Mg–Al hydrotalcite derived mixed metal oxides. Appl. Catal. B Environ. 2017, 207, 385–396. [Google Scholar] [CrossRef]
- Luo, J.Y.; Meng, M.; Li, X.; Li, X.G.; Zha, Y.Q.; Hu, T.D.; Xie, Y.N.; Zhang, J. Mesoporous Co3O4-CeO2 and Pd/Co3O4-CeO2 catalysts: Synthesis characterization and mechanistic study of their catalytic properties for low-temperature CO oxidation. J. Catal. 2008, 254, 310–324. [Google Scholar] [CrossRef]
- Vijay, R.; Sakurai, H.; Snively, C.M.; Lauterbach, J. Mechanistic Investigation of Co Containing NOx Traps. Top. Catal. 2009, 52, 1388–1399. [Google Scholar] [CrossRef]
- Basile, F.; Fornasari, G.; Livi, M.; Tinti, F.; Trifirò, F.; Vaccari, A. Performance of new Pt and Pt-Cu on hydrotalcite-derived materials for NOx storage/reduction. Top. Catal. 2004, 30, 223–227. [Google Scholar] [CrossRef]
- Muñoz, V.; Zotin, F.M.Z.; Palacio, L.A. Copper-aluminum hydrotalcite type precursors for NOx abatement. Catal. Today 2015, 250, 173–179. [Google Scholar] [CrossRef]
- Pereda-Ayo, B.; Duraiswami, D.; Delgado, J.J.; López-Fonseca, R.; Calvino, J.J.; Bernal, S.; González-Velasco, J.R. Tuning operational conditions for efficient NOx storage and reduction over a Pt-Ba/Al2O3 monolith catalyst. Appl. Catal. B Environ. 2010, 96, 329–337. [Google Scholar] [CrossRef]
- Pereda-Ayo, B.; Duraiswami, D.; González-Marcos, J.A.; González-Velasco, J.R. Performance of NOx storage-reduction catalyst in the temperature-reductant concentration domain by response surface methodology. Chem. Eng. J. 2011, 169, 58–67. [Google Scholar] [CrossRef]











| Sample | Main Composition | Other Composition(s) |
|---|---|---|
| a | CuMgFeAlO | Deionized water |
| b 1 | CuMgFeAlO | Deionized water, glacial acetic acid, alumina sol and PEG1000 |
| Sample | D10 (μm) | D50 (μm) | D90 (μm) |
|---|---|---|---|
| a | 2.96 | 6.84 | 303.54 |
| b | 2.82 | 7.08 | 307.13 |
| Sample | Main Composition | BET (m2/g) |
|---|---|---|
| a | Fresh cordierite support | 0.93 |
| b | Hydrotalcite-based washcoat, cordierite support | 86.3 |
| c | Alumina-based washcoat, cordierite support | 123.6 |
| Temperature | Pt/BaO/Al2O3 | Pt/BaO/CuMgFeAlO | ||||
|---|---|---|---|---|---|---|
| 200 °C | 13.56 | 86.44 | 70.66 | 2.78 | 97.22 | 85.64 |
| 300 °C | 20.80 | 79.20 | 74.28 | 3.00 | 97.00 | 90.30 |
| 400 °C | 9.32 | 90.68 | 85.60 | 0.99 | 99.01 | 96.93 |
| 500 °C | 3.59 | 96.41 | 76.69 | 0.15 | 99.85 | 92.80 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Lv, G.; Song, C.; Li, B.; Zhu, Y.; Liu, Y.; Zhang, W.; Wang, Y. Optimization of the Washcoat Slurry for Hydrotalcite-Based LNT Catalyst. Catalysts 2019, 9, 696. https://doi.org/10.3390/catal9080696
Zhu Y, Lv G, Song C, Li B, Zhu Y, Liu Y, Zhang W, Wang Y. Optimization of the Washcoat Slurry for Hydrotalcite-Based LNT Catalyst. Catalysts. 2019; 9(8):696. https://doi.org/10.3390/catal9080696
Chicago/Turabian StyleZhu, Yue, Gang Lv, Chonglin Song, Bo Li, Yantao Zhu, Ye Liu, Wei Zhang, and Yuanhong Wang. 2019. "Optimization of the Washcoat Slurry for Hydrotalcite-Based LNT Catalyst" Catalysts 9, no. 8: 696. https://doi.org/10.3390/catal9080696
APA StyleZhu, Y., Lv, G., Song, C., Li, B., Zhu, Y., Liu, Y., Zhang, W., & Wang, Y. (2019). Optimization of the Washcoat Slurry for Hydrotalcite-Based LNT Catalyst. Catalysts, 9(8), 696. https://doi.org/10.3390/catal9080696




