In-Situ Construction of 2D/2D ZnIn2S4/BiOCl Heterostructure with Enhanced Photocatalytic Activity for N2 Fixation and Phenol Degradation
Abstract
:1. Introduction
2. Results and Discussion
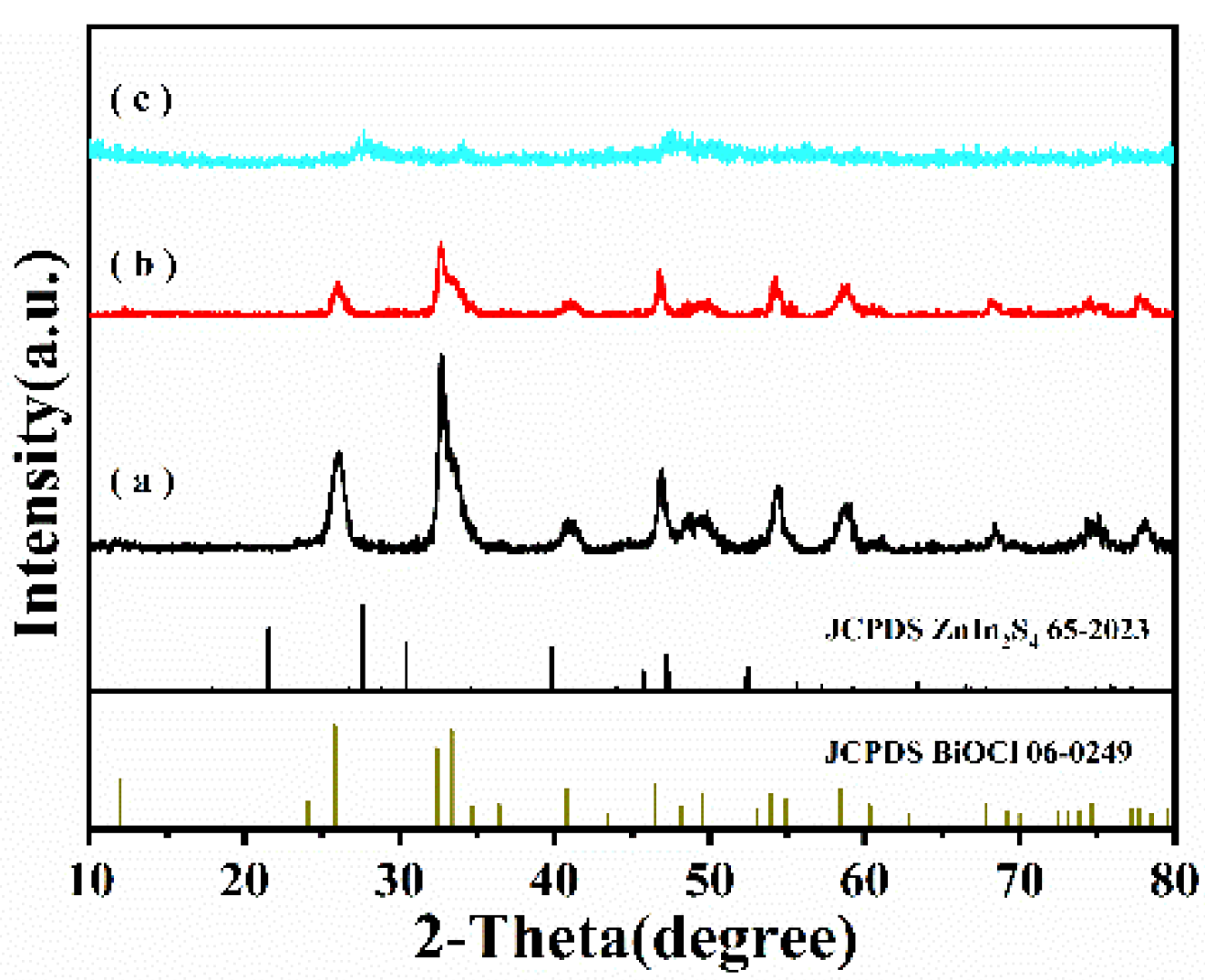
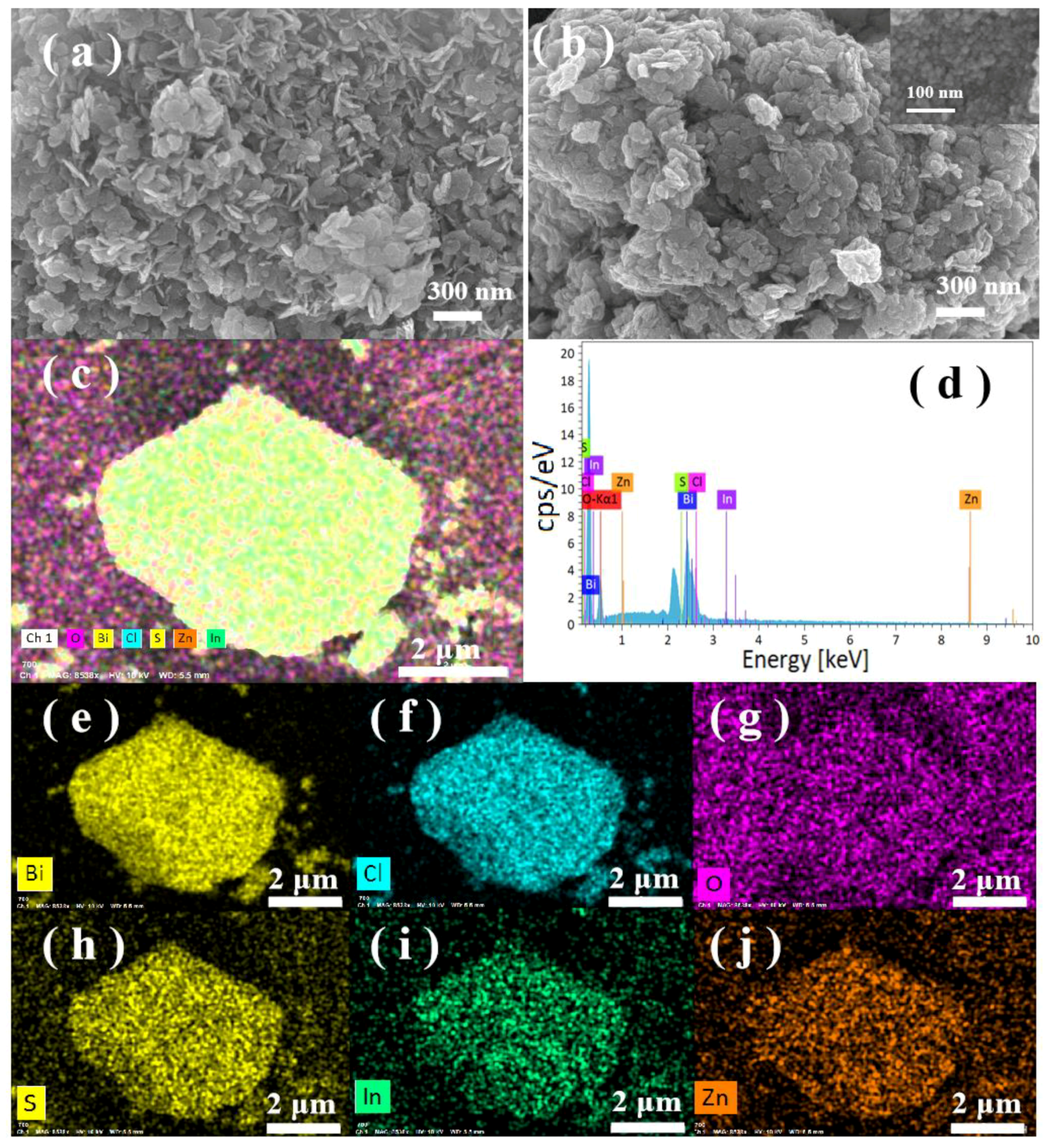
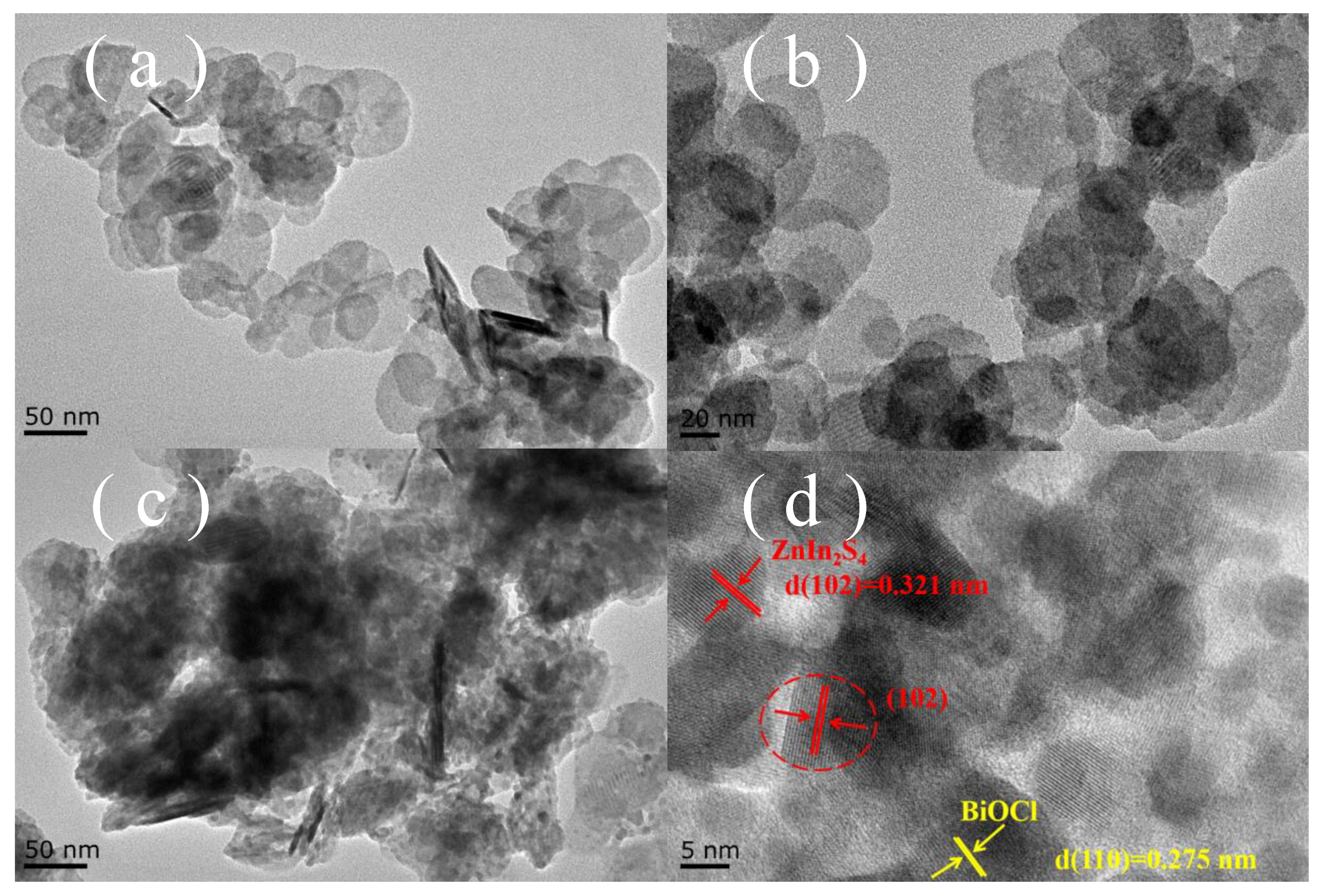
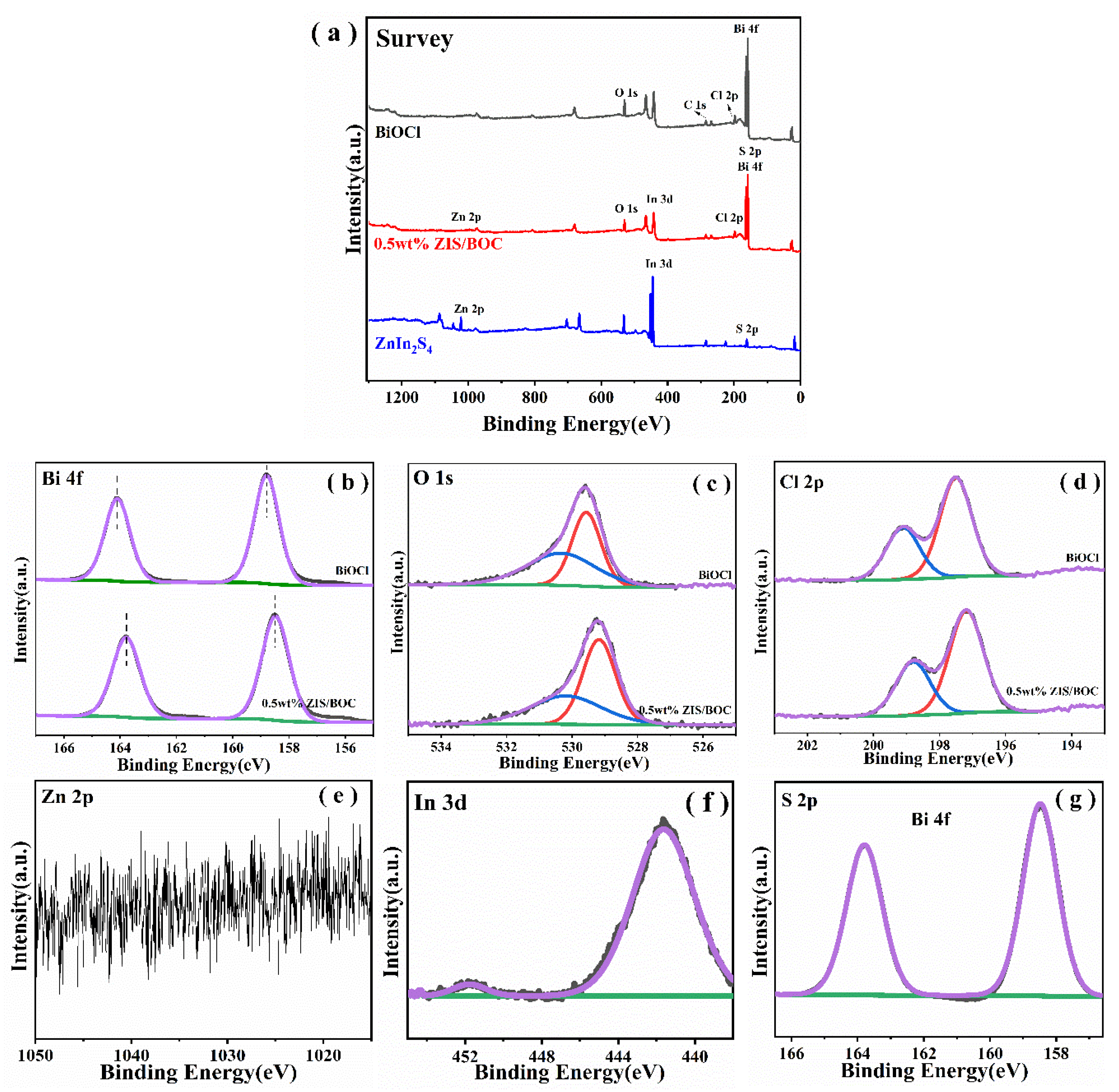
2.1. Characterization of Photocatalysts
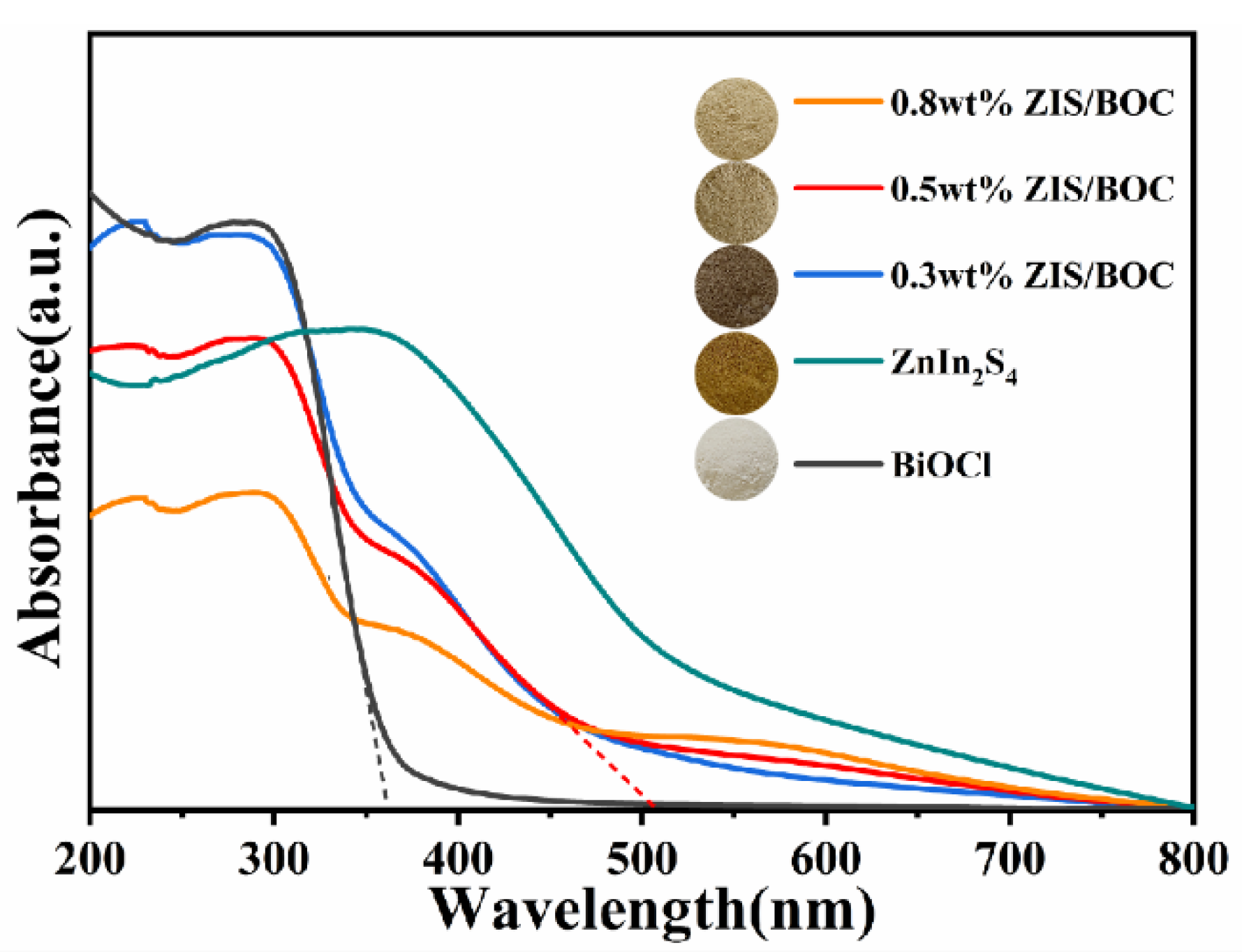
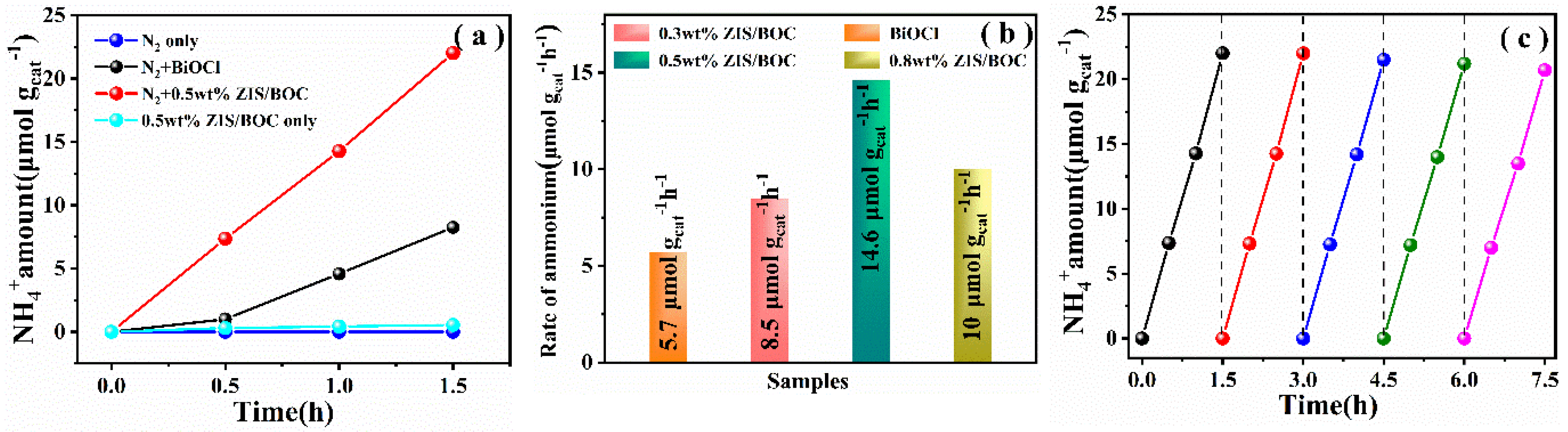
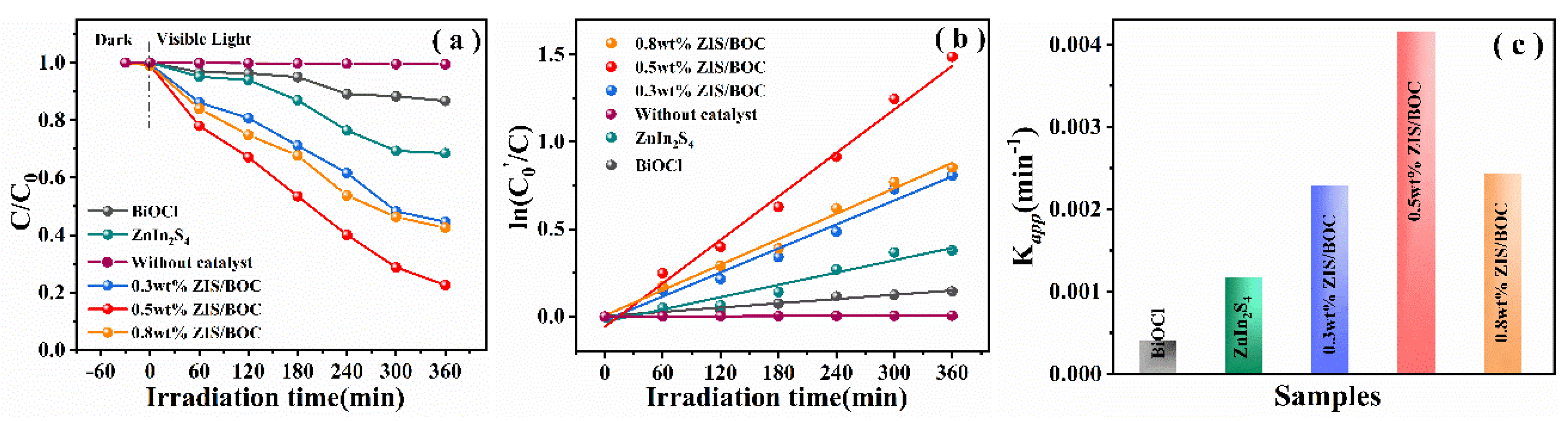
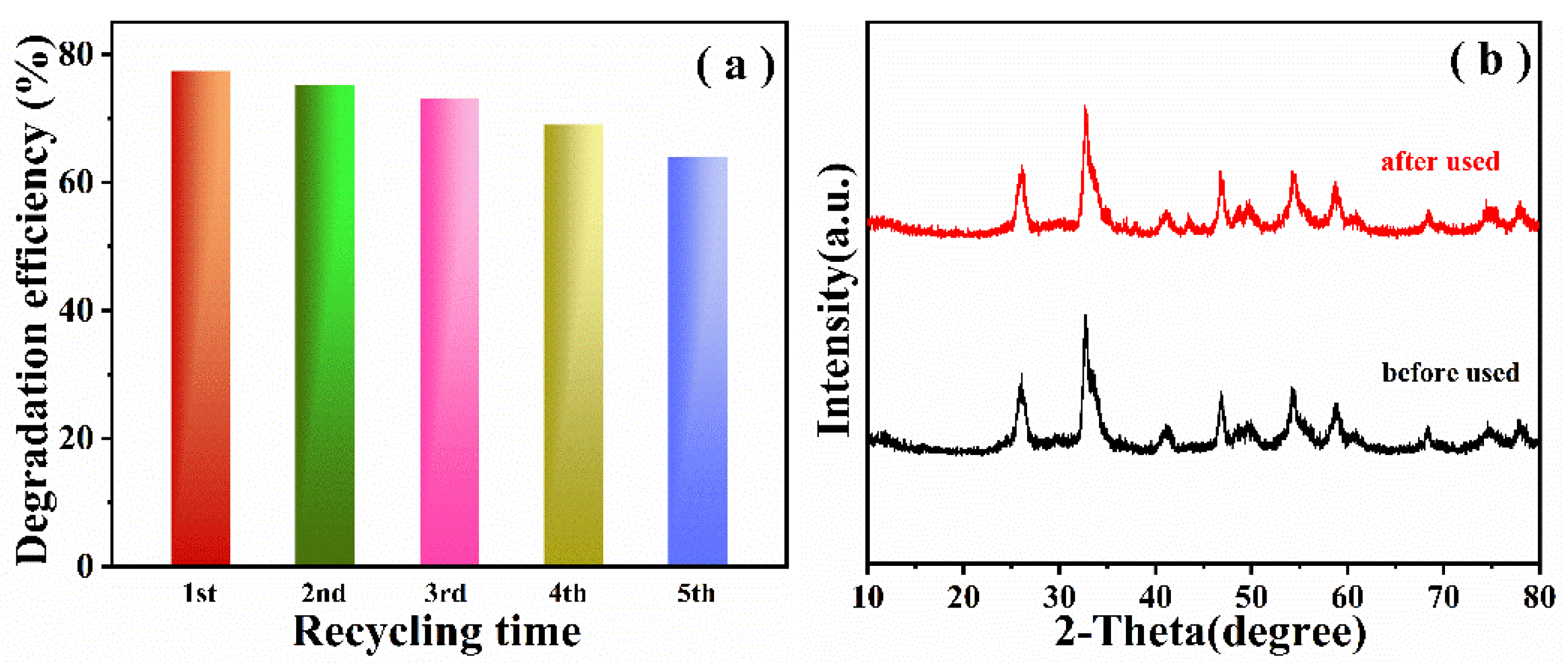
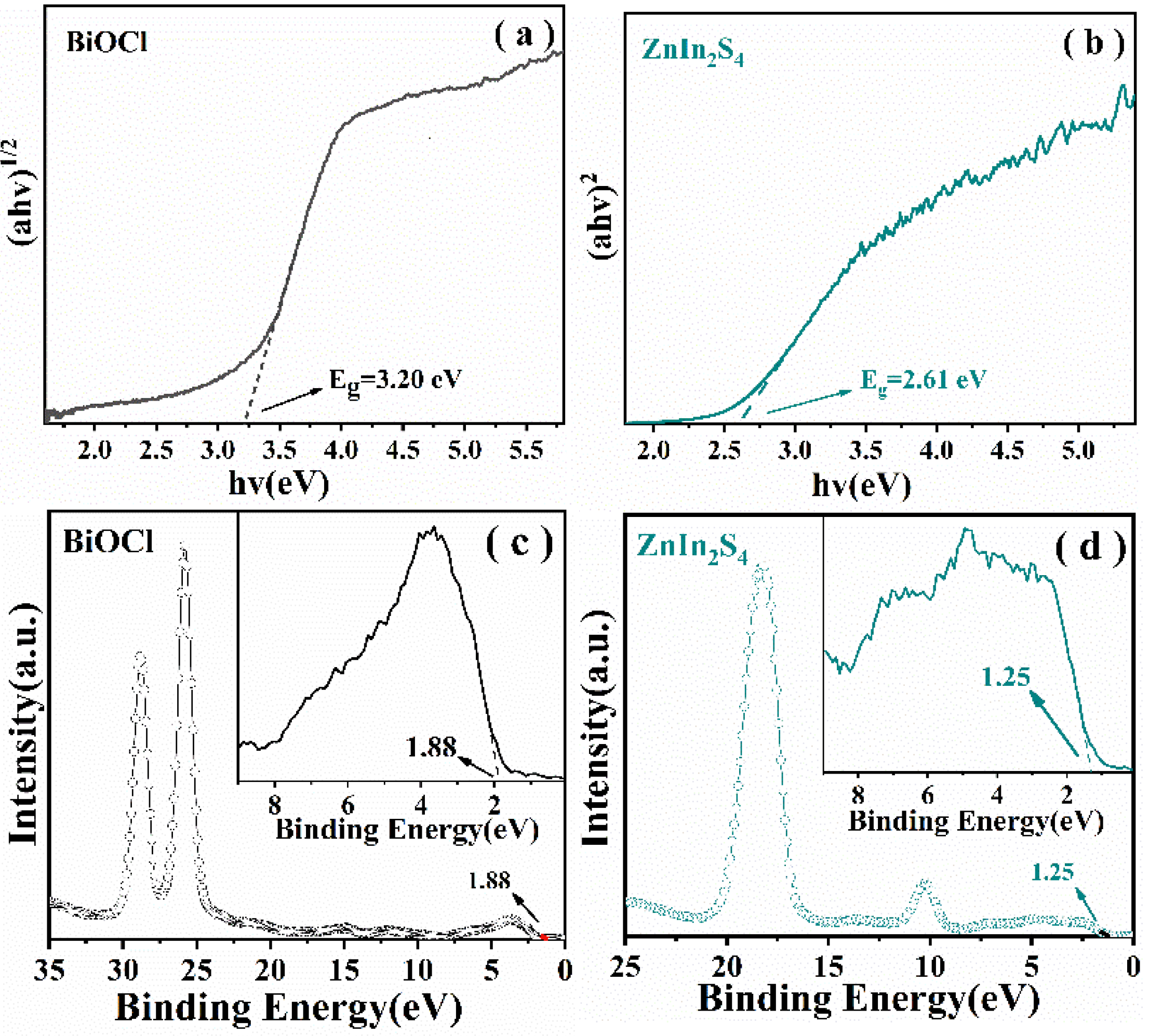
2.2. Optical Properties and Photocatalytic Performances of The Samples
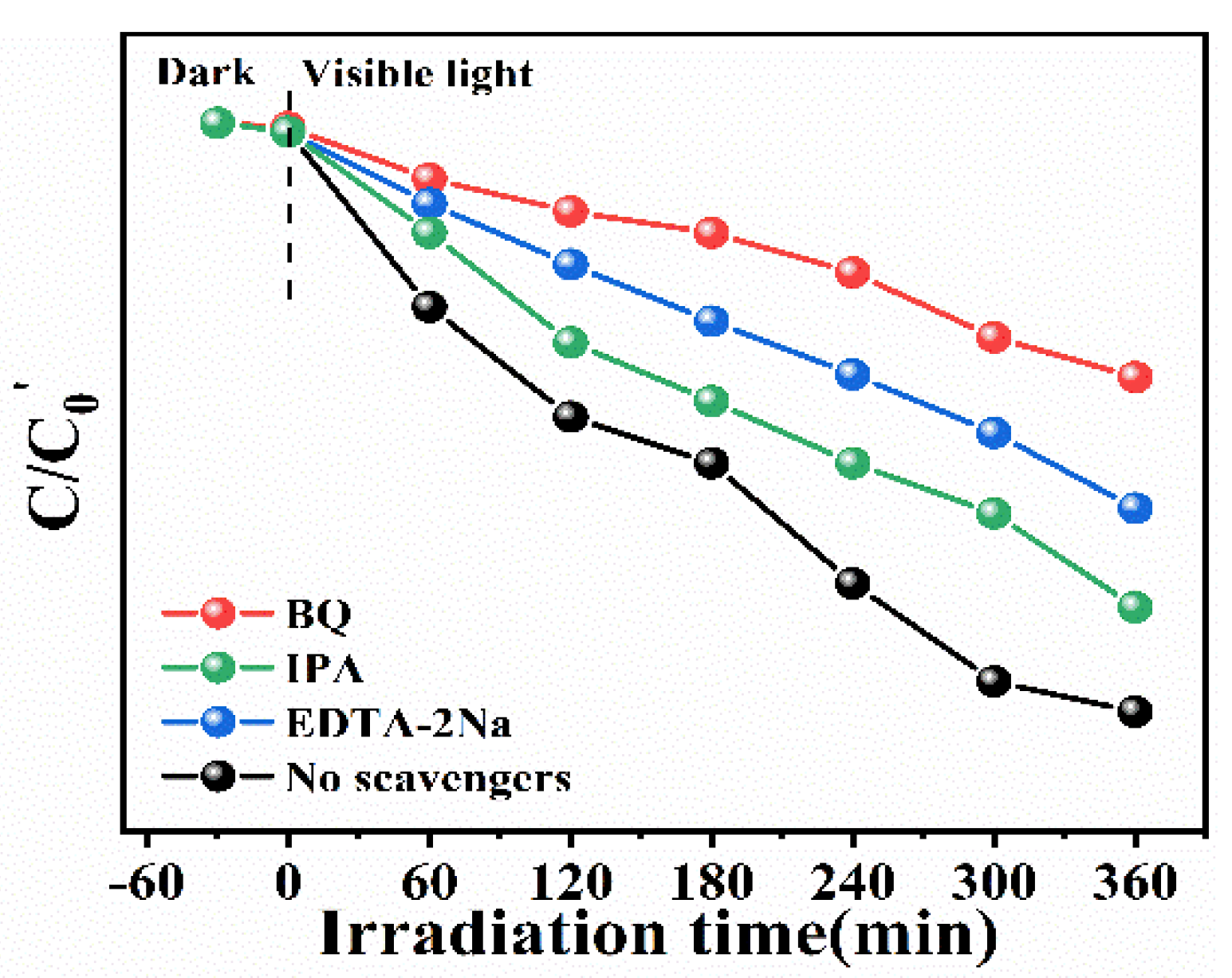
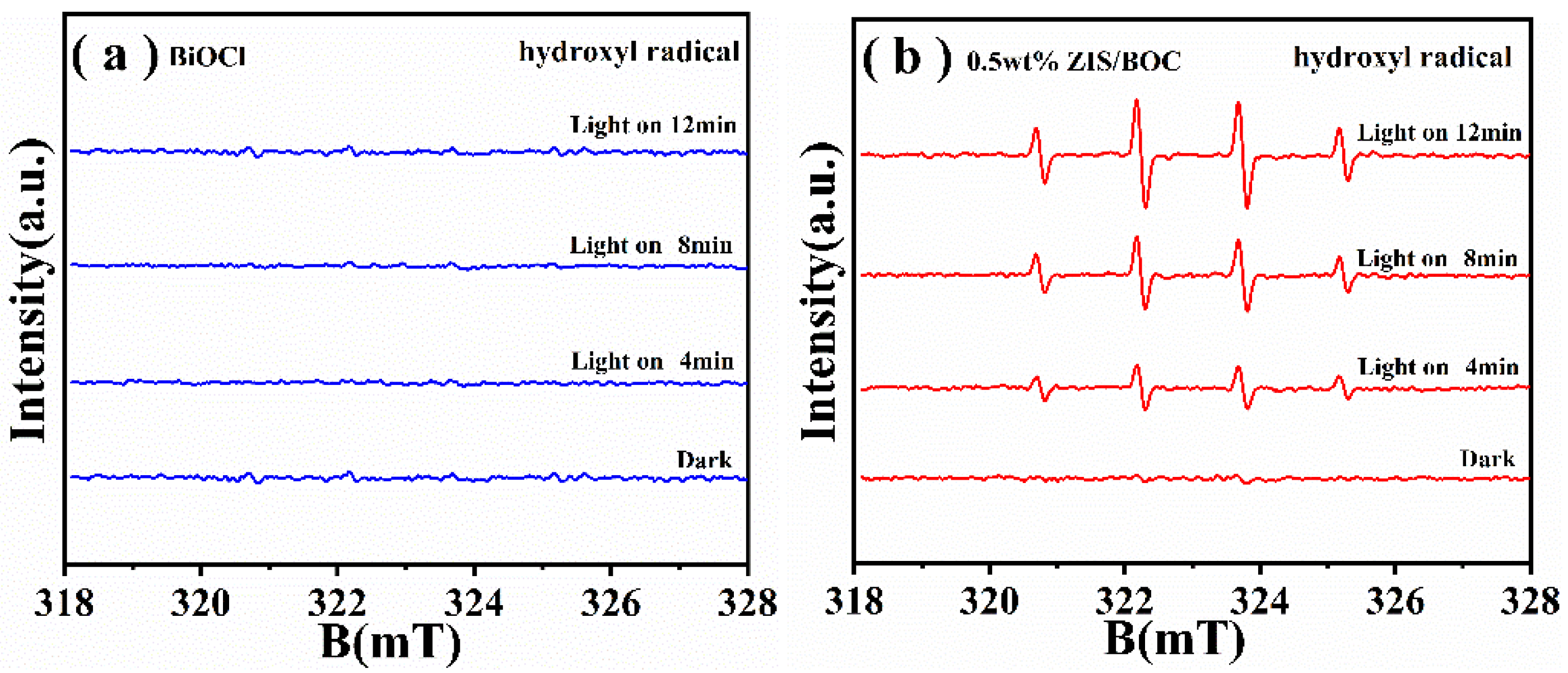
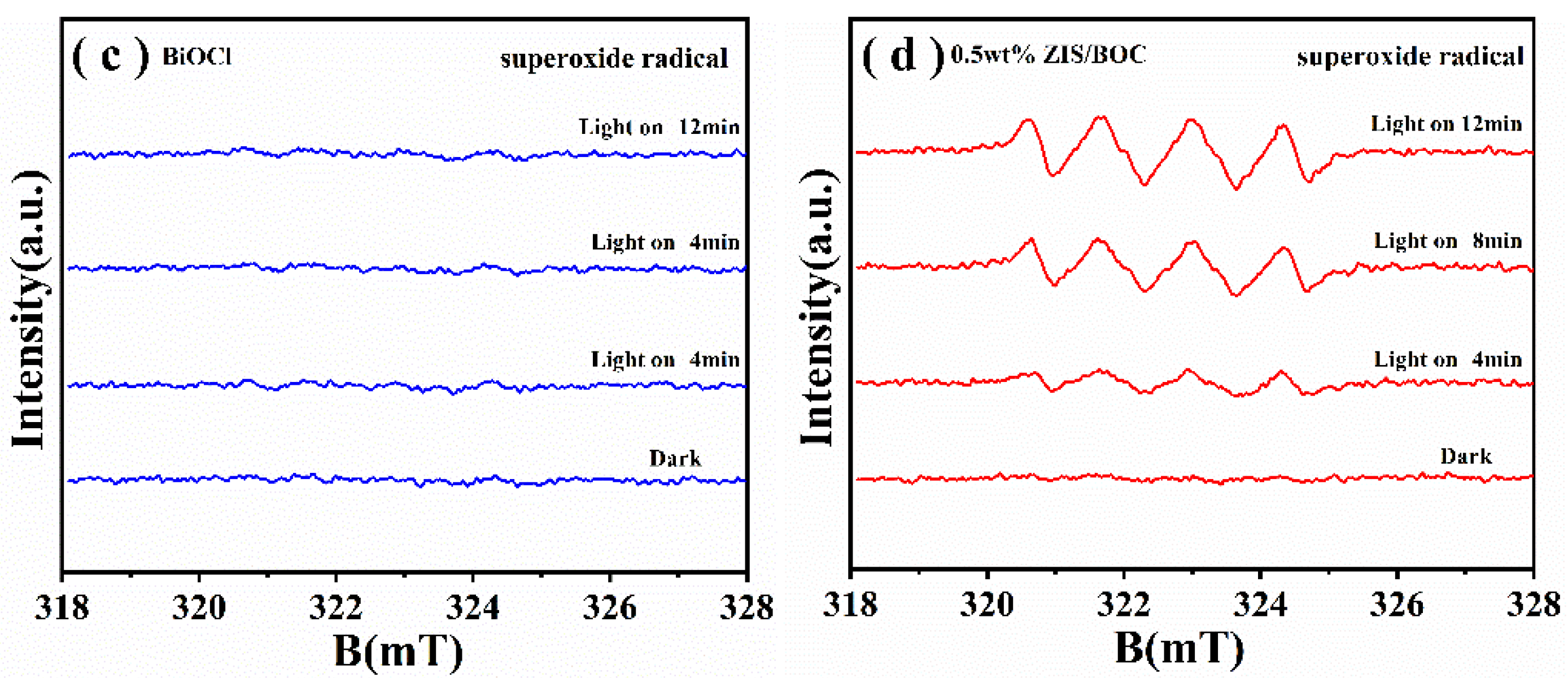
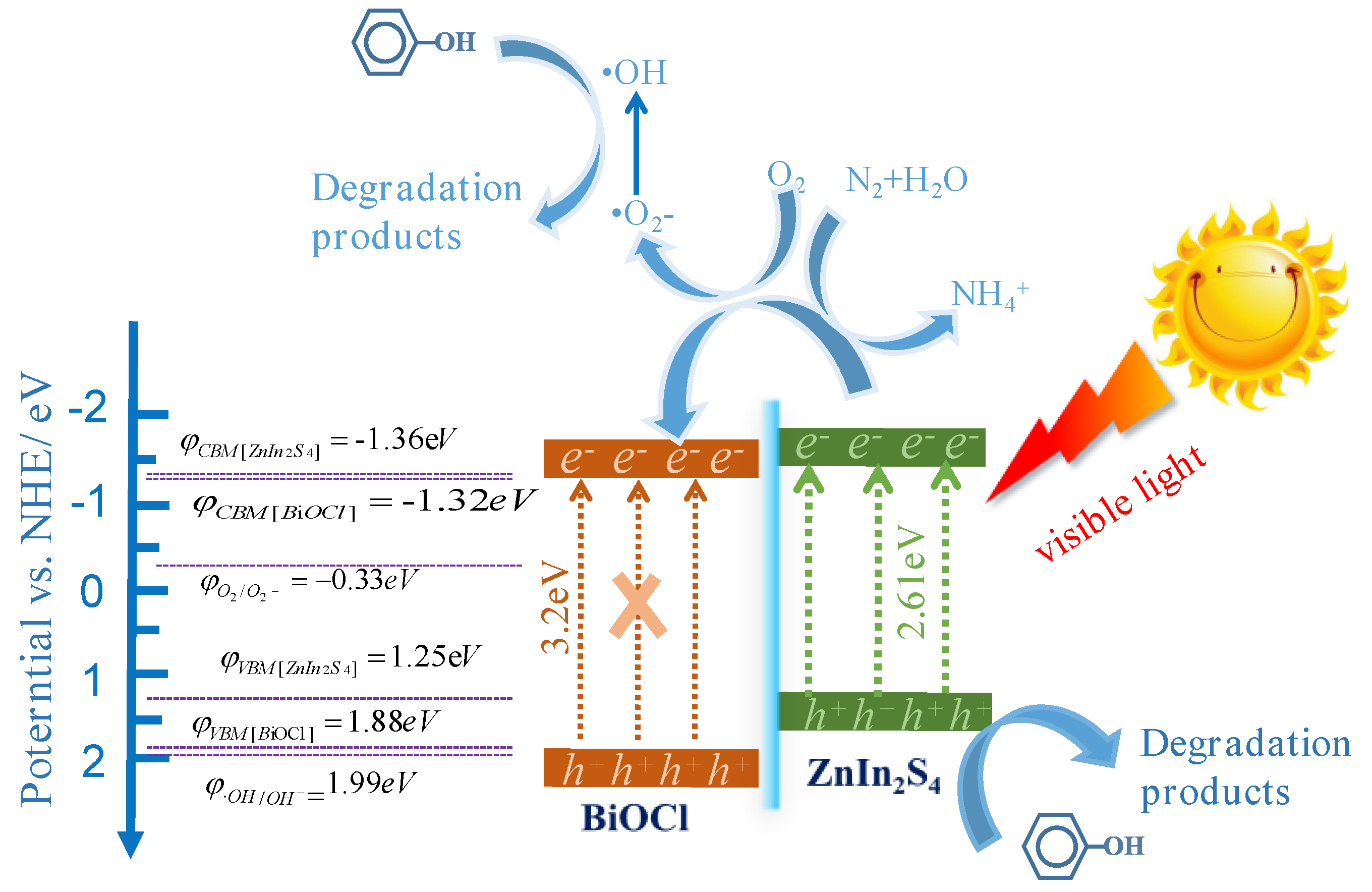
2.3. Photocatalytic Mechanism
3. Experimental Section
3.1. Chemicals
3.2. Sample Preparation
3.3. Characterization
3.4. Photocatalytic Activity Measurement
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, W.; Zhou, J.; Zhang, Z.; Yu, J.; Cai, W. Different Surfactant-Assisted Hydrothermal Synthesis of Hierarchical Gamma-Al2O3 and Its Adsorption Performances for Parachlorophenol. Chem. Eng. J. 2013, 233, 168–175. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, J.; Cai, W.; Zhao, R.; Yuan, J. Hierarchically Porous NiAl-LDH Nanoparticles as Highly Efficient Adsorbent for p-Nitrophenol from Water. Appl. Surf. Sci. 2015, 349, 897–903. [Google Scholar] [CrossRef]
- Avetta, P.; Pensato, A.; Minella, M.; Malandrino, M.; Maurino, V.; Minero, C.; Hanna, K.; Vione, D. Activation of Persulfate by Irradiated Magnetite: Implications for the Degradation of Phenol under Heterogeneous Photo-Fenton-Like Conditions. Environ. Sci. Technol. 2015, 49, 104–1050. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Shen, H.D.; Sun, X.; Xue, W.W.; Shoneyec, A.; Ma, J.N.; Luo, L.; Wang, D.J.; Wang, J.G.; Tang, J.W. Synergistic Effect of Surface Oxygen Vacancies and Interfacial Charge Transfer on Fe(III)/Bi2MoO6 for Efficient Photocatalysis. Appl. Catal. B 2019, 247, 150–162. [Google Scholar] [CrossRef]
- Ding, Z.; Lu, G.Q.; Greenfield, P.F. Role of the Crystallite Phase of TiO2 in Heterogeneous Photocatalysis for Phenol Oxidation in Water. J. Phys. Chem. B 2000, 104, 4815–4820. [Google Scholar] [CrossRef]
- Schrauzer, G.N.; Guth, T.D. Photocatalytic Reactions. 1. Photolysis of Water and Photoreduction of Nitrogen on Titanium Dioxide. J. Am. Chem. Soc. 1977, 99, 7189–7193. [Google Scholar] [CrossRef]
- Zhou, F.; Azofra, L.M.; Ali, M.; Kar, M.; Simonov, A.N.; McDonnell-Worth, C.; Sun, C.; Zhang, X.; MacFarlane, D.R. Electro-Synthesis of Ammonia from Nitrogen at Ambient Temperature and Pressure in Ionic Liquids. Energy Environ. Sci. 2017, 10, 2516–2520. [Google Scholar] [CrossRef]
- Rong, X.; Mao, Y.; Xu, J.; Zhang, X.; Zhang, L.; Zhou, X.; Qiu, F.; Wu, Z. Bi2Te3 Sheet Contributing to the Formation of Flower-Like BiOCl Composite and Its N2 Photofixation Ability Enhancement. Catal. Commun. 2018, 116, 16–19. [Google Scholar] [CrossRef]
- Feng, X.; Chen, H.; Jiang, F.; Wang, X. In-Situ Self-Sacrificial Fabrication of Lanthanide Hydroxycarbonates/Graphitic Carbon Nitride heterostructures: Nitrogen Photofixation under Simulated Solar Light Irradiation. Chem. Eng. J. 2018, 347, 849–859. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, X.; Kong, Z.; Ong, W.J.; Li, N. Unravelling the Electrochemical Mechanisms for Nitrogen Fixation on Single Transition Metal Atoms Embedded in Defective Graphitic Carbon Nitride. J. Mater. Chem. A 2018, 6, 21941–21948. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, L.H.; Wang, D.; Zhao, L.; Wan, B. Light-Induced Efficient Molecular Oxygen Activation on a Cu(II)–Grafted TiO2/Graphene Photocatalyst for Phenol Degradation. ACS Appl. Mater. Interfaces 2015, 7, 1816–1823. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, G.K.; Padhi, D.K.; Parida, K.M. Fabrication of α-Fe2O3 Nanorod/RGO Composite: A Novel Hybrid Photocatalyst for Phenol Degradation. ACS Appl. Mater. Interfaces 2013, 5, 9101–9110. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Shen, H.D.; Xue, W.W.; Zhen, Y.Z.; Soomro, R.A.; Yang, X.X.; Wang, D.J.; Xu, B.; Chi, R.A. Alkali-Assisted Synthesis of Direct Z-Scheme Based Bi2O3/Bi2MoO6 Photocatalyst for Highly Efficient Photocatalytic Degradation of Phenol and Hydrogen Evolution Reaction. J. Catal. 2019, 375, 399–409. [Google Scholar] [CrossRef]
- Saputra, E.; Muhammad, S.; Sun, H.Q.; Ang, H.M.; Tadé, M.O.; Wang, S.B. Manganese Oxides at Different Oxidation States for Heterogeneous Activation of Peroxymonosulfate for Phenol Degradation in Aqueous Solutions. Appl. Catal. B 2013, 142-143, 729–735. [Google Scholar] [CrossRef]
- Saputra, E.; Muhammad, S.; Sun, H.Q.; Ang, H.M.; Tadé, M.O.; Wang, S.B. Shape-Controlled Activation of Peroxymonosulfate by Single Crystal α-Mn2O3 for Catalytic Phenol Degradation in Aqueous Solution. Appl. Catal. B 2014, 154, 246–251. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, G.; Ding, L.; Chen, X.; Ding, L.; Wang, H. Efficient Electrocatalytic N2 Fixation with MXene under ambient conditions. Joule 2019, 3, 1–11. [Google Scholar] [CrossRef]
- Hoffman, B.M.; Lukoyanov, D.; Yang, Z.Y.; Dean, D.R.; Seefeldt, L.C. Mechanism of Nitrogen Fixation by Nitrogenase: The Next Stage. Chem. Rev. 2014, 114, 4041–4062. [Google Scholar] [CrossRef] [PubMed]
- Honkala, K.; Hellman, A.; Remediakis, I.N.; Logadottir, A.; Carlsson, A.; Dahl, S.; Christensen, C.H.; Nørskov, J.K. Ammonia Synthesis from First-Principles Calculations. Science 2005, 307, 555–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanabe, Y.; Nishibayashi, Y. Developing More Dustainable Processes for Ammonia Synthesis. Coord. Chem. Rev. 2013, 257, 2551–2564. [Google Scholar] [CrossRef]
- Sivasankar, C.; Baskaran, S.; Tamizmani, M.; Ramakrishna, K. Lessons Learned and Lessons to be Learned for Developing Homogeneous Transition Metal Complexes Catalyzed Reduction of N2 to Ammonia. J. Organomet. Chem. 2014, 752, 44–58. [Google Scholar] [CrossRef]
- Ma, H.; Shi, Z.; Li, Q.; Li, S. Preparation of Graphitic Carbon Nitride with Large Specifific Surface Area and Outstanding N2 Photofifixation Ability via a Dissolve-Regrowth Process. J. Phys. Chem. Solids 2016, 99, 51–58. [Google Scholar] [CrossRef]
- Qiu, P.; Xu, C.; Zhou, N.; Chen, H.; Jiang, F. Metal-Free Black Phosphorus Nanosheets-Decorated Graphitic Carbon Nitride Nanosheets with C-P Bonds for Excellent Photocatalytic Nitrogen Fixation. Appl. Catal. B 2018, 221, 27–35. [Google Scholar] [CrossRef]
- Liu, Y.; Kong, J.; Yuan, J.; Zhao, W.; Zhu, X.; Sun, C.; Xie, J. Enhanced Photocatalytic Activity over Flower-like Sphere Ag/Ag2CO3/BiVO4 Plasmonic Heterostructure Photocatalyst for Tetracycline Degradation. Chem. Eng. J. 2018, 331, 242–254. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, Y.; Wang, J.; Tian, C.; Li, W.; Feng, Q.; Pan, Q.; Fu, H. In Situ Fabrication of Ag/Ag3PO4/Graphene Triple Heterostructure Visible-Light Photocatalyst Through Graphene-assisted Reduction Strategy. ChemCatChem 2013, 5, 1359–1367. [Google Scholar] [CrossRef]
- Ma, D.K.; Guan, M.L.; Liu, S.S.; Zhang, Y.Q.; Zhang, C.W.; He, Y.X.; Huang, S.M. Controlled Synthesis of Olive-shaped Bi2S3/BiVO4 Microspheres through a Limited Chemical Conversion Route and Enhanced Visible-Light-Responding Photocatalytic Activity. Dalton Trans. 2012, 41, 5581–5586. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, J.; Gao, Z.; Zhu, X.; Liu, Y.; Wei, Z.; Zhao, W.; Sun, C. A Simple and Effective Method for Fabricating Novel p–n Heterostructure Photocatalyst g-C3N4/Bi4Ti3O12 and Its Photocatalytic Performances. Appl. Catal. B 2016, 192, 57–71. [Google Scholar] [CrossRef]
- Miyama, H.; Fujii, N.; Nagae, Y. Heterogeneous Photocatalytic Synthesis of Ammonia from Water and Nitrogen. Chem. Phys. Lett. 1980, 74, 523–524. [Google Scholar] [CrossRef]
- Endoh, E.; Leland, J.K.; Bard, A.J. Heterogeneous Photoreduction of Nitrogen to Ammonia on Tungsten Oxide. J. Phys. Chem. 1986, 90, 6223–6226. [Google Scholar] [CrossRef]
- Hu, S.Z.; Chen, X.; Li, Q.; Li, F.Y.; Fan, Z.P.; Wang, H.; Wang, Y.J.; Zheng, B.H.; Wu, G. Fe3+ Doping Promoted N2 Photofixation Ability of Honeycombed Graphitic Carbon Nitride: The Experimental and Density Functional Theory Simulation Analysis. Appl. Catal. B 2017, 201, 58–69. [Google Scholar] [CrossRef]
- Jiang, J.; Zhao, K.; Xiao, X.Y.; Zhang, L.Z. Synthesis and Facet-Dependent Photoreactivity of BiOCl Single Crystalline Nanosheets. J. Am. Chem. Soc. 2012, 134, 4473–4476. [Google Scholar] [CrossRef]
- Jiang, R.R.; Wu, D.H.; Lu, G.H.; Yan, Z.H.; Liu, J.C.; Zhou, R.R.; Matthew, N. Fabrication of Fe3O4 Quantum Dots Modified BiOCl/BiVO4 p-n Heterostructure to Enhance Photocatalytic Activity for Removing Broad-Spectrum Antibiotics under Visible Light. J. Taiwan Inst. Chem. Eng. 2019, 96, 681–690. [Google Scholar] [CrossRef]
- Guan, M.L.; Xiao, C.; Zhang, J.; Fan, S.J.; An, R.; Cheng, Q.M.; Xie, J.F.; Zhou, M.; Ye, B.J.; Xie, Y. Vacancy Associates Promoting Solar-Driven Photocatalytic Activity of Ultrathin Bismuth Oxychloride Nanosheets. J. Am. Chem. Soc. 2013, 135, 10411–10417. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Zhang, Q.; Yang, L.X.; Luo, X.B.; Wang, A.J.; Luo, S.L.; Dionysiou, D.D. Visible Light-Responsive Graphene-Functionalized Bi-Bridge Z-Scheme Black BiOCl/Bi2O3 Heterostructure with Oxygen Vacancy and Multiple Charge Transfer Channels for Efficient Photocatalytic Degradation of 2-Nitrophenol and Industrial Wastewater Treatment. Appl. Catal. B 2018, 238, 61–69. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, K.; Zhang, G.; Yin, S. Facile Preparation of BiOX (X = Cl, Br, I) Nanoparticles and up-Conversion Phosphors/BiOBr Composites for Efficient Degradation of NO Gas: Oxygen Vacancy Effect and Near Infrared Light Responsive Mechanism. Chem. Eng. J. 2017, 325, 59–70. [Google Scholar] [CrossRef]
- Ye, L.; Liu, J.; Gong, C.; Tian, L.; Peng, T.; Zan, L. Two Different Roles of Metallic Ag on Ag/AgX/BiOX (X = Cl, Br) Visible Light Photocatalysts: Surface Plasmon Resonance and Z-Scheme Bridge. ACS Catal. 2012, 2, 1677–1683. [Google Scholar] [CrossRef]
- Myung, Y.; Wu, F.; Banerjee, S.; Park, J.; Banerjee, P. Electrical Conductivity of p-Type BiOCl Nanosheets. Chem. Commun. 2015, 51, 2629–2632. [Google Scholar] [CrossRef]
- Yao, L.; Wei, D.; Ni, Y.; Yan, D.; Hu, C. Surface Localization of CdZnS Quantum Dots onto 2D g-C3N4 Ultrathin Microribbons: Highly Efficient Visible Light-Induced H2-Generation. Nano Energy 2016, 26, 248–256. [Google Scholar] [CrossRef]
- Jing, L.; Xu, Y.; Xie, M.; Liu, J.; Deng, J.; Huang, L.; Xu, H.; Li, H. Three Dimensional Polyaniline/MgIn2S4 Nanoflower Photocatalysts Accelerated Interfacial Charge Transfer for the Photoreduction of Cr(VI), Photodegradation of Organic Pollution and Photocatalytic H2 Production. Chem. Eng. J. 2019, 360, 1601–1612. [Google Scholar] [CrossRef]
- Gou, X.L.; Cheng, F.Y.; Shi, Y.H.; Zhang, L.; Peng, S.J.; Chen, J.; Shen, P.W. Shape-Controlled Synthesis of Ternary Chalcogenide ZnIn2S4 and CuIn(S,Se)2 Nano-/Microstructures via Facile Solution Route. J. Am. Chem. Soc. 2006, 128, 7222–7229. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, Z.H.; Song, Q.G.; Li, X.F.; Chen, B.W.; Liu, Z.F. Hybrid 0D/2D Edamame Shaped ZnIn2S4 Photoanode Modified by Co-Pi and Pt for Charge Management towards Efficient Photoelectrochemical Water Splitting. Appl. Catal. B 2019, 244, 188–196. [Google Scholar] [CrossRef]
- Fan, B.; Chen, Z.; Liu, Q.; Zhang, Z.; Fang, X. One-Pot Hydrothermal Synthesis of Ni-Doped ZnIn2S4 Nanostructured Film Photoelectrodes with Enhanced Photoelectrochemical Performance. Appl. Surf. Sci. 2016, 370, 252–259. [Google Scholar] [CrossRef]
- Guan, Z.; Pan, J.; Li, Q.; Li, G.; Yang, J. Boosting Visible-Light Photocatalytic Hydrogen Evolution with an Efficient CuInS2/ZnIn2S4 2D/2D Heterostructure. ACS Sustain. Chem. Eng. 2019, 7, 7736–7742. [Google Scholar] [CrossRef]
- Kale, S.B.; Kalubarme, R.S.; Mahadadalkar, M.A.; Jadhav, H.S.; Bhirud, A.P.; Ambekar, J.D.; Park, C.J.; Kale, B.B. Hierarchical 3D ZnIn2S4/Graphene Nano-Heterostructures: Their in Situ Fabrication with Dual Functionality in Solar Hydrogen Production and As Anodes for Lithium Ion Batteries. Phys. Chem. Chem. Phys. 2015, 17, 31850–31861. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, Q.; Xia, Y.; Lv, K.; Li, M. Enhanced Visible-Light Photocatalytic CO2 Reduction Performance of Znln2S4 Microspheres by Using CeO2 as Cocatalyst. Appl. Surf. Sci. 2019, 464, 388–395. [Google Scholar] [CrossRef]
- Yang, G.; Chen, D.M.; Ding, H.; Feng, J.J.; Zhang, J.Z.; Zhu, Y.F.; Hamid, S.; Bahnemann, D.W. Well-Designed 3D ZnIn2S4 Nanosheets/TiO2 Nanobelts as Direct Z-Scheme photocatalysts for CO2 Photoreduction into Renewable Hydrocarbon Fuel with High Efficiency. Appl. Catal. B 2017, 219, 611–618. [Google Scholar] [CrossRef]
- Chai, B.; Peng, T.Y.; Zeng, P.; Zhang, X.H. Preparation of a MWCNTs/ZnIn2S4 Composite and Its Enhanced Photocatalytic Hydrogen Production under Visible-Light Irradiation. Dalton Trans. 2012, 41, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Z.; Huang, L.; Zhang, J.J.; Wang, F.J.; Xie, Y.Y.; Shang, X.T.; Go, Y.Y.; Zhao, H.B.; Wang, X.X. In Situ Constructing Interfacial Contact MoS2/ZnIn2S4 Heterostructure for Enhancing Solar Photocatalytic Hydrogen Evolution. Appl. Catal. B 2018, 233, 112–119. [Google Scholar] [CrossRef]
- Yuan, Y.J.; Chen, D.Q.; Zhong, J.S.; Yang, L.X.; Wang, J.J.; Liu, M.J.; Tu, W.G.; Yu, Z.T.; Zou, Z.G. Interface Engineering of a Noble-metal-free 2D–2D MoS2/Cu-ZnIn2S4 Photocatalyst for Enhanced Photocatalytic H2 Production. J. Mater. Chem. A 2017, 5, 15771. [Google Scholar] [CrossRef]
- Sun, W.X.; Wei, N.; Cui, H.Z.; Lin, Y.; Wang, X.Z.; Tian, J.; Li, J.; Wen, J. 3D ZnIn2S4 Nanosheet/TiO2 Nanowire Arrays and Their Efficient Photocathodic Protection for 304 Stainless Steel. Appl. Surf. Sci. 2018, 434, 1030–1039. [Google Scholar] [CrossRef]
- Gao, X.Y.; Tang, G.B.; Peng, W.; Guo, Q.; Luo, Y.M. Surprise in the Phosphate Modification of BiOCl with Oxygen Vacancy: In Situ Construction of Hierarchical Z-Scheme BiOCl-OV-BiPO4 Photocatalyst for the Degradation of Carbamazepine. Chem. Eng. J. 2019, 360, 1320–1329. [Google Scholar] [CrossRef]
- Wang, D.J.; Guo, L.; Zhen, Y.Z.; Yue, L.L.; Xue, G.L.; Fu, F. AgBr Quantum Dots Decorated Mesoporous Bi2WO6 Architectures with Enhanced Photocatalytic Activities for Methylene Blue. J. Mater. Chem. A 2014, 2, 11716–11727. [Google Scholar] [CrossRef]
- Ruan, X.W.; Hu, H.; Che, H.N.; Jiang, E.H.; Zhang, X.X.; Liu, C.B.; Che, G.B. A Visible-Light-Driven Z-scheme CdS/Bi12GeO20 Heterostructure with Enhanced Photocatalytic Degradation of Various Organics and the Reduction of Aqueous Cr(VI). J. Colloid Interface Sci. 2019, 543, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Bhoi, Y.P.; Mishra, B.G. Single Step Combustion Synthesis, Characterization and Photoctalytic Application of α-Fe2O3-Bi2S3 Heterojunction for Efficient and Selective Reduction of Structurally Diverse Nitroarenes. Chem. Eng. J. 2017, 316, 70–81. [Google Scholar] [CrossRef]
- Luo, S.; Ke, J.; Yuan, M.Q.; Zhang, Q.; Xie, P.; Deng, L.D.; Wang, S.B. CuInS2 Quantum Dots Embedded in Bi2WO6 Nanoflowers for Enhanced Visible Light Photocatalytic Removal of Contaminants. Appl. Catal. B 2017, 221, 215–222. [Google Scholar] [CrossRef]
- Zhang, N.; Jalil, A.; Wu, D.X.; Chen, S.M.; Liu, Y.F.; Gao, C.; Ye, W.; Qi, Z.M.; Ju, H.X.; Wang, C.M.; et al. Refining Defect States in W18O49 by Mo Doping: A Strategy for Tuning N2 Activation towards Solar-Driven Nitrogen Fixation. J. Am. Chem. Soc. 2018, 140, 9434–9443. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.R.; Guo, W.W.; Wang, H.L.; Zhu, B.C.; Yu, J.G.; Qiao, S.Z. Metal-Free 2D/2D Phosphorene/g-C3N4 Van der Waals Heterojunction for Highly Enhanced Visible-Light Photocatalytic H2 Production. Adv. Mater. 2018, 30, 1800128. [Google Scholar] [CrossRef] [PubMed]
- Dewidar, H.; Nosier, S.A.; El-Shazly, A.H. Photocatalytic Degradation of Phenol Solution using Zinc Oxide/UV. J. Chem. Health Saf. 2018, 25, 2–11. [Google Scholar] [CrossRef]
- Chiou, C.H.; Juang, R.S. Photocatalytic Degradation of Phenol in Aqueous Solutions by Pr-Doped TiO2 Nanoparticles. J. Hazard. Mater. 2007, 149, 1–7. [Google Scholar] [CrossRef]
- Al-Hamdi, A.M.; Sillanpää, M.; Dutta, J. Photocatalytic Degradation of Phenol in Aqueous Solution by Rare Earth-Doped SnO2 Nanoparticles. J. Mater. Sci. 2014, 49, 5151–5159. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, D.; Medrano, M.G.M.; Remita, H.; Escobar-Barrios, V. Photocatalytic Properties of BiOCl-TiO2 Composites for Phenol Photodegradation. J. Environ. Chem. Eng. 2018, 6, 1601–1612. [Google Scholar] [CrossRef]
- Li, Q.B.; Zhao, X.; Yang, J.; Jia, C.J.; Jin, Z.; Fan, W.L. Exploring the Effects of Nanocrystal Facet Orientations in g-C3N4/BiOCl Heterostructures on Photocatalytic Performance. Nanoscale 2015, 7, 18971–18983. [Google Scholar] [CrossRef] [PubMed]
- Cai, L. Enhanced Visible Light Photocatalytic Activity of BiOCl by Compositing with g-C3N4. Mater. Res. Innov. 2015, 19, 392–396. [Google Scholar] [CrossRef]
- Liu, H.; Jin, Z.T.; Xu, Z.Z.; Zhang, Z.; Ao, D. Fabrication of ZnIn2S4-G-C3N4 Sheet-On-Sheet Nanocomposites for Efficient Visible-Light Photocatalytic H2 Evolution and Degradation of Organic Pollutants. RSC Adv. 2015, 5, 97951–97961. [Google Scholar] [CrossRef]
- Liu, H.; Jia, M.Q.; Sun, N.; Cao, B.; Chen, R.J.; Zhu, Q.Z.; Wu, F.; Qiao, N.; Xu, B. Nitrogen-Rich Mesoporous Carbon as Anode Material for High Performance Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 27124–27130. [Google Scholar] [CrossRef] [PubMed]
- Li, B.X.; Shao, L.Z.; Wang, R.S.; Dong, X.P.; Zhao, F.G.; Gao, P.; Li, Z.Q. Interfacial Synergism of Pd-Decorated BiOCl Ultrathin Nanosheets for the Selective Oxidation of Aromatic Alcohols. J. Mater. Chem. A 2018, 6, 6344–6355. [Google Scholar] [CrossRef]
- Lin, B.; Li, H.; An, H.; Hao, W.B.; Wei, J.J.; Dai, Y.Z.; Ma, C.S.; Yang, G.D. Preparation of 2D/2D g-C3N4 Nanosheet@ZnIn2S4 Nanoleaf Heterostructures with Well-Designed High-Speed Charge Transfer Nanochannels towards High-Efficiency Photocatalytic Hydrogen Evolution. Appl. Catal. B 2018, 220, 542–552. [Google Scholar] [CrossRef]

| Samples | Light Source | Organic Pollutants | Ref. | ||||
|---|---|---|---|---|---|---|---|
| Type (a) | Power (W) | Conc.(b) (mg·L−1) | Catalyst (g/L) | t (c) (time/min) | η (d) (%) | ||
| ZnO | UV lamp | 15 | 25 | 1.5 | 120 | 78.2 | [57] |
| Pr-doped-TiO2 | high-pressure mercury lamp | 400 | 50 | 1.0 | 120 | 94.4 | [58] |
| La-doped-SnO2 | Mercury lamp | 8 | 10 | 1.3 | 90 | 100 | [59] |
| BiOCl/TiO2 | Xe lamp | 300 | 50 | 1.0 | 360 | 43 | [60] |
| g-C3N4/BiOCl | Metal halide lamp | 300 | 30 | 0.6 | 480 | 50 | [61] |
| g-C3N4/BiOCl | LED lamp | 5×24 | 10 | 1.0 | 150 | 94 | [62] |
| ZnIn2S4/g-C3N4 | Xe lamp | 500 | 10 | 0.4 | 240 | 72.3 | [63] |
| 0.5% wt % ZIS/BOC | Metal halide lamp | 300 | 10 | 1.0 | 360 | 77.4 | This work |
| Semiconductor | Eg (eV) | CB (eV) | VB (eV) |
|---|---|---|---|
| BiOCl | 3.20 | −1.32 | 1.88 |
| ZnIn2S4 | 2.61 | −1.36 | 1.25 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, L.; Han, X.; Zhang, K.; Zhang, Y.; Zhao, Q.; Wang, D.; Fu, F. In-Situ Construction of 2D/2D ZnIn2S4/BiOCl Heterostructure with Enhanced Photocatalytic Activity for N2 Fixation and Phenol Degradation. Catalysts 2019, 9, 729. https://doi.org/10.3390/catal9090729
Guo L, Han X, Zhang K, Zhang Y, Zhao Q, Wang D, Fu F. In-Situ Construction of 2D/2D ZnIn2S4/BiOCl Heterostructure with Enhanced Photocatalytic Activity for N2 Fixation and Phenol Degradation. Catalysts. 2019; 9(9):729. https://doi.org/10.3390/catal9090729
Chicago/Turabian StyleGuo, Li, Xuanxuan Han, Kailai Zhang, Yuanyuan Zhang, Qiang Zhao, Danjun Wang, and Feng Fu. 2019. "In-Situ Construction of 2D/2D ZnIn2S4/BiOCl Heterostructure with Enhanced Photocatalytic Activity for N2 Fixation and Phenol Degradation" Catalysts 9, no. 9: 729. https://doi.org/10.3390/catal9090729
APA StyleGuo, L., Han, X., Zhang, K., Zhang, Y., Zhao, Q., Wang, D., & Fu, F. (2019). In-Situ Construction of 2D/2D ZnIn2S4/BiOCl Heterostructure with Enhanced Photocatalytic Activity for N2 Fixation and Phenol Degradation. Catalysts, 9(9), 729. https://doi.org/10.3390/catal9090729





