Microwave-Assisted Furfural Production Using Hectorites and Fluorohectorites as Catalysts
Abstract
1. Introduction
2. Results and Discussion
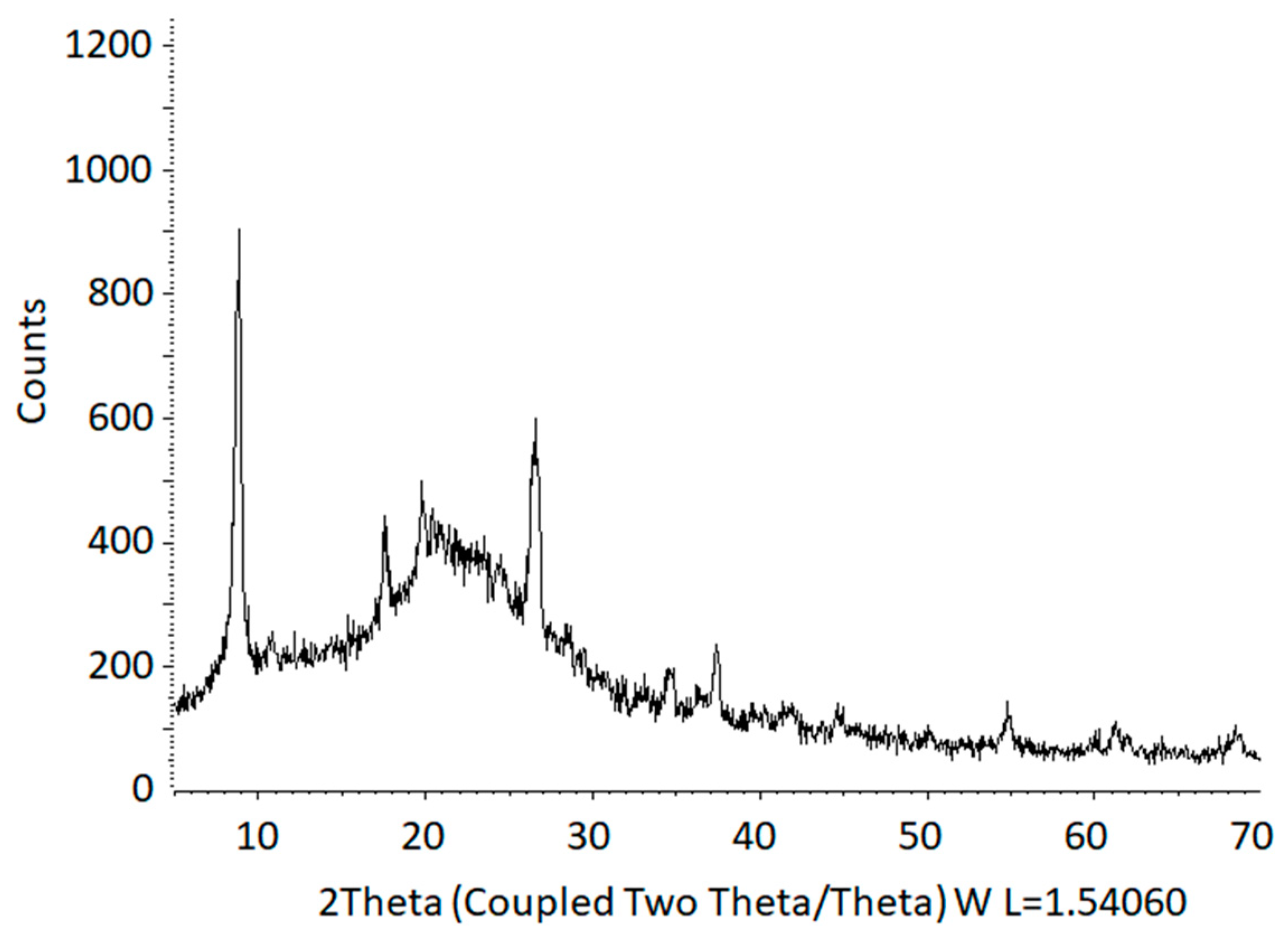
2.1. Preparation of Fluorohectorites
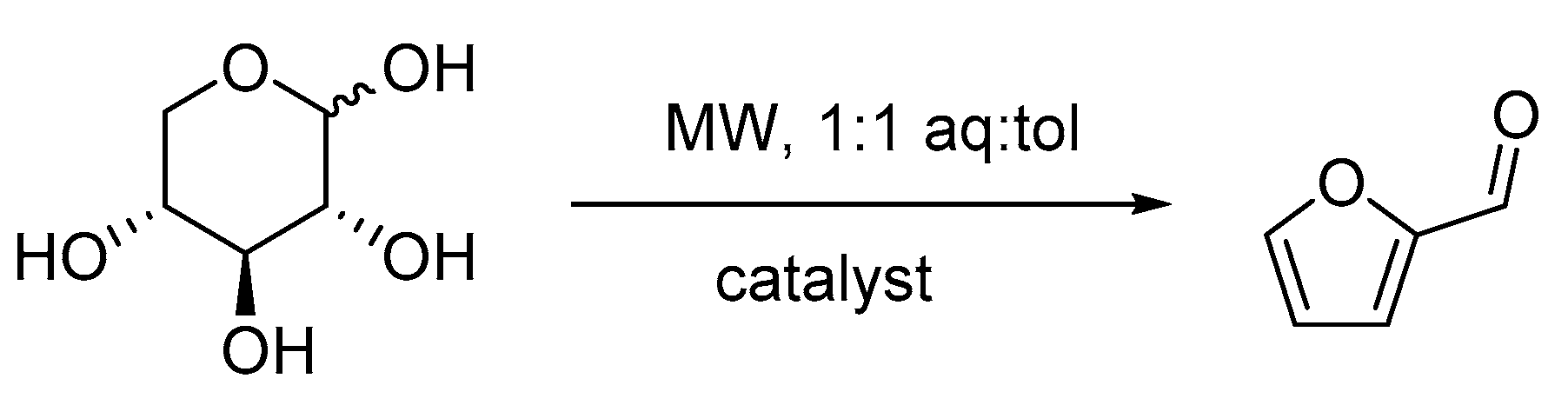
2.2. Catalytic Tests
2.2.1. Preliminary Experiments
2.2.2. Effect of the Catalyst
3. Materials and Methods
3.1. Materials
3.2. Fluorohectorite Preparation
3.3. Catalyst Preparation
3.4. Characterization and Analytic Techniques
3.5. Extraction of Xylose from Biomass
3.6. Catalytic Reaction
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nakagawa, Y.; Tamura, M.; Tomishige, K. Catalytic conversions of furfural to pentanediols. Catal. Surv. Asia 2015, 19, 249–256. [Google Scholar] [CrossRef]
- Qing, Q.; Guo, Q.; Zhou, L.; Wan, Y.; Xu, Y.; Ji, H.; Gao, X.; Zhang, Y. Catalyitc conversion of corncob and corncob pretreatment hydrolysate to furfural in a biphasic system with addition of sodium chloride. Biores. Tech. 2017, 226, 247–254. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Tamura, M.; Tomishige, K. Catalytic reduction of biomass-derived furanic compounds with hydrogen. ACS Catal. 2013, 3, 2655–2668. [Google Scholar] [CrossRef]
- Cai, C.M.; Zhang, T.; Kumar, R.; Wyman, C.E. Integrated furfural production as a renewable fuel and chemical platform from lignocellulosic biomass. J. Chem. Technol. Biotechnol. 2014, 89, 2–10. [Google Scholar] [CrossRef]
- Weingarten, R.; Tompsett, G.; Conner, W.; Huber, G. Design of solid acid catalysts for aqueous-phasedehydration of carbohydrates: The role of Lewis and Brønsted acid sites. J. Catal. 2011, 279, 174–182. [Google Scholar] [CrossRef]
- Choudhary, V.; Sandler, S.; Vlachos, D. Conversion of xylose to furfural using lewis and brønsted acid catalysts in aqueous media. ACS Catal. 2012, 2, 2022–2028. [Google Scholar] [CrossRef]
- Karinen, R.; Vilonen, K.; Niemelä, M. Biorefining: Heterogeneously catalyzed reactions of carbohydrates for the production of furfural and hydroxymethylfurfural. ChemSusChem 2011, 4, 1002–1016. [Google Scholar] [CrossRef]
- Bhaumik, P.; Dhepe, P.L. Exceptionally high yields of furfural from assorted raw biomass over solid acids. RSC Adv. 2014, 4, 26215–26221. [Google Scholar] [CrossRef]
- Ji, L.Q. An assessment of agricultural residue resources for liquid biofuel production in China. Renew. Sust. Energy Rev. 2015, 44, 561–575. [Google Scholar] [CrossRef]
- Dias, A.S.; Pillinger, M.; Valente, A.A. Liquid phase dehydration of d-Xylose in the presence of Keggin-type heteropolyacids. Appl. Catal. A Gen. 2005, 285, 126–131. [Google Scholar] [CrossRef]
- Dias, A.S.; Lima, S.; Carriazo, D.; Rives, V.; Pillinger, M.; Valente, A.A. Exfoliated titanate, niobate and titanoniobatenanosheets as solid acid catalysts for the liquid-phase dehydration of d-Xylose into furfural. J. Catal. 2006, 244, 230–237. [Google Scholar] [CrossRef]
- Lima, S.; Pillinger, M.; Valente, A.A. Dehydration of d-Xylose into furfural catalysed by solid acids derived from the layered zeolite Nu-6(1). Catal. Commun. 2008, 9, 2144–2148. [Google Scholar] [CrossRef]
- Qi, X.; Watanabe, M.; Aida, T.M.; Smith Jr, R.L. Catalytic dehydration of fructose into 5-hydroxymethylfurfural by ion-exchange resin in mixed-aqueous system by microwave heating. Catal. Commun. 2008, 10, 799–805. [Google Scholar] [CrossRef]
- Qi, X.; Watanabe, M.; Aida, T.M.; Smith, R.L. Catalytical conversion of fructose and glucose into 5-hydroxymethylfurfural in hot compressed water by microwave heating. Catal. Commun. 2008, 9, 2244–2249. [Google Scholar] [CrossRef]
- Yemis, O.; Mazza, G. Acid-catalyzed conversion of sylose, xylan and straw into furfural by microwave-assisted reaction. Biores. Tech. 2011, 102, 7371–7378. [Google Scholar] [CrossRef]
- Yemos, O.; Mazza, G. Catalytic performances of various solid catalysts and metal halides for microwave-assisted hydrotermal conversion of xylose, xylan, and straw to furfural. Waste Biomass Valorization 2019, 10, 1343–1353. [Google Scholar] [CrossRef]
- Gómez Millán, G.; El Assal, Z.; Nieminen, K.; Hellsten, S.; Llorca, J.; Sixta, H. Fast furfural formation from xylose using solid acid catalysts assisted by a microwave reactor. Fuel Process. Technol. 2018, 182, 56–67. [Google Scholar] [CrossRef]
- Delbecq, F.; Wang, Y.; Len, C. Conversion of xylose, xylan and rice husk into furfural via betaine and formic acid mixture as novel homogeneous catalyst in biphasic system by microwave-assisted dehydration. J. Mol. Catal. A Chem. 2016, 423, 520–525. [Google Scholar] [CrossRef]
- Le Guenic, S.; Gergela, D.; Ceballos, C.; Delbecq, F.; Len, C. Furfural production from d-xylose and xylan by using stable nafion nr50 and nacl in a microwave-assisted biphasic reaction. Molecules 2016, 21, 1102. [Google Scholar] [CrossRef]
- Wang, Y.; Delbecq, F.; Kwapinski, W.; Len, C. Application of sulfonated carbon-based catalyst for the furfural production from d-xylose and xylan in a microwave-assisted biphasic reaction. Mol. Catal. 2017, 438, 167–172. [Google Scholar] [CrossRef]
- Wang, Y.; Delbecq, F.; Varma, R.S.; Len, C. Comprehensive study on expeditious conversion of pre-hydrolyzed alginic acid to furfural in Cu(II) biphasic systems using microwaves. Mol. Catal. 2018, 445, 73–79. [Google Scholar] [CrossRef]
- Delbecq, F.; Takahashi, Y.; Kondo, T.; Corbas, C.C.; RuizRamos, E.; Len, C. Microwave assisted efficient furfural production using nano-sized surface-sulfonated diamond powder. Catal. Commun. 2018, 110, 74–78. [Google Scholar] [CrossRef]
- Xiouras, C.; Radacsi, N.; Sturm, G.; Stefanidis, G.D. Furfural Synthesis form D-Xylose in the Presence of Sodium Chloride: Microwave versus Conventional Heating. ChemSusChem 2016, 9, 20159–20166. [Google Scholar] [CrossRef]
- Conner, W.C., Jr.; Tompsett, G.A. How Could and Do Microwaves Influence Chemistry at Interfaces? J. Phys. Chem. B 2008, 112, 2110–2118. [Google Scholar] [CrossRef]
- Lourvanij, K.; Rorrer, G.L. Reaction Rates for the Partial Dehydration of Glucose to Organic Acids in Solic-Acid, Molecular-Sieving Catalyst Powders. J. Chem. Technol. Biotechnol. 1997, 69, 35–44. [Google Scholar] [CrossRef]
- Lourvanij, K.; Rorrer, G.L. Dehydration of glucose to organic acids in microporous pillared clay catalysts. Appl. Catal. A Gen. 1994, 109, 147–165. [Google Scholar] [CrossRef]
- Sánchez, T.; Salagre, P.; Cesteros, Y.; Bueno-López, A. Use of delaminated hectorites as supports of copper catalysts for the hydrogenolysis of glycerol to 1,2-propanediol. Chem. Eng. J. 2012, 179, 302–311. [Google Scholar] [CrossRef]
- Majid, A.; Argue, S.; Kingston, D.; Lang, S. Controlled fluorination of clays. J. Fluor. Chem. 2007, 128, 1012–1018. [Google Scholar] [CrossRef][Green Version]
- Kalo, H.; Möller, M.W.; Kunz, D.A.; Breu, J. How to maximize the aspect ratio of clay nanoplatelets. Nanoscale 2012, 4, 5633–5639. [Google Scholar] [CrossRef]
- Barrer, R.M.; Jones, D.L. Chemistry of Soil Minerals. Part VIII. Synthesis and Properties of Fluorhectorites. J. Chem. Soc. A 1970, 1531–1537. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Marques, J.P.; Gener, I.; Ayrault, P.; Bordado, J.C.; Lopes, J.M.; Ribeiro, F.R.; Guisnet, M. Dealumination of HBEA zeolite by steaming and acid leaching: distribution of the various aluminic species and identification of the hydroxyl groups. C. R. Chimie 2005, 8, 399–410. [Google Scholar] [CrossRef]
- Bergaya, F.; Vayer, M. CEC of clays: measurement by adsorption of a copper ethylenediamine complex. Appl. Clay Sci. 1997, 12, 275–280. [Google Scholar] [CrossRef]








| FH 800 °C (s) | FH 850 °C (ns) | ||||||
|---|---|---|---|---|---|---|---|
| Synthesis Time (h) | 3 | 6 | 12 | 24 | 0.5 | 1 | 2 |
| Sample name | FH800s3 | FH800s6 | FH800s12 | FH800s24 | FH850ns0.5 | FH850ns1 | FH850ns2 |
| Li0.7(Mg6−x)LixSi8O20F4 | X | X | X | ||||
| SiO2 | X | X | X | X | X | X | X |
| Li2SiO3/Li2Si2O5 | X | X | X | X | X | ||
| MgO | X | X | X | ||||
| LiF | X | X | X | ||||
| Mg7Si8O22(OH)2 | X | X | X | X | X | X | X |
| Sample | BET Area (m2/g) | Porosity (Å) | C.E.C. (meq/100 g) | Si/F (ESEM-EDS) | Si/Mg (ESEM-EDS) |
|---|---|---|---|---|---|
| Li-FH | 16 | 20 *, 105 | 105 | 1.8 | 1.5 |
| Na-DH | 327 | 17 | 60 | - | - |
| H-β | 584 | 17 | 65 | - | - |
| Atom | Mg 2p | Si 2p | Li 1s | F 1s | O 1s |
|---|---|---|---|---|---|
| % | 18.8 | 26.7 | 0.0 | 4.7 | 49.8 |
| B.E(eV) | 50.6 s; 48.6 w | 103 s; 101.3 w | - | 684.2 | 531.8 s; 529.2 w |
| Ratios | Si/Mg | Si/O | Si/F | ||
| 1.42 (1.5 *) | 0.54 (0.33 *) | 5.7 (2 *) | |||
| Catalyst | H-β | Na-DH | H-DH | Li-FH | H-FH | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Acidity Strength | T °C | meq/g | T °C | meq/g | T °C | meq/g | T °C | meq/g | T °C | meq/g |
| 190 | 0.83 | 190 | 0.9 | 224 | 0.67 | 221 | 0.08 | 318 | 0.05 | |
| 342 | 0.55 | 316 | 0.09 | 721 | 0.47 | |||||
| 512 | 0.05 | |||||||||
| 627 | 0.02 | |||||||||
| Total acidity (meq/g) | 1.38 | 0.9 | 0.67 | 0.23 | 0.53 | |||||
| meq/m2 | 2.36 × 10−3 | 2.7 × 10−3 | 2.05 × 10−3 | 14.4 × 10−3 | 33.1 × 10−3 | |||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez, V.; Dafinov, A.; Salagre, P.; Llorca, J.; Cesteros, Y. Microwave-Assisted Furfural Production Using Hectorites and Fluorohectorites as Catalysts. Catalysts 2019, 9, 706. https://doi.org/10.3390/catal9090706
Sánchez V, Dafinov A, Salagre P, Llorca J, Cesteros Y. Microwave-Assisted Furfural Production Using Hectorites and Fluorohectorites as Catalysts. Catalysts. 2019; 9(9):706. https://doi.org/10.3390/catal9090706
Chicago/Turabian StyleSánchez, Vladimir, Anton Dafinov, Pilar Salagre, Jordi Llorca, and Yolanda Cesteros. 2019. "Microwave-Assisted Furfural Production Using Hectorites and Fluorohectorites as Catalysts" Catalysts 9, no. 9: 706. https://doi.org/10.3390/catal9090706
APA StyleSánchez, V., Dafinov, A., Salagre, P., Llorca, J., & Cesteros, Y. (2019). Microwave-Assisted Furfural Production Using Hectorites and Fluorohectorites as Catalysts. Catalysts, 9(9), 706. https://doi.org/10.3390/catal9090706