Synthesis of Sulfonic Acid-Functionalized Zirconium Poly(Styrene-Phenylvinyl-Phosphonate)-Phosphate for Heterogeneous Epoxidation of Soybean Oil
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Acidity of the Samples of Solid Particles
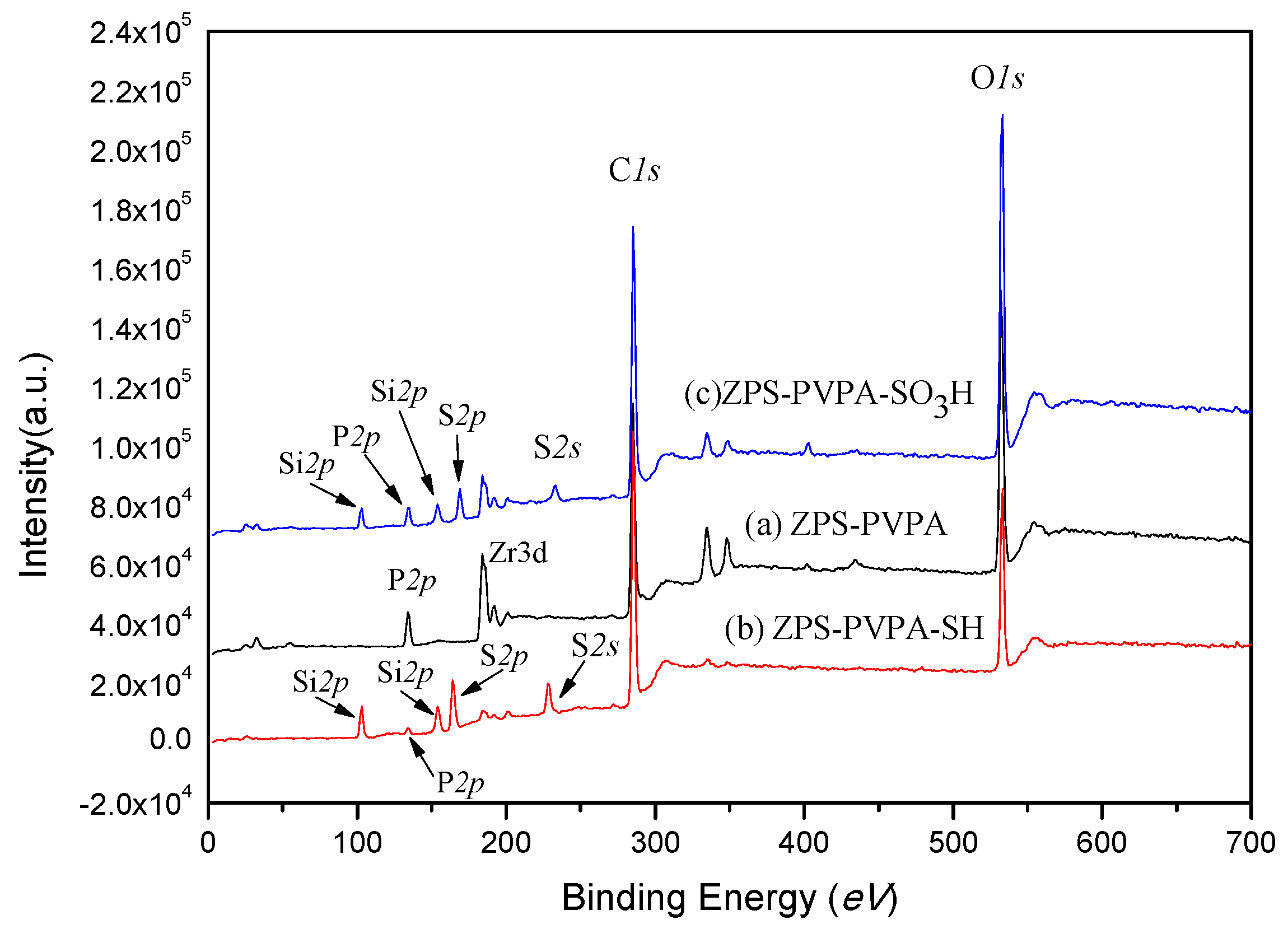
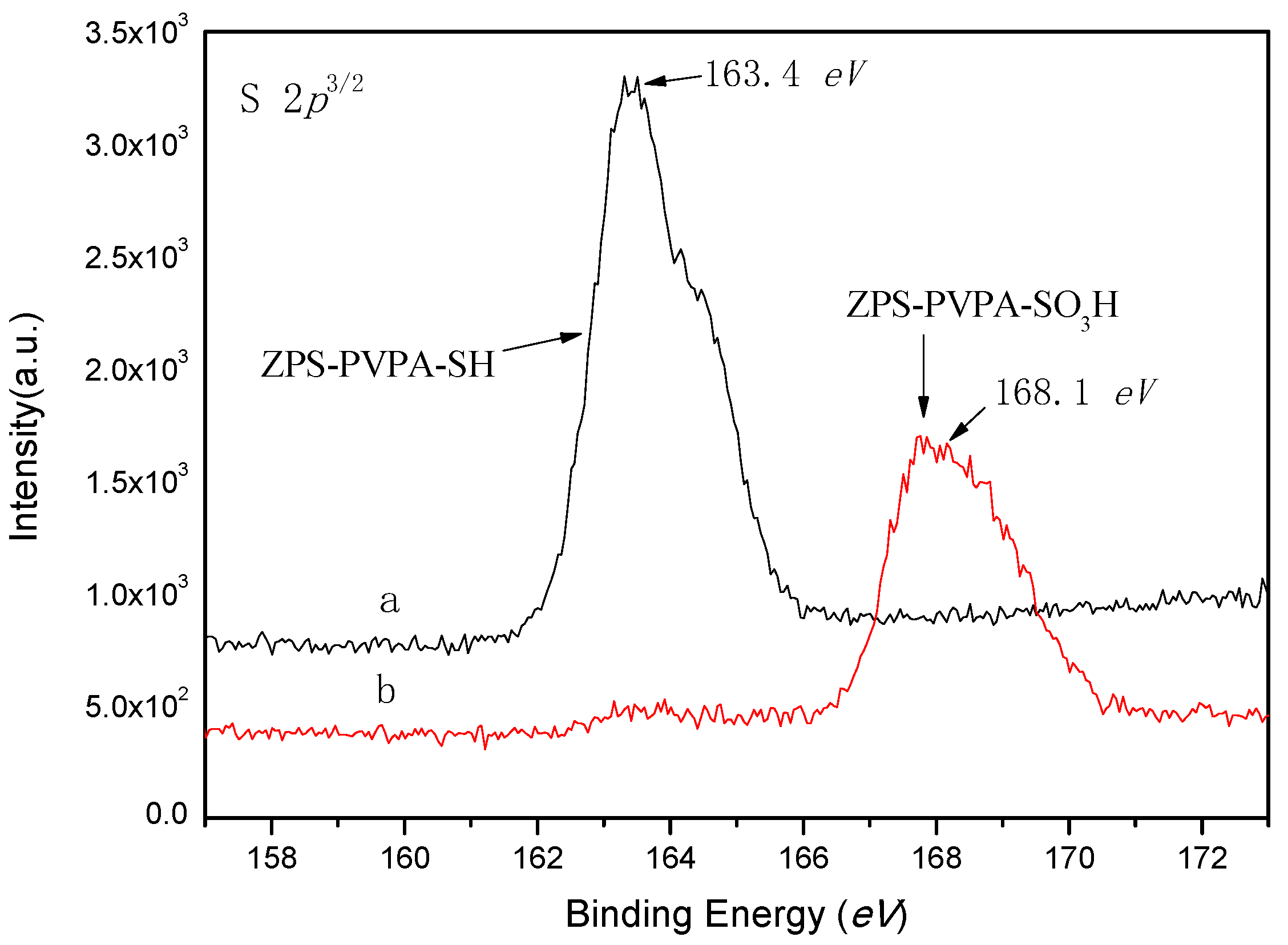
2.2. X-Ray Photoelectron Spectroscopy
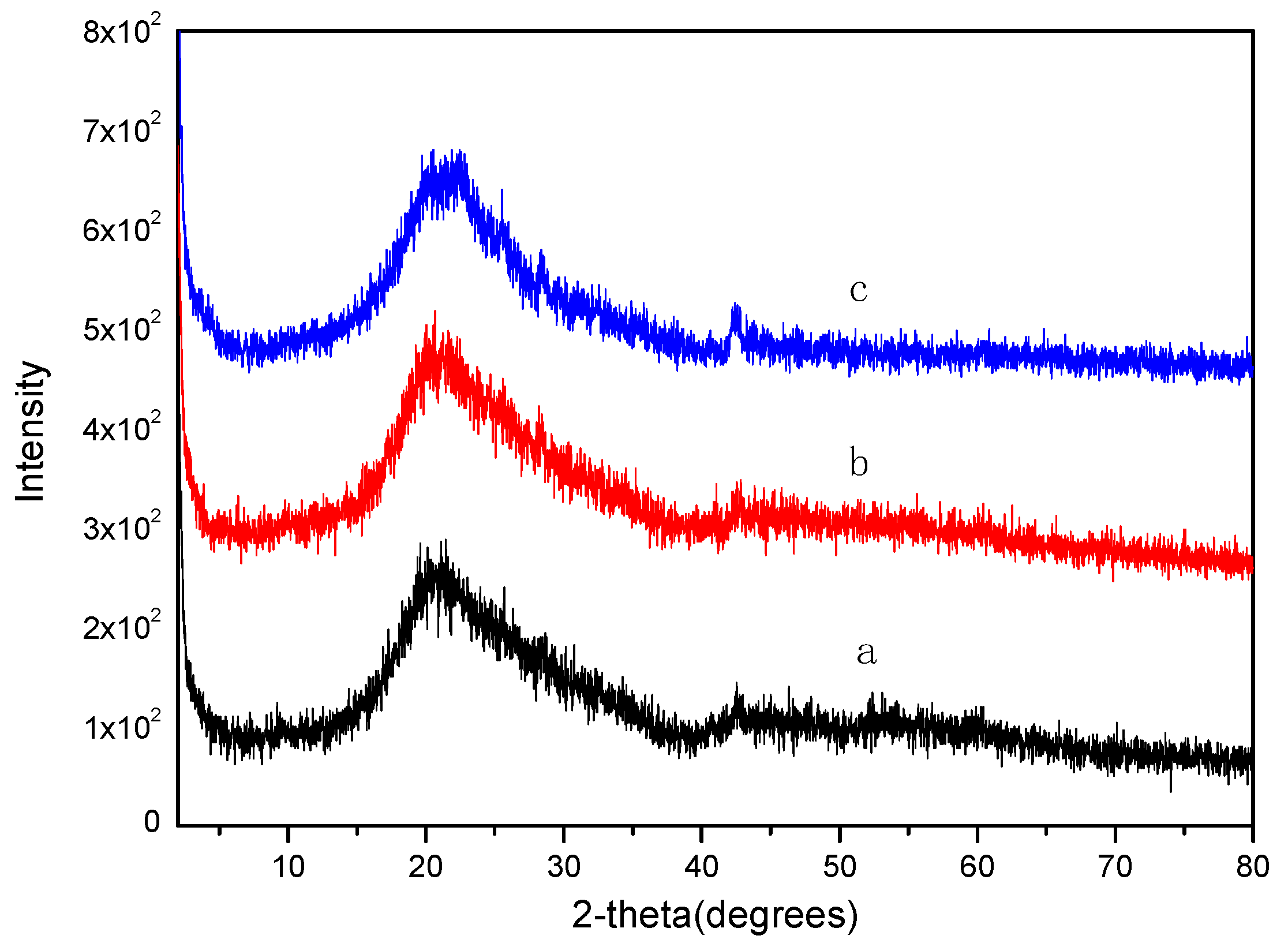
2.3. XRD Analysis
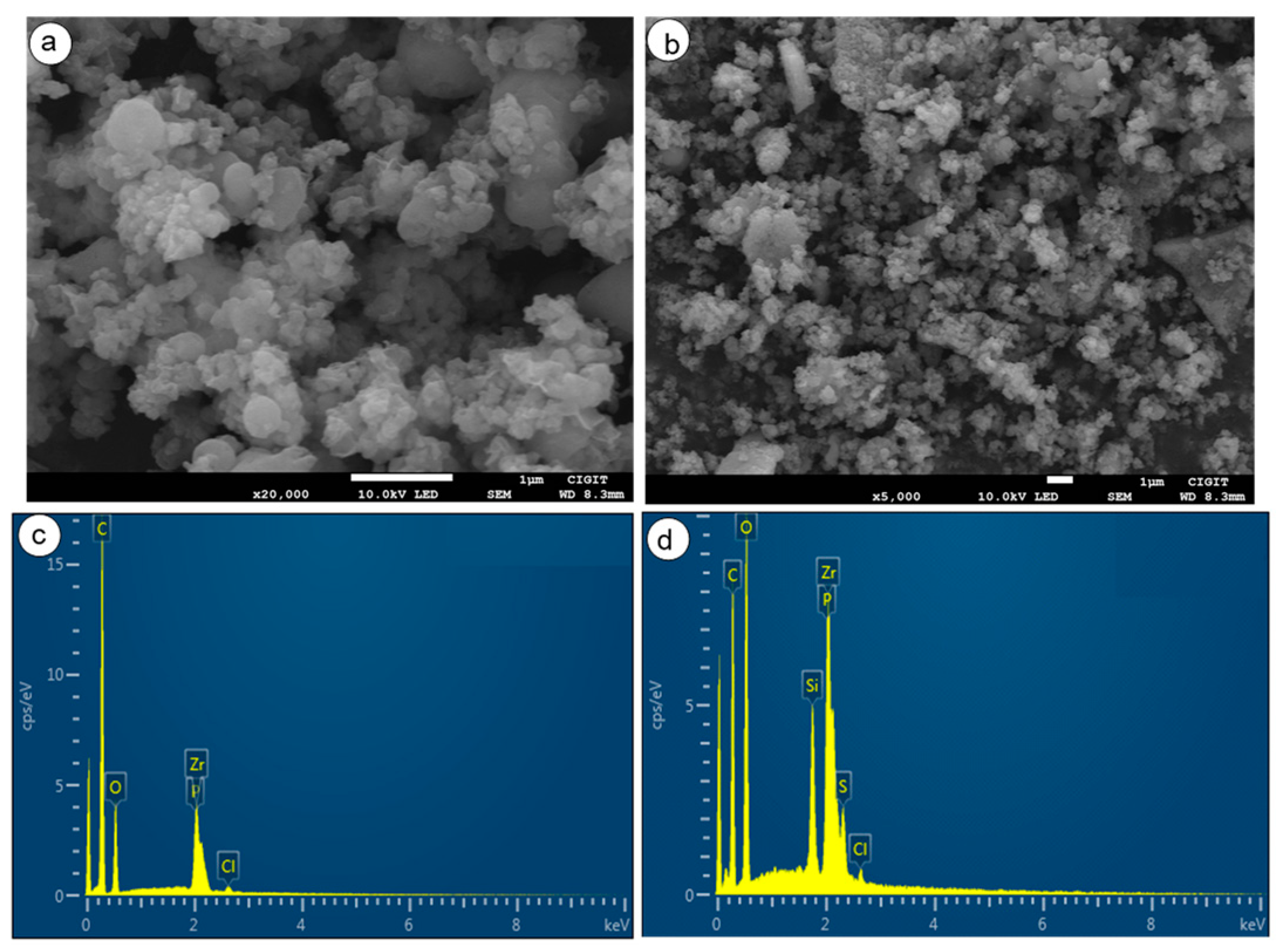
2.4. Microscopic Analysis
2.5. Thermal Gravimetric Analysis
2.6. Catalytic Reaction
2.7. Reusability of the ZPS–PVPA–SO3H
3. Material and Methods
3.1. Materials
3.2. Methods
3.3. Preparation of Catalysts
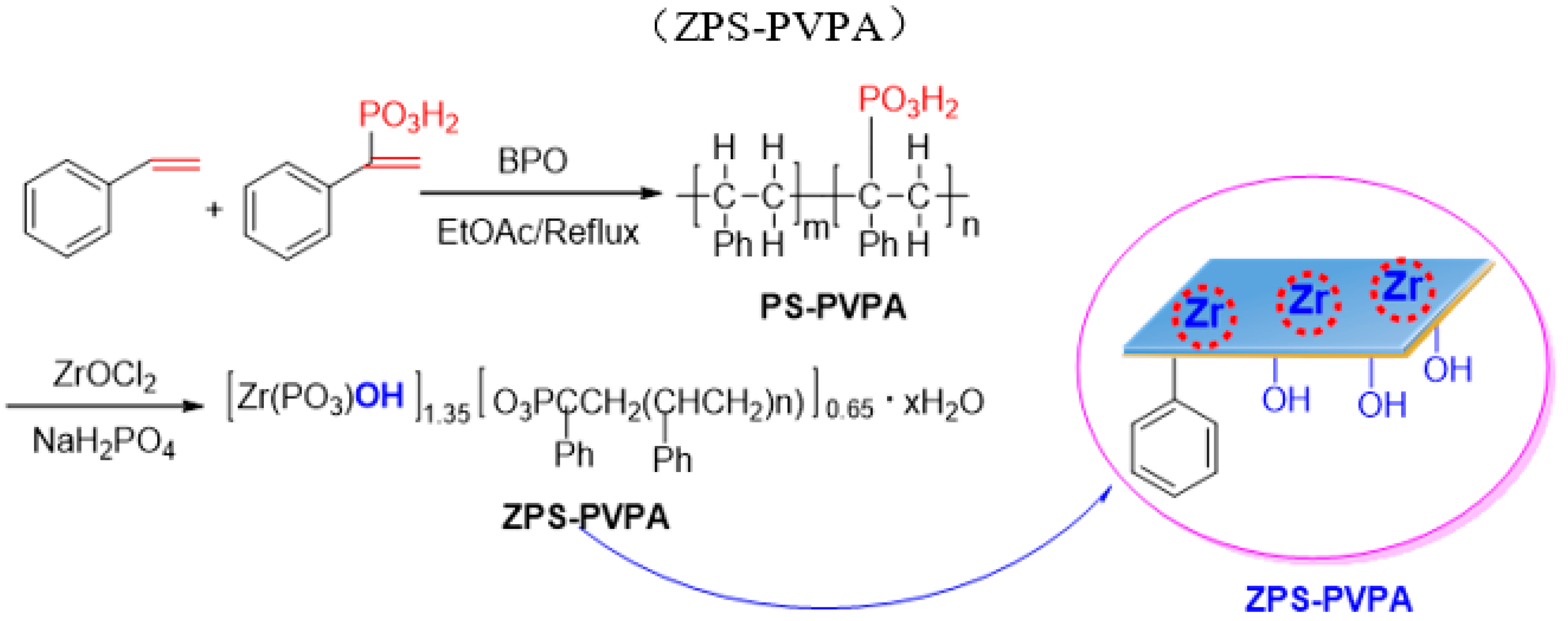
3.3.1. Synthesis of Zirconium poly(styrene-phenylvinyl-phosphonate)-phosphate (ZPS–PVPA)
3.3.2. Synthesis of Sulfonic Acid-Functionalized Zirconium Poly(styrene-phenylvinyl-phosphonate)-phosphate
3.3.3. Epoxidation of Soybean Oil
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goud, V.V.; Patwardhan, A.V.; Dinda, S.; Pradhan, N. Kinetics of epoxidation of jatropha oil with peroxyacetic and peroxyformic acid catalysed by acidic ion exchange resin. Chem. Eng. Sci. 2007, 62, 4065–4076. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Jiang, P.P.; Leng, Y.; Xu, Y.C.; Mo, G.T.; Bian, G. Synthesis of cis-dioxomolybdenum(VI)-tridentate schiff base complexes and its catalytic activity on the epoxidation of soybean oil. Chem. J. Chin. Univ. 2013, 34, 1703–1708. [Google Scholar]
- Turco, R.; Pischetola, C.; Di Serio, M.; Vitiello, R.; Tesser, R.; Santacesaria, E. Selective Epoxidation of Soybean Oil in the Presence of H-Y Zeolite. Ind. Eng. Chem. Res. 2017, 56, 7930–7936. [Google Scholar] [CrossRef]
- Vianello, C.; Piccolo, D.; Lorenzetti, A.; Salzano, E.; Maschio, G. Study of soybean oil epoxidation: Effects of sulfuric acid and the mixing program. Ind. Eng. Chem. Res. 2018, 57, 11517–11525. [Google Scholar] [CrossRef]
- Varyambath, A.; Kim, M.R.; Kim, I. Sulfonic acid-functionalized organic knitted porous polyaromatic microspheres as heterogeneous catalysts for biodiesel production. New J. Chem. 2018, 42, 12745–12753. [Google Scholar] [CrossRef]
- Chang, J.M.; Guan, X.Y.; Pan, S.Y.; Jia, M.L.; Chen, Y.; Fan, H.J. Sulfonated poly(styrene-divinylbenzene-glycidyl methacrylate)-capsulated magnetite nanoparticles as a recyclable catalyst for one-step biodiesel production from high free fatty acid-containing feedstocks. New J. Chem. 2018, 42, 13074–13080. [Google Scholar] [CrossRef]
- Cano, S.E.; Campos, M.J.M.; Fierro, J.L.G. Sulfonic acid-functionalized silica through quantitative oxidation of thiol groups. Chem. Commun. 2003, 2, 246–247. [Google Scholar] [CrossRef]
- Rhee, C.H.; Kim, H.K.; Chang, H.; Lee, J.S. Nafion/sulfonated montmorillonite composite: A new concept electrolyte membrane for direct methanol fuel cells. Chem. Mater. 2005, 17, 1691–1697. [Google Scholar] [CrossRef]
- Smuleac, V.; Butterfield, D.A.; Sikdar, S.K.; Varma, R.S.; Bhattacharyya, D. Polythiol-functionalized alumina membranes for mercury capture. J. Membr. Sci. 2005, 251, 169–178. [Google Scholar] [CrossRef]
- Felice, V.; Ntais, S.; Tavares, A.C. Propyl sulfonic acid functionalization of faujasite-type zeolites: Effect on water and methanol sorption and on proton conductivity. Microporous Mesoporous Mater. 2013, 169, 128–136. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Huang, R.C.; Ding, F.C.; Brittain, A.D.; Liu, J.J.; Zhang, M.; Xiao, M.; Meng, Y.Z.; Sun, L.Y. Sulfonic acid-functionalized α-zirconium phosphate single-layer nanosheets as a strong solid acid for heterogeneous catalysis applications. ACS Appl. Mater. Interfaces 2014, 6, 7417–7425. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.C.; Wang, Y.; Wang, C.; Shi, K.Y.; Ren, Y.R.; Zhao, X. Chiral MnIII (Salen) immobilized on organic polymer/inorganic zirconium hydrogen phosphate functionalized with 3-aminopropyltrimethoxysilane as an efficient and recyclable catalyst for enantioselective epoxidation of styrene. Polymers 2019, 11, 212. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.C.; Wang, C.; Wang, Y.; Shi, K.Y.; Wang, Z.M.; Li, D.W.; Fu, X.K. Chiral MnIII (Salen) covalently bonded on modified ZPS–PVPA and ZPS-IPPA as efficient catalysts for enantioselective epoxidation of unfunctionalized olefins. Polymers 2017, 9, 108. [Google Scholar] [CrossRef]
- Zou, X.C.; Shi, K.Y.; Li, J.; Wang, Y.; Wang, C.; Deng, C.F.; Ren, Y.R.; Tan, J.; Fu, X.K. Research progress on epoxidation of olefins catalyzed by Mn(II, III, V) in different valence states. Chin. J. Org. Chem. 2016, 36, 1765–1778. [Google Scholar] [CrossRef]
- Luo, Y.F.; Zou, X.C.; Fu, X.K.; Huang, X.M.; Jia, Z.Y. The advance in asymmetric epoxidation of olefins catalyzed by chiral Mn (salen). Sci. China Chem. 2011, 41, 433–450. [Google Scholar]
- Zou, X.C.; Huang, L.Y.; Quan, W.X.; Wang, C.; Wang, Y.; Zhao, X.; Tan, Z.W. Sulfonic-functionalized organic polystyrene/inorganic hydrogen zirconium phosphate catalyzed epoxidation of soybean oil. Chin. J. Inorg. Chem. 2019, 35, 1349–1356. [Google Scholar]
- Alberti, G.; Casciola, M.; Palombari, R. Acid zirconium phosphates and phosphonates as proton conductors and their use for solid-state gas sensors. Russ. J. Electrochem. 1993, 29, 1257–1264. [Google Scholar]
- Sun, S.Q.; Yang, F.C.; Yi, Y.J.; Chang, Y.; Zhou, Y.S.; Zha, F. Catalytic synthesis of epoxidized soybean oil by tridentate pyridine schif-base molybdenum (Ⅵ) complex. Fine Chem. 2016, 33, 792–796. [Google Scholar]
- Wu, F.J.; Jiang, P.P.; Zhang, P.B.; Dong, Y.M.; Li, X.L. Cu-salen complex as a catalyst for epoxidation of soybean oil to synthesize an environmental friendly plasticizer. China Oils Fats 2012, 37, 56–60. [Google Scholar]
- Ren, W.S.; Fu, X.K.; Bao, H.B.; Bai, R.F.; Ding, P.P.; Sui, B.L. Enantioselective epoxidation of unfunctionalized olefins catalyzed by chiral salen Mn(III) catalyst immobilized on zirconium oligostyrenylphosphonate-phosphate. Catal. Commun. 2009, 10, 788–793. [Google Scholar] [CrossRef]
- Wu, X.J.; Ma, X.B.; Ji, Y.L.; Wang, Q.; Jia, X.; Fu, X.K. Synthesis and characterization of a novel type of self-assembled chiral zirconium phosphonates and its application for heterogeneous asymmetric catalysis. J. Mol. Catal. A Chem. 2007, 265, 316–322. [Google Scholar] [CrossRef]
- Zou, X.C.; Shi, K.Y.; Wang, C. Chiral MnIII (Salen) supported on tunable phenoxyl group modified zirconium poly(styrene-phenylvinylphosphonate)-phosphate as an efficient catalyst for epoxidation of unfunctionalized olefins. Chin. J. Catal. 2014, 35, 1446–1455. [Google Scholar] [CrossRef]
- Park, J.W.; Park, Y.J.; Jun, C.H. Post-grafting of silica surfaces with pre-functionalized organosilanes: New synthetic equivalents of conventional trialkoxysilanes. Chem. Commun. 2011, 47, 4860–4871. [Google Scholar] [CrossRef]
- Alberti, G.; Casciola, M.; Costantino, U. Inorganic ion-exchange pellicles obtained by delamination of alpha-zirconium phosphate crystals. J. Colloid Interface Sci. 1985, 107, 256–263. [Google Scholar] [CrossRef]

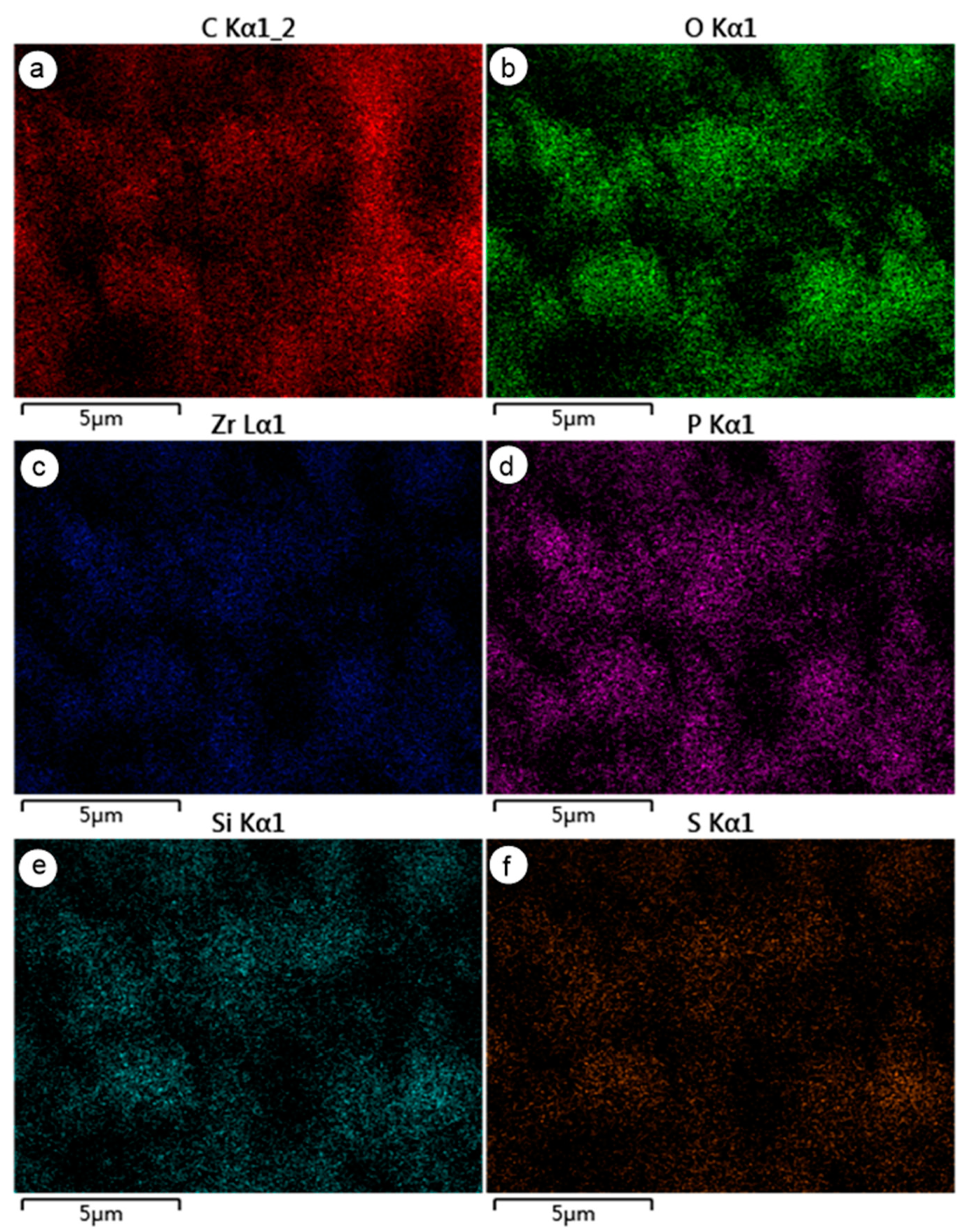


| Number | Sample | Acidity (μmol/g) |
|---|---|---|
| 1 | ZPS–PVPA | 7.2 |
| 2 | ZPS–PVPA–SH | 0.12 |
| 3 | ZPS–PVPA–SO3H | 418.9 |
| Entry | ZPS–PVPA–SO3H (wt%) (h) | Temperature (°C) | Conversion (%) [b] | Selectivity (%) [b] | Yield (%) |
|---|---|---|---|---|---|
| 1 | 1 (6) | 80 | 32.1 | 50.7 | 16.27 |
| 2 | 2 (6) | 80 | 53.7 | 57.2 | 30.72 |
| 3 | 5 (6) | 80 | 69.1 | 61.6 | 42.57 |
| 4 | 10 (6) | 80 | 78.2 | 76.8 | 60.06 |
| 5 | 20 (6) | 80 | 81.6 | 73.2 | 59.73 |
| 6 | No catalyst (6) | 80 | 10.6 | 3.2 | 0.34 |
| 7 | 10 (6) | 40 | 36.8 | 43.7 | 16.08 |
| 8 | 10 (6) | 100 | 81.6 | 53.7 | 43.82 |
| 9 | 10 (2) | 80 | 47.3 | 56.2 | 26.58 |
| 10 | 10 (4) | 80 | 59.7 | 63.7 | 38.03 |
| 11 | 10 (10) | 80 | 83.1 | 57.0 | 47.37 |
| Run | Conversion (%) [b] (h) | Selectivity (%) [b] | Yield (%) |
|---|---|---|---|
| 1 | 78.2 (6) | 76.8 | 60.06 |
| 2 | 77.9 (6) | 75.4 | 58.74 |
| 3 | 77.1 (6) | 74.2 | 57.21 |
| 4 | 76.8 (6) | 73.8 | 56.68 |
| 5 | 76.0 (6) | 72.7 | 55.25 |
| 6 | 70.1 (6) | 66.3 | 46.48 |
| 7 | 61.3 (6) | 50.9 | 31.20 |
| 8 | 76.3 (6) | 69.4 | 52.95 |
| 9 | 75.8 (6) | 67.8 | 51.39 |
| 10 | 70.6 (6) | 62.9 | 44.41 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, X.; Nie, X.; Tan, Z.; Shi, K.; Wang, C.; Wang, Y.; Zhao, X. Synthesis of Sulfonic Acid-Functionalized Zirconium Poly(Styrene-Phenylvinyl-Phosphonate)-Phosphate for Heterogeneous Epoxidation of Soybean Oil. Catalysts 2019, 9, 710. https://doi.org/10.3390/catal9090710
Zou X, Nie X, Tan Z, Shi K, Wang C, Wang Y, Zhao X. Synthesis of Sulfonic Acid-Functionalized Zirconium Poly(Styrene-Phenylvinyl-Phosphonate)-Phosphate for Heterogeneous Epoxidation of Soybean Oil. Catalysts. 2019; 9(9):710. https://doi.org/10.3390/catal9090710
Chicago/Turabian StyleZou, Xiaochuan, Xuyuan Nie, Zhiwen Tan, Kaiyun Shi, Cun Wang, Yue Wang, and Xin Zhao. 2019. "Synthesis of Sulfonic Acid-Functionalized Zirconium Poly(Styrene-Phenylvinyl-Phosphonate)-Phosphate for Heterogeneous Epoxidation of Soybean Oil" Catalysts 9, no. 9: 710. https://doi.org/10.3390/catal9090710





