Effects of Basic Promoters on the Catalytic Performance of Cu/SiO2 in the Hydrogenation of Dimethyl Maleate
Abstract
:1. Introduction
2. Results and Discussion
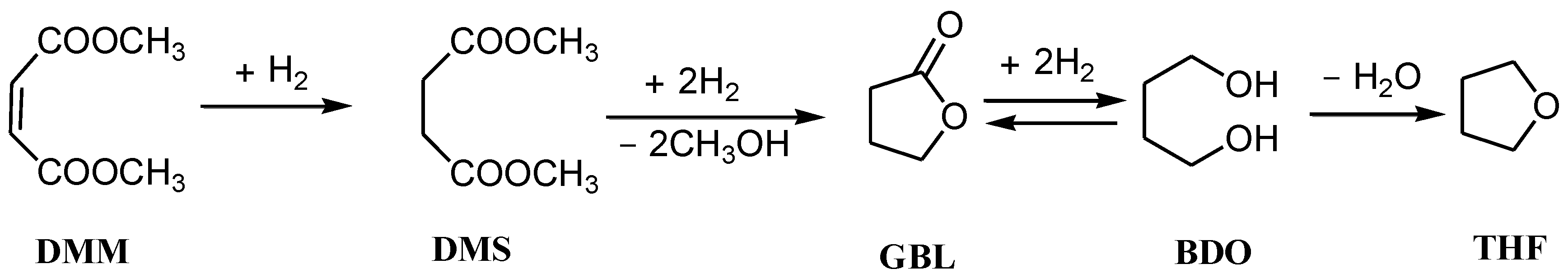
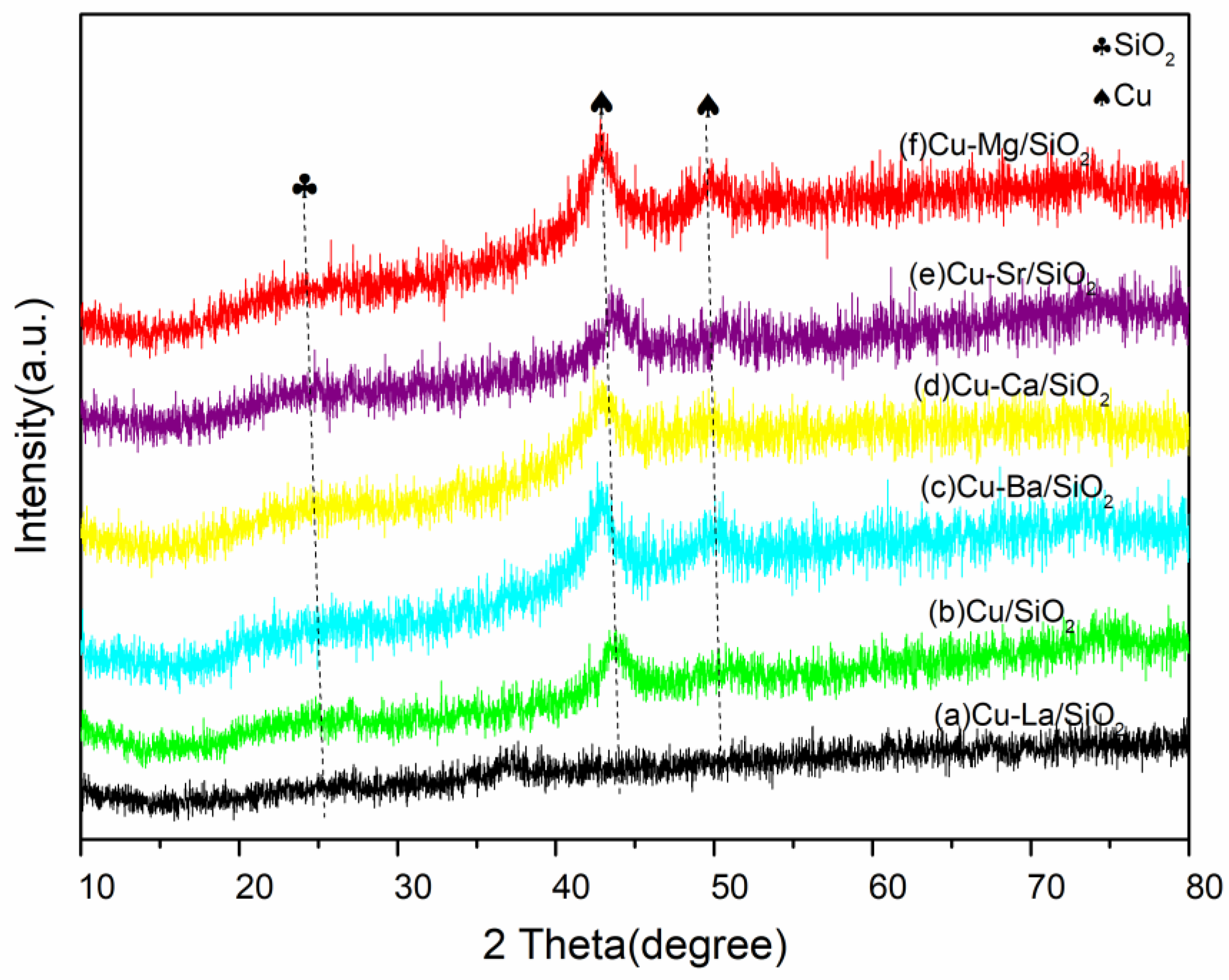
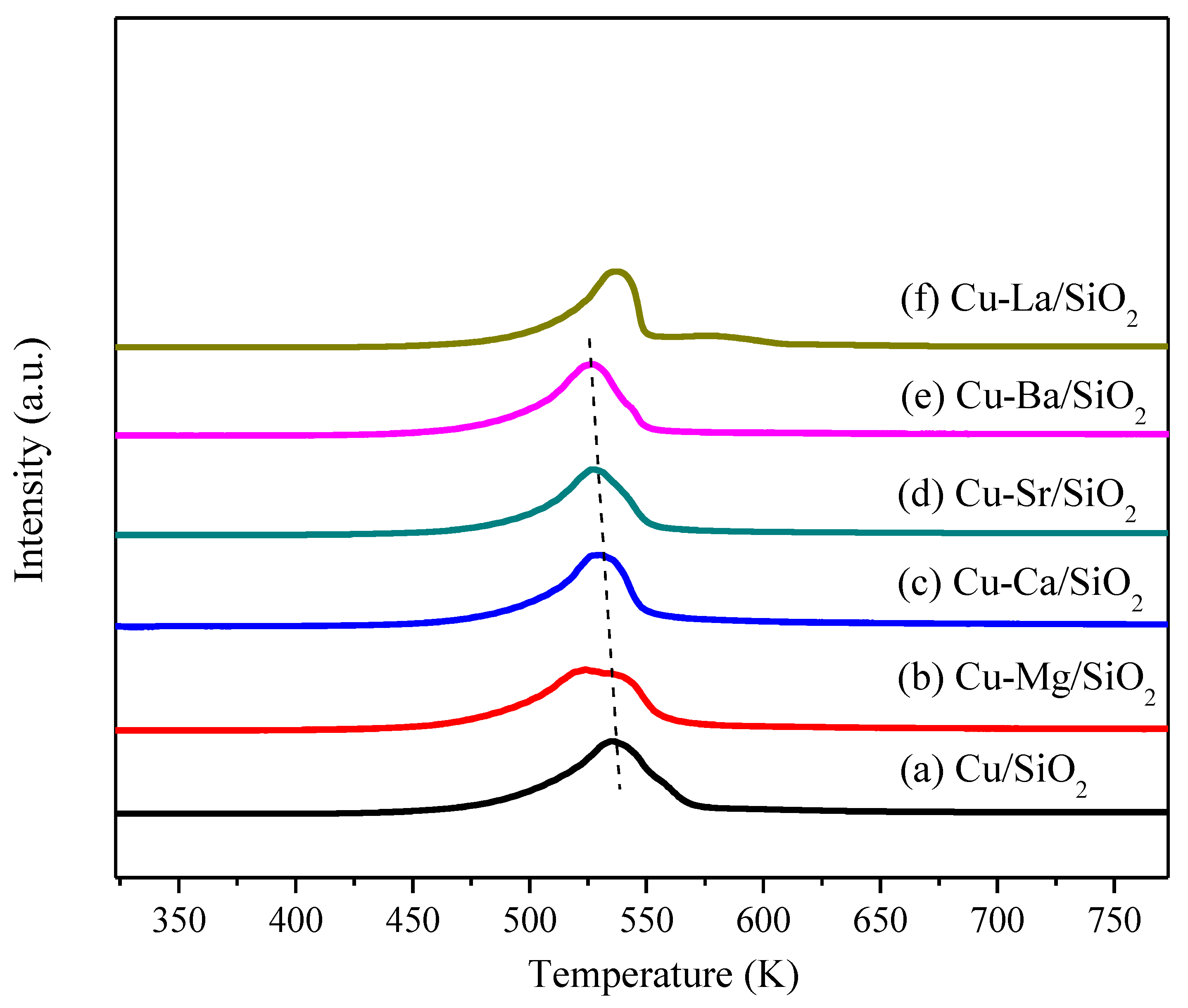
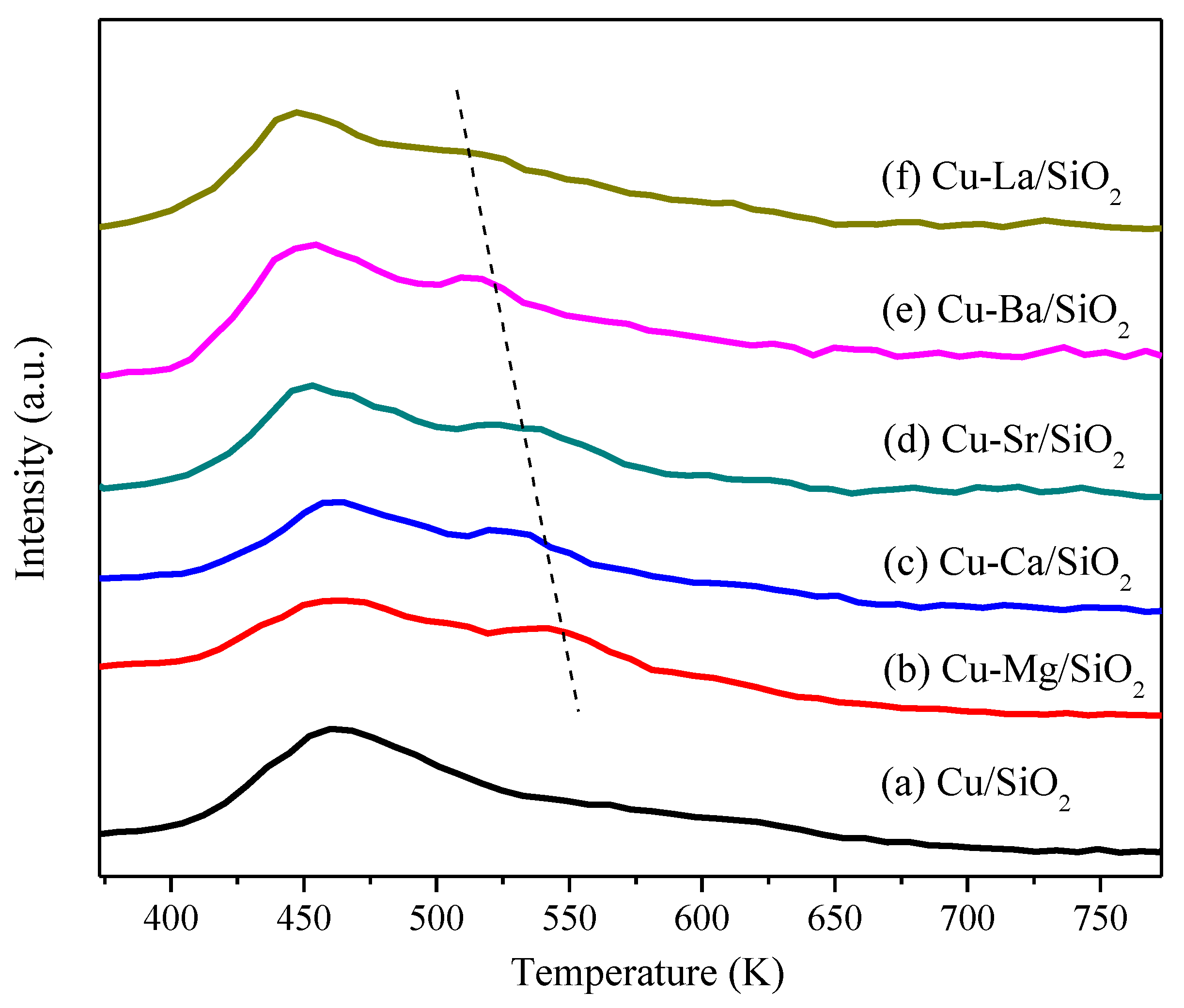
2.1. Catalysts Characterization
2.2. Hydrogenation of DMM
3. Experimental
3.1. Catalysts Preparation
3.2. Catalyst Characterization
3.3. Hydrogenation Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Muller, S.P.; Kucher, M.; Ohlinger, C.; Kraushaar-Czarnetzki, B. Extrusion of Cu/ZnO catalysts for the single-stage gas-phase processing of dimethyl maleate to tetrahydrofuran. J. Catal. 2003, 218, 419–426. [Google Scholar] [CrossRef] [Green Version]
- Ohlinger, C.; Kraushaar-Czarnetzki, B. Improved processing stability in the hydrogenation of dimethyl maleate to gamma-butyrolactone, 1,4-butanediol and tetrahydrofuran. Chem. Eng. Sci. 2003, 58, 1453–1461. [Google Scholar] [CrossRef]
- Zellmer, D.; Niewa, R.; Kreher, R.P. A functionalized dimethyl maleate (maleic acid dimethyl ester). Acta Cryst. 2014, 55, 823–825. [Google Scholar] [CrossRef]
- Emig, G.; Martin, F. Economics of maleic anhydride production from C-4 feedstocks. Catal. Today 1987, 1, 477–498. [Google Scholar] [CrossRef]
- Centi, G.; Trifiro, F.; Ebner, J.R.; Franchetti, V.M. Mechanistic aspects of maleic anhydride synthesis from C4 hydrocarbons over phosphorus vanadium oxide. Chem. Rev. 1988, 88, 55–80. [Google Scholar] [CrossRef]
- Wang, L.; Abudukelimu, N.; Ma, Y.B.; Qing, S.; Gao, Z.; Wumanjiang, E. Catalytic performance of Fe-modified Ru/Al2O3 in the hydrogenation of dimethyl maleate. J. Fuel Chem. Technol. 2014, 42, 839–844. [Google Scholar]
- Guo, P.J.; Chen, L.F.; Yan, S.R.; Dai, W.L.; Qiao, M.H.; Xu, H.L.; Fan, K.N. One-step hydrogenolysis of dimethyl maleate to tetrahydrofuran over chromium-modified Cu–B/γ-Al2O3 catalysts. J. Mol. Catal. A Chem. 2006, 256, 164–170. [Google Scholar] [CrossRef]
- Pritchard, J.; Filonenko, G.A.; van Putten, R.; Hensen, E.J.M.; Pidko, E.A. Heterogeneous and homogeneous catalysis for the hydrogenation of carboxylic acid derivatives: History, advances and future directions. Chem. Soc. Rev. 2015, 44, 3808–3833. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.C.; Bing, Q.M.; Kong, J.C.; Liu, J.Y.; Zhao, C. Mechanism of supported Ru3Sn7 nanocluster-catalyzed selective hydrogenation of coconut oil to fatty alcohols. Catal. Sci. Technol. 2018, 8, 1322–1332. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Kaburagi, W.; Fujitani, T. Catalytic transfer hydrogenation of levulinate esters to gamma-valerolactone over supported ruthenium hydroxide catalysts. RSC Adv. 2014, 4, 45848–45855. [Google Scholar] [CrossRef]
- Fang, X.L.; Zhang, C.Y.; Chen, J.; Zhu, H.P.; Yuan, Y.Z. Synthesis and catalytic performance of ruthenium complexes ligated with rigid o-(diphenylphosphino)aniline for chemoselective hydrogenation of dimethyl oxalate. RSC Adv. 2016, 6, 45512–45518. [Google Scholar] [CrossRef]
- Stathis, P.; Stavroulaki, D.; Kaika, N.; Krommyda, K.; Papadogianakis, G. Low trans-isomers formation in the aqueous-phase Pt/TPPTS-catalyzed partial hydrogenation of methyl esters of linseed oil. Appl. Catal. B Environ. 2017, 209, 579–590. [Google Scholar] [CrossRef]
- Numwong, N.; Luengnaruemitchai, A.; Chollacoop, N.; Yoshimura, Y. Partial hydrogenation of polyunsaturated fatty acid methyl esters over Pd/activated carbon: Effect of type of reactor. Chem. Eng. J. 2012, 210, 173–181. [Google Scholar] [CrossRef]
- Pérez-Cadenas, A.F.; Kapteijn, F.; Zieverink, M.M.P.; Moulijn, J.A. Selective hydrogenation of fatty acid methyl esters over palladium on carbon-based monoliths: Structural control of activity and selectivity. Catal. Today 2007, 128, 13–17. [Google Scholar] [CrossRef]
- Li, F.; Lu, C.S.; Li, X.N. The effect of the amount of ammonia on the Cu0/Cu+ ratio of Cu/SiO2 catalyst for the hydrogenation of dimethyl oxalate to ethylene glycol. Chin. Chem. Lett. 2014, 25, 1461–1465. [Google Scholar] [CrossRef]
- Huang, H.; Cao, G.; Wang, S. An evaluation of alkylthiols and dialkyl disulfides on deactivation of Cu/Zn catalyst in hydrogenation of dodecyl methyl ester to dodecanol. J. Ind. Eng. Chem. 2014, 20, 988–993. [Google Scholar] [CrossRef]
- Gong, J.; Yue, H.; Zhao, Y.; Zhao, S.; Zhao, L.; Lv, J.; Wang, S.; Ma, X. Synthesis of Ethanol via Syngas on Cu/SiO2 Catalysts with Balanced Cu0–Cu+ Sites. J. Am. Chem. Soc. 2012, 134, 13922–13925. [Google Scholar] [CrossRef]
- Ma, X.; Yang, Z.; Liu, X.; Tan, X.; Ge, Q. Dynamic redox cycle of Cu0 and Cu+ over Cu/SiO2 catalyst in ester hydrogenation. RSC Adv. 2015, 5, 37581–37584. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, K.; Xiang, Y.; Zhou, Y.; Wang, Q.; Guo, L.; Ma, L.; Xu, X.; Lu, C.; Feng, F.; et al. Sulfur-doped porous carbon supported palladium catalyst for high selective o-chloro-nitrobenzene hydrogenation. Appl. Catal. A Gen. 2019, 581, 74–81. [Google Scholar] [CrossRef]
- Sadjadi, S. Palladium nanoparticles immobilized on cyclodextrin-decorated halloysite nanotubes: Efficient heterogeneous catalyst for promoting copper- and ligand-free Sonogashira reaction in water–ethanol mixture. Appl. Organomet. Chem. 2018, 32. [Google Scholar] [CrossRef]
- Brands, D.S.; Poels, E.K.; Bliek, A. Ester hydrogenolysis over promoted Cu/SiO2 catalysts. Appl. Catal. A Gen. 1999, 184, 279–289. [Google Scholar] [CrossRef]
- Toupance, T.; Kermarec, M.; Louis, C. Metal Particle Size in Silica-Supported Copper Catalysts. Influence of the Conditions of Preparation and of Thermal Pretreatments. J. Phys. Chem. B 2000, 104, 965–972. [Google Scholar] [CrossRef]
- Munnik, P.; Wolters, M.; Gabrielsson, A.; Pollington, S.D.; Headdock, G.; Bitter, J.H.; de Jongh, P.E.; de Jong, K.P. Copper Nitrate Redispersion To Arrive at Highly Active Silica-Supported Copper Catalysts. J. Phys. Chem. C 2011, 115, 14698–14706. [Google Scholar] [CrossRef] [Green Version]
- Shimokawabe, M.; Takezawa, N.; Kobayashi, H. Characterization of copper-silica catalysts prepared by ion exchange. Appl. Catal. 1982, 2, 379–387. [Google Scholar] [CrossRef]
- Kohler, M.A.; Curry-Hyde, H.E.; Hughes, A.E.; Sexton, B.A.; Cant, N.W. The structure of CuSiO2 catalysts prepared by the ion-exchange technique. J. Catal. 1987, 108, 323–333. [Google Scholar] [CrossRef]
- Shi, L.; Zeng, C.; Jin, Y.; Wang, T.; Tsubaki, N. A sol–gel auto-combustion method to prepare Cu/ZnO catalysts for low-temperature methanol synthesis. Catal. Sci. Technol. 2012, 2, 2569–2577. [Google Scholar] [CrossRef]
- Ye, R.P.; Lin, L.; Chen, C.C.; Yang, J.X.; Li, F.; Zhang, X.; Li, D.J.; Qin, Y.Y.; Zhou, Z.; Yao, Y.G. Synthesis of Robust MOF-Derived Cu/SiO2 Catalyst with Low Copper Loading via Sol–Gel Method for the Dimethyl Oxalate Hydrogenation Reaction. ACS Catal. 2018, 8, 3382–3394. [Google Scholar] [CrossRef]
- Kasinathan, P.; Hwang, D.W.; Lee, U.H.; Hwang, Y.K.; Chang, J.S. Effect of Cu particle size on hydrogenation of dimethyl succinate over Cu–SiO2 nanocomposite. Catal. Commun. 2013, 41, 17–20. [Google Scholar] [CrossRef]
- Miyazaki, H.; Hirai, K.; Uda, T.; Nakamura, Y.; Ikezawa, H.; Tsuchie, T. Process for Producing Ethylene Glycol and/or Glycolic Acid Ester, Catalyst Composition Used Therefor, and Process for the Production of This Composition. Patent EP0064241A1, 4 September 1985. [Google Scholar]
- Chen, L.F.; Guo, P.J.; Qiao, M.H.; Yan, S.R.; Li, H.X.; Shen, W.; Xu, H.L.; Fan, K.N. Cu/SiO2 catalysts prepared by the ammonia-evaporation method: Texture, structure, and catalytic performance in hydrogenation of dimethyl oxalate to ethylene glycol. J. Catal. 2008, 257, 172–180. [Google Scholar] [CrossRef]
- Han, X.Q.; Zhang, Q.F.; Feng, F.; Lu, C.S.; Ma, L.; Li, X.N. Selective hydrogenation of dimethyl maleate to tetrahydrofuran over Cu/SiO2 catalyst: Effect of Cu+ on the catalytic performance. Chin. Chem. Lett. 2015, 26, 1150–1154. [Google Scholar] [CrossRef]
- Yin, A.; Wen, C.; Guo, X.; Dai, W.L.; Fan, K. Influence of Ni species on the structural evolution of Cu/SiO2 catalyst for the chemoselective hydrogenation of dimethyl oxalate. J. Catal. 2011, 280, 77–88. [Google Scholar] [CrossRef]
- Ungureanu, A.; Dragoi, B.; Chirieac, A.; Royer, S.; Duprez, D.; Dumitriu, E. Synthesis of highly thermostable copper-nickel nanoparticles confined in the channels of ordered mesoporous SBA-15 silica. J. Mater. Chem. 2011, 21, 12529–12541. [Google Scholar] [CrossRef]
- He, Z.; Lin, H.; He, P.; Yuan, Y. Effect of boric oxide doping on the stability and activity of a Cu–SiO2 catalyst for vapor-phase hydrogenation of dimethyl oxalate to ethylene glycol. J. Catal. 2011, 277, 54–63. [Google Scholar] [CrossRef]
- Zhao, S.; Yue, H.; Zhao, Y.; Wang, B.; Geng, Y.; Lv, J.; Wang, S.; Gong, J.; Ma, X. Chemoselective synthesis of ethanol via hydrogenation of dimethyl oxalate on Cu/SiO2: Enhanced stability with boron dopant. J. Catal. 2013, 297, 142–150. [Google Scholar] [CrossRef]
- Zhu, S.; Gao, X.; Zhu, Y.L.; Zhu, Y.F.; Zheng, H.; Li, Y. Promoting effect of boron oxide on Cu/SiO2 catalyst for glycerol hydrogenolysis to 1,2-propanediol. J. Catal. 2013, 303, 70–79. [Google Scholar] [CrossRef]
- Wang, L.; Abudukelimu, N.; Ma, Y.; Qing, S.; Gao, Z.; Eli, W. Enhanced Ru/Alumina catalyst via the adsorption-precipitation (AP) method for the hydrogenation of dimethyl maleate. React. Kinet. Mech. Catal. 2014, 112, 117–129. [Google Scholar] [CrossRef]
- Chen, L.; Guo, P.; Zhu, L.; Qiao, M.; Shen, W.; Xu, H.; Fan, K. Preparation of Cu/SBA-15 catalysts by different methods for the hydrogenolysis of dimethyl maleate to 1,4-butanediol. Appl. Catal. A Gen. 2009, 356, 129–136. [Google Scholar] [CrossRef]
- Van Der Grift, C.J.G.; Elberse, P.A.; Mulder, A.; Geus, J.W. Preparation of silica-supported copper catalysts by means of deposition-precipitation. Appl. Catal. 1990, 59, 275–289. [Google Scholar] [CrossRef]
- Zheng, X.; Lin, H.; Zheng, J.; Duan, X.; Yuan, Y. Lanthanum Oxide-Modified Cu/SiO2 as a High-Performance Catalyst for Chemoselective Hydrogenation of Dimethyl Oxalate to Ethylene Glycol. ACS Catal. 2013, 3, 2738–2749. [Google Scholar] [CrossRef]
- Zhang, G.; Hattori, H.; Tanabe, K. Aldol Addition of Acetone, Catalyzed by Solid Base Catalysts: Magnesium Oxide, Calcium Oxide, Strontium Oxide, Barium Oxide, Lanthanum (III) Oxide and Zirconium Oxide. Appl. Catal. 1988, 36, 189–197. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, Y.; Ding, G.; Zheng, H.; Li, Y. Modification of the supported Cu/SiO2 catalyst by alkaline earth metals in the selective conversion of 1,4-butanediol to γ-butyrolactone. Appl. Catal. A Gen. 2012, 443–444, 191–201. [Google Scholar] [CrossRef]
| Sample | Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Size (nm) | Elemental Composition (wt%) | |
|---|---|---|---|---|---|
| Cu | M | ||||
| SiO2 | 167 | 0.50 | 11.8 | 0 | 0 |
| Cu/SiO2 | 117 | 0.42 | 14.4 | 19.8 | 0 |
| Cu-Mg/SiO2 | 108 | 0.39 | 14.3 | 18.8 | 3.2 |
| Cu-Ca/SiO2 | 95 | 0.33 | 13.9 | 19.0 | 3.1 |
| Cu-Sr/SiO2 | 102 | 0.36 | 14.1 | 18.9 | 2.9 |
| Cu-Ba/SiO2 | 99 | 0.35 | 14.0 | 19.1 | 2.8 |
| Cu-La/SiO2 | 112 | 0.40 | 14.4 | 18.9 | 3.0 |
| Catalysts | LHSV | DMM Conversion/% | Selectivity/% | ||
|---|---|---|---|---|---|
| (h−1) | GBL | BDO | THF | ||
| Cu/SiO2 | 0.3 | 100 | 0 | 0 | 100 |
| Cu/SiO2 | 0.6 | 100 | 0 | 0 | 100 |
| Cu/SiO2 | 0.9 | 83.65 | 0 | 0 | 100 |
| Cu-Mg/SiO2 | 0.3 | 84.21 | 24.43 | 4.82 | 70.75 |
| Cu-Ca/SiO2 | 0.3 | 88.38 | 27.42 | 0 | 72.58 |
| Cu-Sr/SiO2 | 0.3 | 83.17 | 32.17 | 3.43 | 64.40 |
| Cu-Ba/SiO2 | 0.3 | 64.21 | 25.43 | 5.01 | 69.56 |
| Cu-La/SiO2 | 0.3 | 100 | 24.54 | 4.29 | 71.17 |
| Reaction Time (h) | DMM Conversion (%) | Selectivity (%) | ||
|---|---|---|---|---|
| GBL | BDO | THF | ||
| 0 | 100 | 24.54 | 4.29 | 71.17 |
| 5 | 100 | 25.12 | 4.04 | 70.84 |
| 10 | 100 | 24.98 | 4.16 | 70.86 |
| 15 | 100 | 24.67 | 4.28 | 71.05 |
| 20 | 100 | 24.38 | 4.35 | 71.27 |
| 25 | 100 | 24.87 | 4.31 | 70.82 |
| 30 | 100 | 25.22 | 4.17 | 70.61 |
| 35 | 100 | 25.16 | 4.42 | 70.42 |
| 40 | 100 | 24.45 | 4.55 | 71 |
| 45 | 100 | 24.23 | 4.26 | 71.51 |
| 50 | 100 | 24.79 | 4.13 | 71.08 |
| 55 | 100 | 25.72 | 4.59 | 69.69 |
| 60 | 100 | 25.48 | 4.26 | 70.26 |
| 65 | 100 | 25.56 | 4.37 | 70.07 |
| 70 | 100 | 24.94 | 4.14 | 70.92 |
| 75 | 100 | 24.42 | 4.3 | 71.28 |
| 80 | 100 | 24.1 | 4.17 | 71.73 |
| 85 | 100 | 23.87 | 4.46 | 71.67 |
| 90 | 100 | 24.71 | 4.58 | 70.71 |
| 95 | 100 | 25.66 | 4.27 | 70.07 |
| 100 | 100 | 25.34 | 4.33 | 70.33 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ying, J.; Han, X.; Ma, L.; Lu, C.; Feng, F.; Zhang, Q.; Li, X. Effects of Basic Promoters on the Catalytic Performance of Cu/SiO2 in the Hydrogenation of Dimethyl Maleate. Catalysts 2019, 9, 704. https://doi.org/10.3390/catal9090704
Ying J, Han X, Ma L, Lu C, Feng F, Zhang Q, Li X. Effects of Basic Promoters on the Catalytic Performance of Cu/SiO2 in the Hydrogenation of Dimethyl Maleate. Catalysts. 2019; 9(9):704. https://doi.org/10.3390/catal9090704
Chicago/Turabian StyleYing, Juntao, Xueqing Han, Lei Ma, Chunshan Lu, Feng Feng, Qunfeng Zhang, and Xiaonian Li. 2019. "Effects of Basic Promoters on the Catalytic Performance of Cu/SiO2 in the Hydrogenation of Dimethyl Maleate" Catalysts 9, no. 9: 704. https://doi.org/10.3390/catal9090704





