Sn-Based Porous Coordination Polymer Synthesized with Two Ligands for Tandem Catalysis Producing 5-Hydroxymethylfurfural
Abstract
1. Introduction
2. Results and Discussion
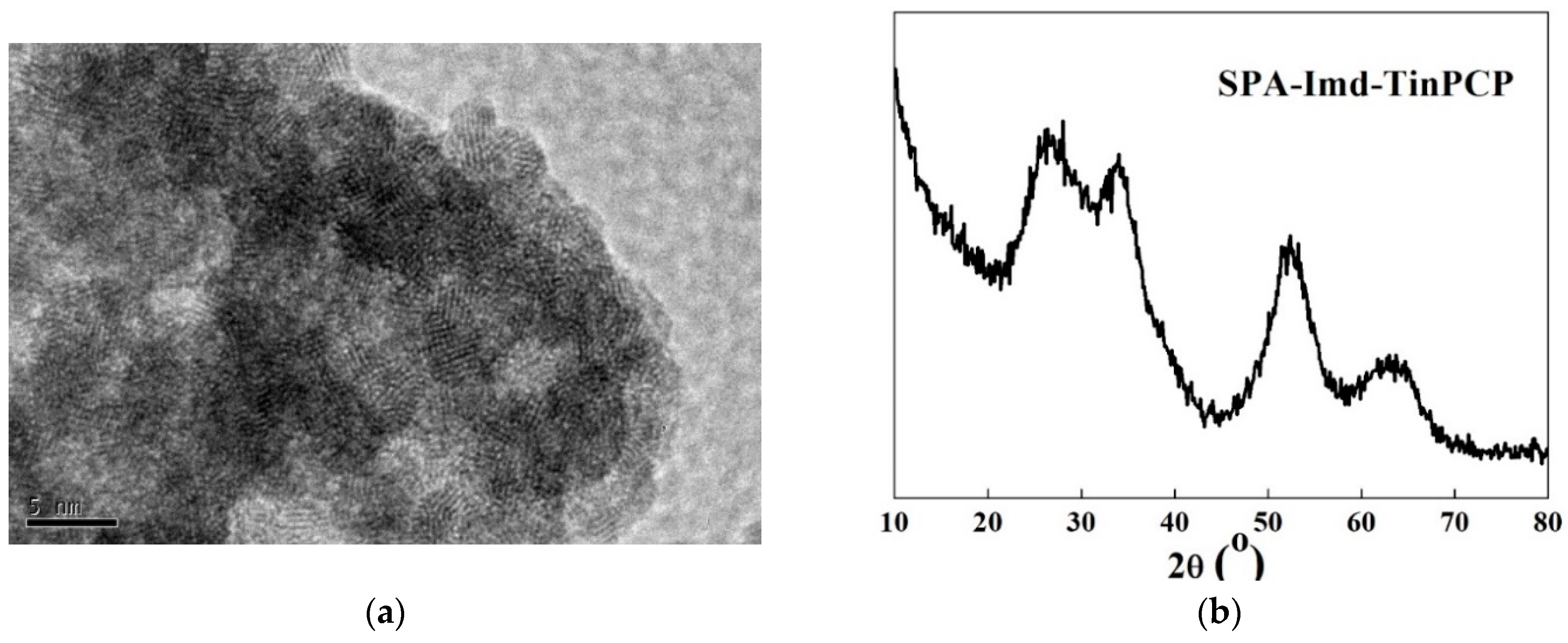
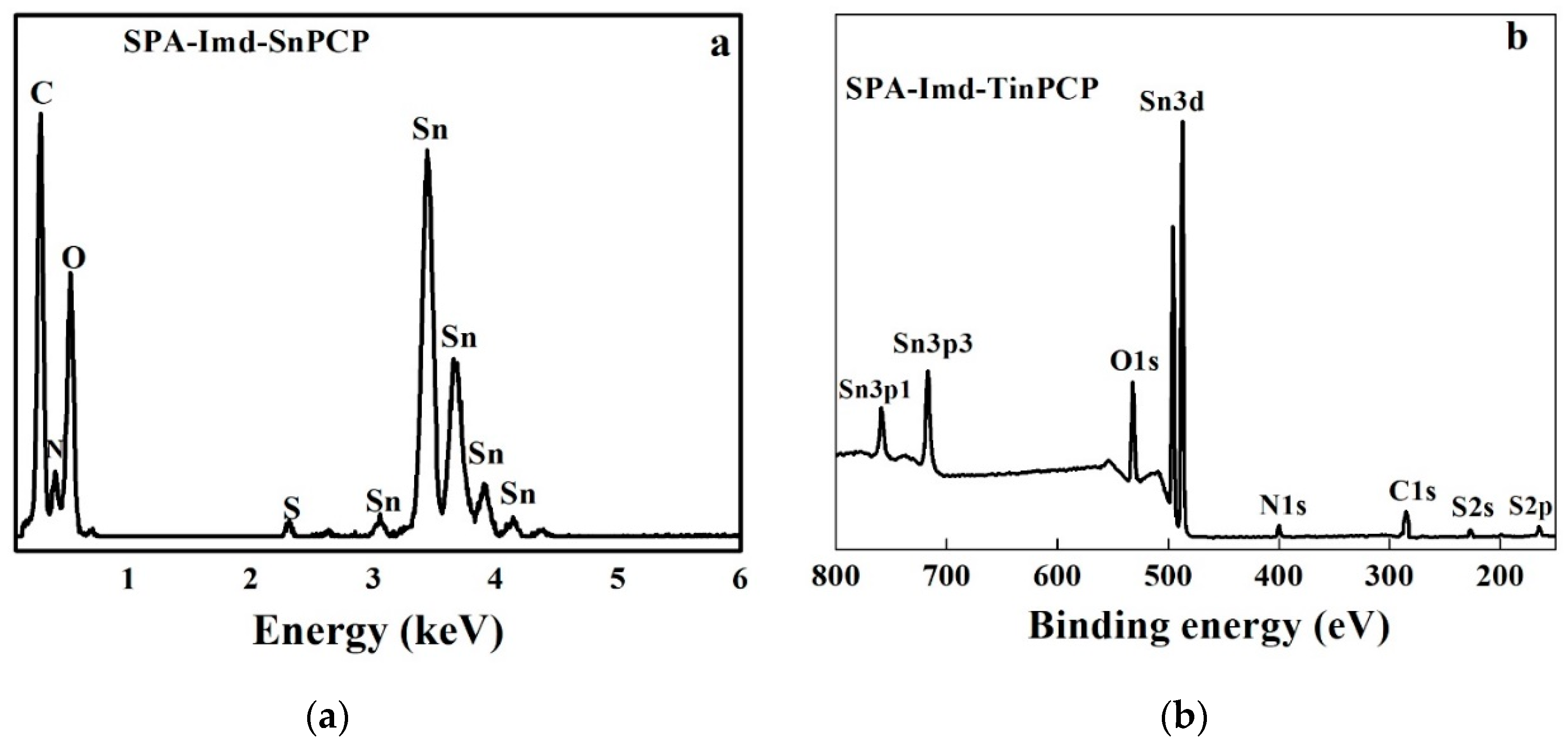
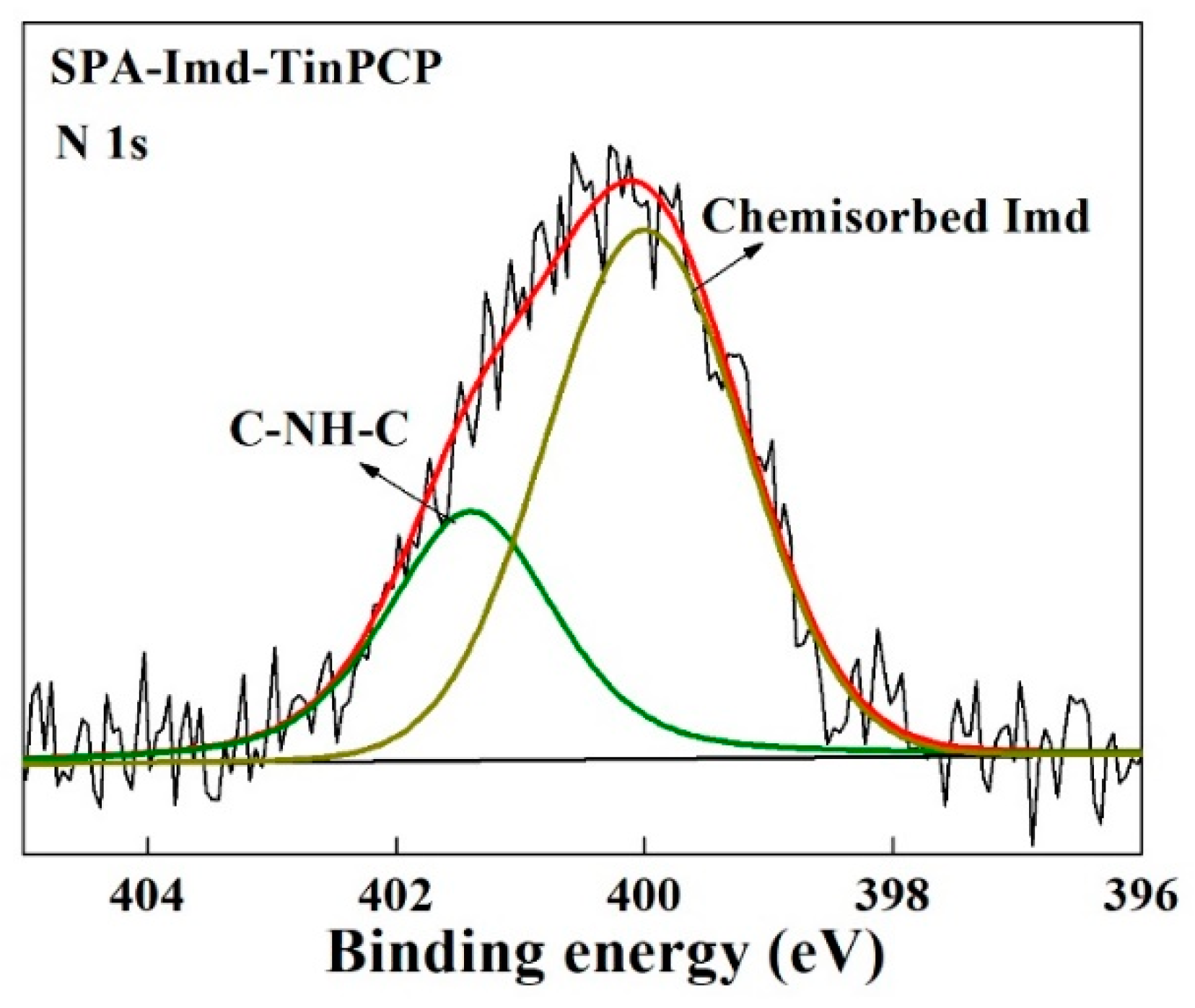
2.1. Characterization of SPA-Imd-TinPCP
2.2. The Roles 5-Sulfoisophthalic Acid and Imidazole Play in Catalysis
2.2.1. 5-Sulfoisophthalic Acid
2.2.2. Imidazole
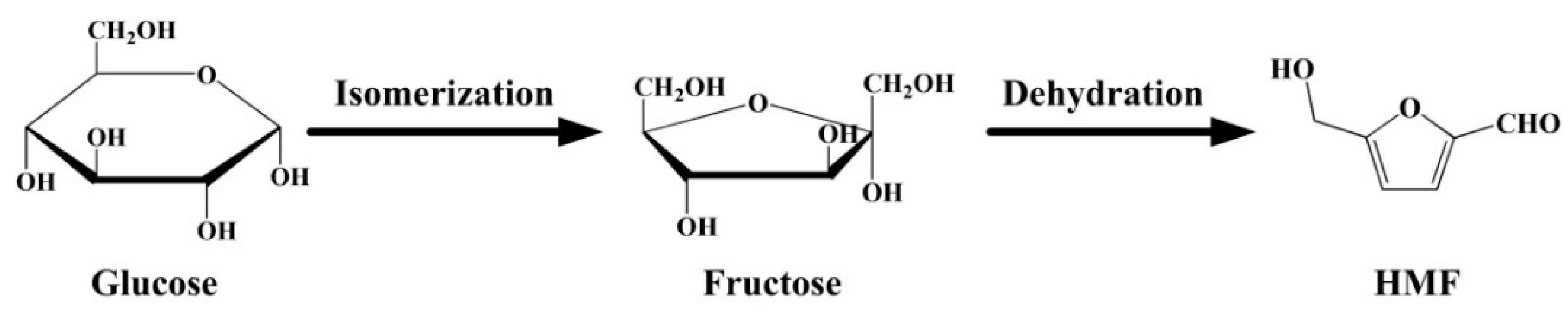
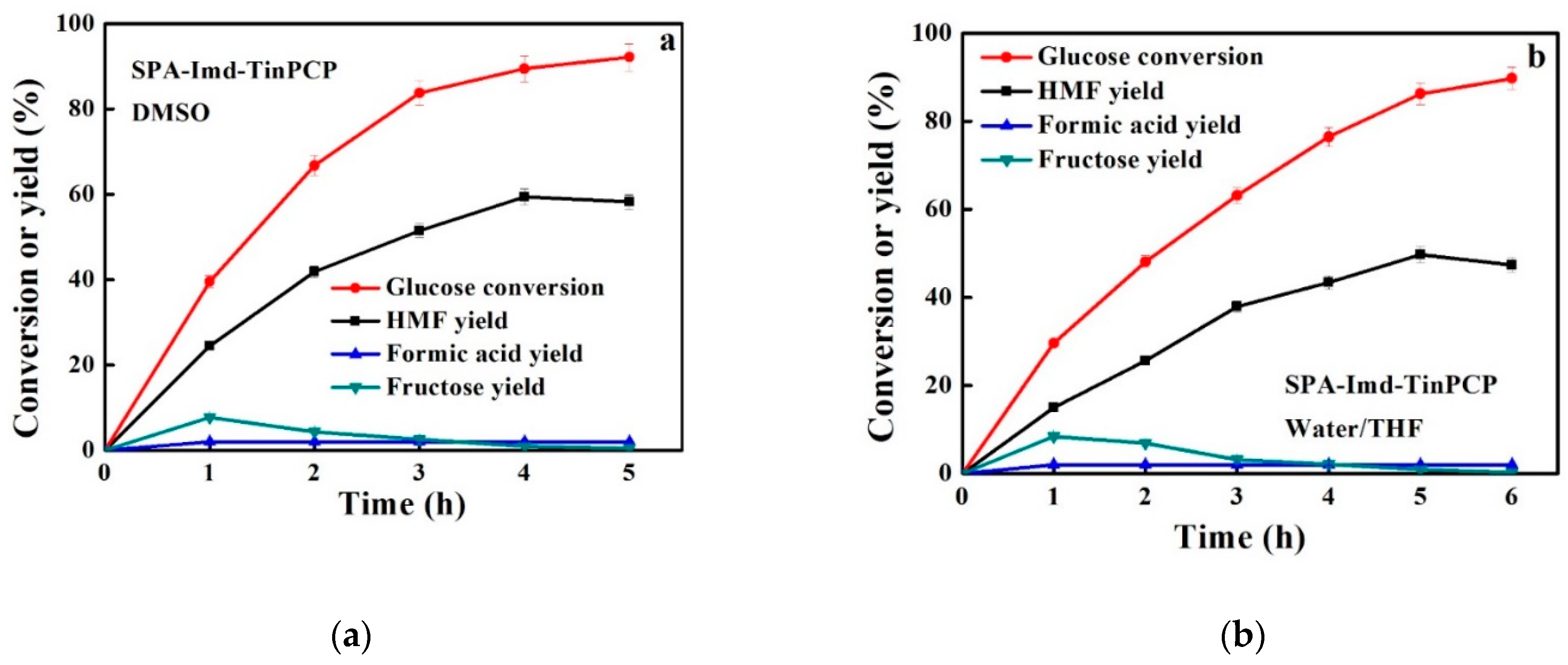
2.3. Catalysis of Conversion of Glucose to HMF
3. Experimental Section
3.1. Chemicals and Materials
3.2. Synthesis of SPA-Imd-TinPCP
3.3. Characterization and Measurement
3.4. Conversion of Glucose to HMF
3.5. Substrate and Product Analysis
3.6. Reuse of Catalyst
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Herbst, A.; Janiak, C. Selective glucose conversion to 5-hydroxymethylfurfural (5-HMF) instead of levulinic acid with MIL-101Cr MOF-derivatives. New J. Chem. 2016, 40, 7958–7967. [Google Scholar] [CrossRef]
- Yabushita, M.; Li, P.; Islamoglu, T.; Kobayashi, H.; Fukuoka, A.; Farha, O.K.; Katz, A. Selective Metal−Organic framework catalysis of glucose to 5‑hydroxymethylfurfural using phosphate-modified NU-1000. Ind. Eng. Chem. Res. 2017, 56, 7141–7148. [Google Scholar] [CrossRef]
- Liu, L.; Chang, H.; Jameel, H.; Park, J.; Park, S. Catalytic conversion of biomass hydrolysate into 5‑hydroxymethylfurfural. Ind. Eng. Chem. Res. 2017, 56, 14447–14453. [Google Scholar] [CrossRef]
- Mamo, W.; Chebude, Y.; Márquez-Álvarez, C.; Díaz, I.; Sastre, E. Comparison of glucose conversion to 5-HMF using different modified mordenites in ionic liquid and biphasic media. Catal. Sci. Technol. 2016, 6, 2766–2774. [Google Scholar] [CrossRef]
- Nikolla, E.; Román-Leshkov, Y.; Moliner, M.; Davis, M.E. “One-Pot” synthesis of 5-(hydroxymethyl)-furfural from carbohydrates using Tin-beta zeolite. ACS Catal. 2011, 1, 408–410. [Google Scholar] [CrossRef]
- Yu, S.; Kim, E.; Park, S.; Song, I.K.; Jung, C. Isomerization of glucose into fructose over Mg-Al hydrotalcite catalysts. Catal. Commum. 2012, 29, 63–67. [Google Scholar] [CrossRef]
- Yang, Q.; Sherbahn, M.; Runge, T. Basic amino acids as green catalysts for isomerization of glucose to fructose in water. ACS Sustain. Chem. Eng. 2016, 4, 3526–3534. [Google Scholar] [CrossRef]
- Liu, C.; Carraher, J.M.; Swedberg, J.L.; Herndon, C.R.; Fleitman, C.N.; Tessonnier, J. Selective base-catalyzed isomerization of glucose to fructose. ACS Catal. 2014, 4, 4295–4298. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Sharma, M.M. Cationic ion exchange resins as catalyst. React. Polym. 1993, 20, 1–45. [Google Scholar] [CrossRef]
- Moliner, M.; Román-Leshkov, Y.; Davis, M.E. Tin-containing zeolites are highly active catalysts for the isomerization of glucose in water. Proc. Natl. Acad. Sci. USA 2010, 107, 6164–6168. [Google Scholar] [CrossRef]
- Liu, M.; Jia, S.; Li, C.; Zhang, A.; Song, C.; Guo, X. Facile preparation of Sn-β zeolites by post-synthesis (isomorphous substitution) method for isomerization of glucose to fructose. Chin. J. Catal. 2014, 35, 723–732. [Google Scholar] [CrossRef]
- Guo, T.; Tong, X.L.; Cheng, Y.; Xue, S. Tin-catalyzed efficient conversion of carbohydrates for the production of 5-hydroxymethylfurfural in the presence of quaternary ammonium salts. Carbohydr. Res. 2013, 370, 33–37. [Google Scholar]
- Deval, R.B.; Assary, R.S.; Nikolla, E.; Moliner, M.; Román-Leshkov, Y.; Hwang, S.; Palsdottir, A.; Silverman, D.; Lobo, R.F.; Curtiss, L.A.; et al. Metalloenzyme-like catalyzed isomerizations of sugars by Lewis acid zeolites. Proc. Natl. Acad. Sci. USA 2012, 109, 9727–9732. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhang, Z.; Song, J.; Zhou, Y.; Han, B. Efficient conversion of glucose into 5-hydroxymethylfurfural catalyzed by a common Lewis acid SnCl₄ in an ionic liquid. Green Chem. 2009, 11, 1746–1749. [Google Scholar] [CrossRef]
- Chang, C.; Wang, Z.; Dornath, P.; Cho, H.J.; Fan, W. Rapid synthesis of Sn-Beta for the isomerization of cellulosic sugars. RSC Adv. 2012, 2, 10475–10477. [Google Scholar] [CrossRef]
- Ferrini, P.; Dijkmans, J.; Clercq, R.D.; Van de Vyver, S.; Dusselier, M.; Jacobs, P.A.; Sels, B.F. Lewis acid catalysis on single site Sn centers incorporated into silica hosts. Coord. Chem. Rev. 2017, 343, 220–255. [Google Scholar] [CrossRef]
- Neilson, J.R.; Kurzman, J.A.; Seshadri, R.; Morse, D.E. Ordering double perovskite hydroxides by kinetically controlled aqueous hydrolysis. Inorg. Chem. 2011, 50, 3003–3009. [Google Scholar] [CrossRef] [PubMed]
- Dijkmans, J.; Dusselier, M.; Gabriëls, D.; Houthoofd, K.; Magusin, P.C.M.M.; Huang, S.; Pontikes, Y.; Trekels, M.; Vantomme, A.; Giebeler, L.; et al. Cooperative catalysis for multistep biomass conversion with Sn/Al Beta zeolite. ACS Catal. 2015, 5, 928–940. [Google Scholar] [CrossRef]
- Liu, F.; Li, B.; Liu, C.; Kong, W.; Yi, X.; Zheng, A.; Qi, C. Template-free synthesis of porous carbonaceous solid acids with controllable acid sites and their excellent activity for catalyzing the synthesis of biofuels and fine chemicals. Catal. Sci. Technol. 2016, 6, 2995–3007. [Google Scholar] [CrossRef]
- Vekariya, R.H.; Patel, H.D. Sulfonated polyethylene glycol (PEG–OSO₃H) as a polymer supported biodegradable and recyclable catalyst in green organic synthesis: Recent advances. RSC Adv. 2015, 5, 49006–49030. [Google Scholar] [CrossRef]
- Hou, Q.; Li, W.; Ju, M.; Liu, L.; Chen, Y.; Yang, Q. One-pot synthesis of sulfonated graphene oxide for efficient conversion of fructose into HMF. RSC Adv. 2016, 6, 104016–104024. [Google Scholar] [CrossRef]
- Hou, Q.; Zhen, M.; Liu, L.; Chen, Y.; Huang, F.; Zhang, S.; Li, W.; Ju, M. Tin phosphate as a heterogeneous catalyst for efficient dehydration of glucose into 5-hydroxymethylfurfural in ionic liquid. Appl. Catal. B Environ. 2018, 224, 183–193. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Ma, J.; Ma, Z. Bifunctional Brønsted–Lewis solid acid as a recyclable catalyst for conversion of glucose to 5-hydroxymethylfurfural and its hydrophobicity effect. RSC Adv. 2016, 6, 43152–43158. [Google Scholar] [CrossRef]
- Reche, M.T.; Osatiashtiani, A.; Durndell, L.J.; Isaacs, M.A.; Silva, Â.; Lee, A.F.; Wilson, K. Niobic acid nanoparticle catalysts for the aqueous phase transformation of glucose and fructose to 5-hydroxymethylfurfural. Catal. Sci. Technol. 2016, 6, 7334–7341. [Google Scholar] [CrossRef]
- Huang, Y.; Liang, J.; Wang, X.; Cao, R. Multifunctional metal–organic framework catalysts: Synergistic catalysis and tandem reactions. Chem. Soc. Rev. 2017, 46, 126–157. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, G.; Matsuda, R.; Sato, H.; Takata, M.; Kitagawa, S. Cellulose hydrolysis by a new porous coordination polymer decorated with sulfonic acid functional groups. Adv. Mater. 2011, 23, 3294–3297. [Google Scholar] [CrossRef]
- Dong, X.Y.; Wang, R.; Wang, J.Z.; Zang, S.Q.; Mak, T.C.W. Highly selective Fe3⁺ sensing and proton conduction in a water-stable sulfonate carboxylate Tb–organic-framework. J. Mater. Chem. A 2015, 3, 641–647. [Google Scholar] [CrossRef]
- Ramaswamy, P.; Matsuda, R.; Kosaka, W.; Akiyama, G.; Jeon, H.J.; Kitagawa, S. Highly proton conductive nanoporous coordination polymers with sulfonic acid groups on the pore surface. Chem. Commun. 2014, 50, 1144–1146. [Google Scholar] [CrossRef]
- Chen, S.S. The roles of imidazole ligands in coordination supramolecular systems. CrystEngComm 2016, 18, 6543–6565. [Google Scholar] [CrossRef]
- Zhang, F.M.; Dong, L.Z.; Qin, J.S.; Guan, W.; Liu, J.; Li, S.L.; Lu, M.; Lan, Y.Q.; Su, Z.M.; Zhou, H.C. Effect of imidazole arrangements on proton-conductivity in metal−organic frameworks. J. Am. Chem. Soc. 2017, 139, 6183–6189. [Google Scholar] [CrossRef]
- Xue, G.; Dai, Q.; Jiang, S. Chemical reactions of imidazole with metallic silver studied by the use of sers and xps techniques. J. Am. Chem. Soc. 1988, 110, 2393–2395. [Google Scholar] [CrossRef]
- Zangmeister, R.A.; Morris, T.A.; Tarlov, M.J. Characterization of polydopamine thin films deposited at short times by autoxidation of dopamine. Langmuir 2013, 29, 8619–8628. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Shan, J.; Hu, J.; Liu, X.; Zhao, J.; Tong, Z. Co-curing effect of imidazole grafting graphene oxide synthesized by one-pot method to reinforce epoxy nanocomposites. Compos. Sci. Technol. 2016, 128, 161–168. [Google Scholar] [CrossRef]
- Dai, W.; Wang, C.; Tang, B.; Wu, G.; Guan, N.; Xie, Z.; Hunger, M.; Li, L. Lewis acid catalysis confined in zeolite cages as a strategy for sustainable heterogeneous hydration of epoxides. ACS Catal. 2016, 6, 2955–2964. [Google Scholar] [CrossRef]
- Weng, H.; Yan, B. Cadmium metal–organic frameworks: Ln3+ ion functionalized assembly, fluorescence tuning and polymer film preparation. New J. Chem. 2016, 40, 3732–3737. [Google Scholar] [CrossRef]
- Kurc, T.; Janczak, J.; Hoffmann, J.; Videnova-Adrabinska, V. New heterometallic hybrid polymers constructed with aromatic sulfonate-carboxylate ligands: Synthesis, layered structures, and properties. Cryst. Growth Des. 2012, 12, 2613–2624. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Q.; Zheng, Y.; Wang, S.; Yan, Y.; Yang, Y. Extraction behaviour and mechanism of Pt(IV) and Pd(II) by liquid–liquid extraction with an ionic liquid [HBBIm]Br. Dalton Trans. 2017, 46, 7210–7218. [Google Scholar] [CrossRef] [PubMed]
- Morzyk-Ociepa, B.; Rózycka-Sokołowska, E.; Michalska, D. Revised crystal and molecular structure, FT-IR spectra and DFT studies of chlorotetrakis (imidazole) copper(II) chloride. J. Mol. Struct. 2012, 1028, 49–56. [Google Scholar] [CrossRef]
- Chen, D.; Liang, F.; Feng, D.; Xian, M.; Zhang, H.; Liu, H.; Du, F. An efficient route from reproducible glucose to 5-hydroxymethylfurfural catalyzed by porous coordination polymer heterogeneous catalysts. Chem. Eng. J. 2016, 300, 177–184. [Google Scholar] [CrossRef]
- Kitano, M.; Wada, E.; Nakajima, K.; Hayashi, S.; Miyazaki, S.; Kobayashi, H.; Hara, M. Protonated titanate nanotubes with Lewis and Brønsted acidity: Relationship between nanotube structure and catalytic activity. Chem. Mater. 2013, 25, 385–393. [Google Scholar] [CrossRef]
- Atanda, L.; Mukundan, S.; Shortri, A.; Ma, Q.; Beltramini, J. Catalytic conversion of glucose to 5-hydroxymethylfurfural with a phosphated TiO2 catalyst. ChemCatChem 2015, 7, 781–790. [Google Scholar] [CrossRef]
- Nakajima, K.; Noma, R.; Kitano, M.; Hara, M. Selective glucose transformation by titania as a heterogeneous Lewis acid catalyst. J. Mol. Catal. A Chem. 2014, 388, 100–105. [Google Scholar] [CrossRef]
- Wang, J.; Ren, J.; Liu, X.; Xi, J.; Xia, Q.; Zu, Y.; Lu, G.; Wang, Y. Direct conversion of carbohydrates to 5-hydroxymethylfurfural using Sn-Mont catalyst. Green Chem. 2012, 14, 2506–2512. [Google Scholar] [CrossRef]
- Silahua-Pavóna, A.A.; Espinosa-González, C.G.; Ortiz-Chi, F.; Pacheco-Sosa, J.G.; Pérez-Vidal, H.; Arévalo-Pérez, J.C.; Godavarthi, S.; Torres-Torres, J.G. Production of 5-HMF from glucose using TiO₂–ZrO₂ catalysts: Effect of the sol-gel synthesis additive. Catal. Commun. 2019, 129, 105723. [Google Scholar] [CrossRef]
- Liang, F.; Chen, D.; Liu, H.; Liu, W.; Xian, M.; Feng, D. One-pot synthesis of 5‑hydroxymethylfurfural from glucose by Brønsted acid-free bifunctional porous coordination polymers in water. ACS Omega 2019, 4, 9316–9323. [Google Scholar] [CrossRef] [PubMed]

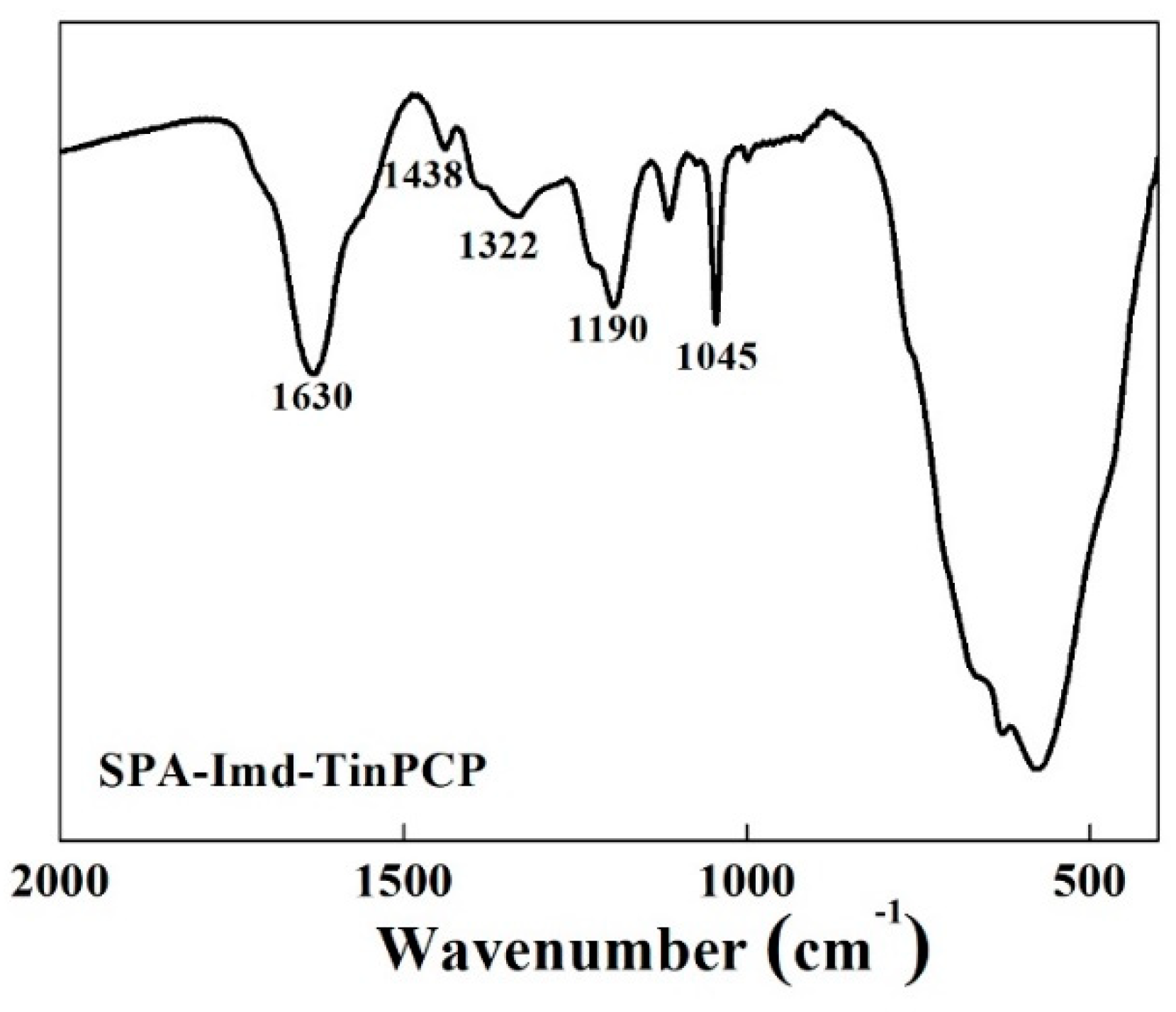
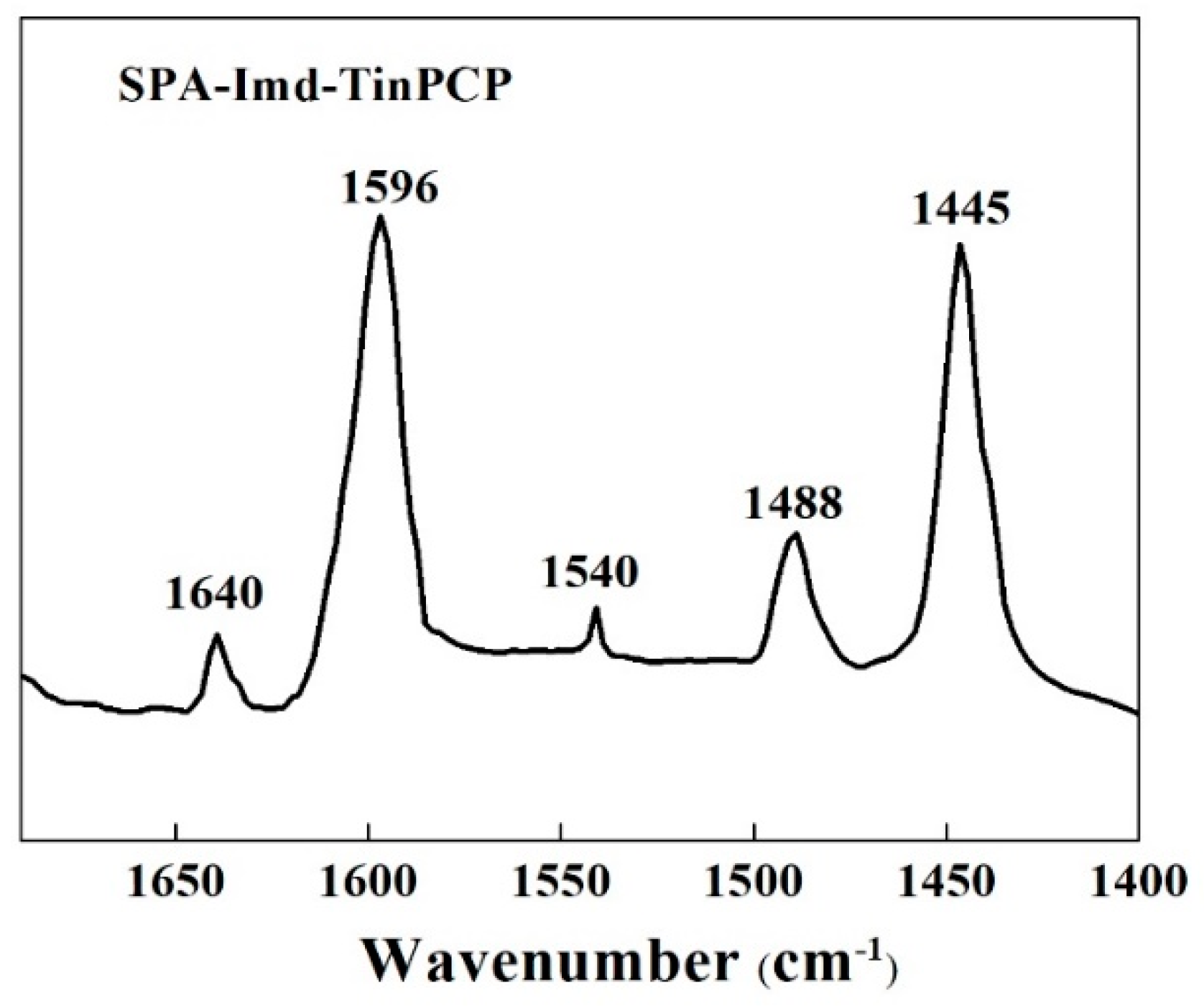

 imidazole; and
imidazole; and  5-sulfoisophthanic acid.
5-sulfoisophthanic acid.
 imidazole; and
imidazole; and  5-sulfoisophthanic acid.
5-sulfoisophthanic acid.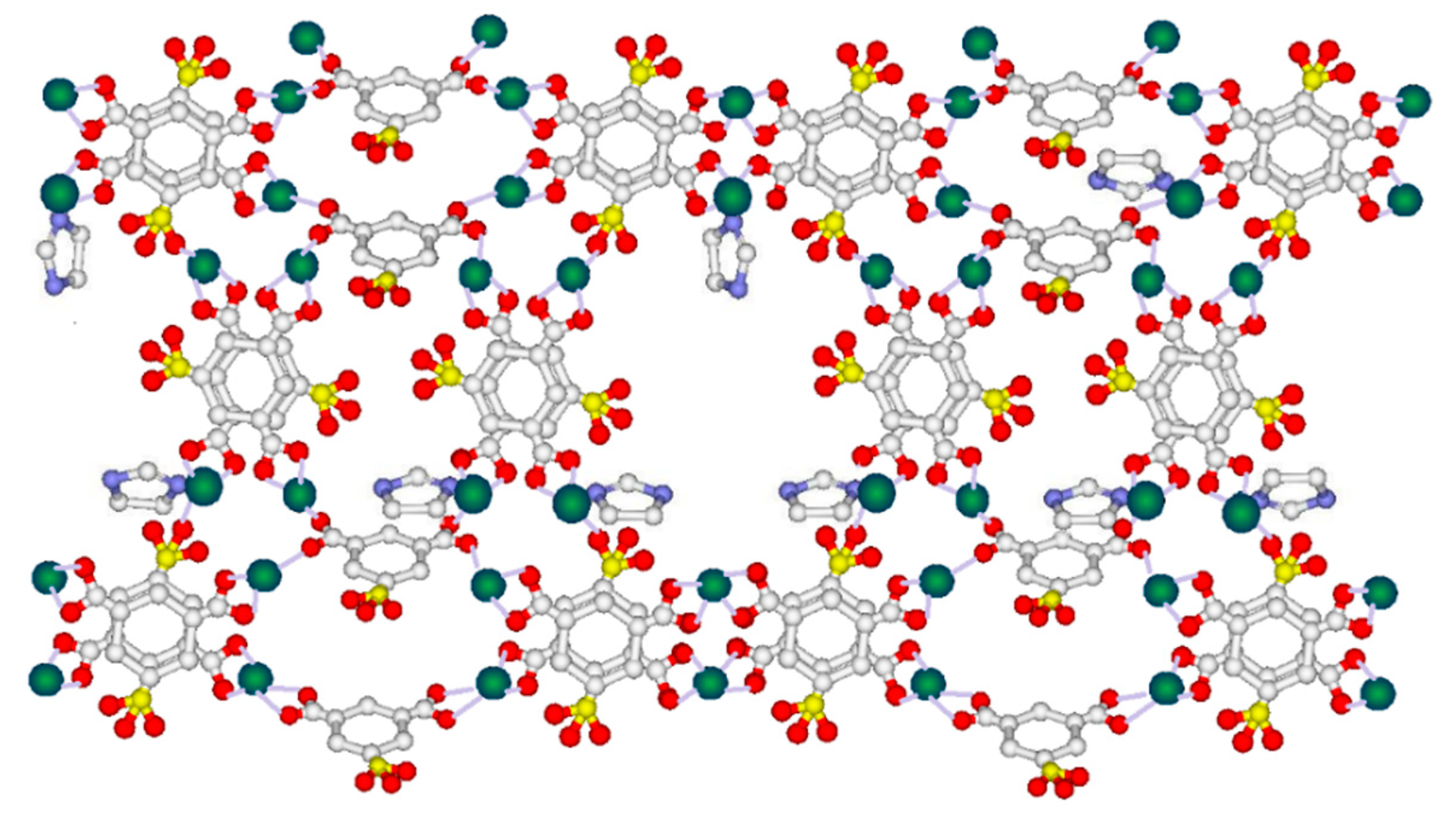
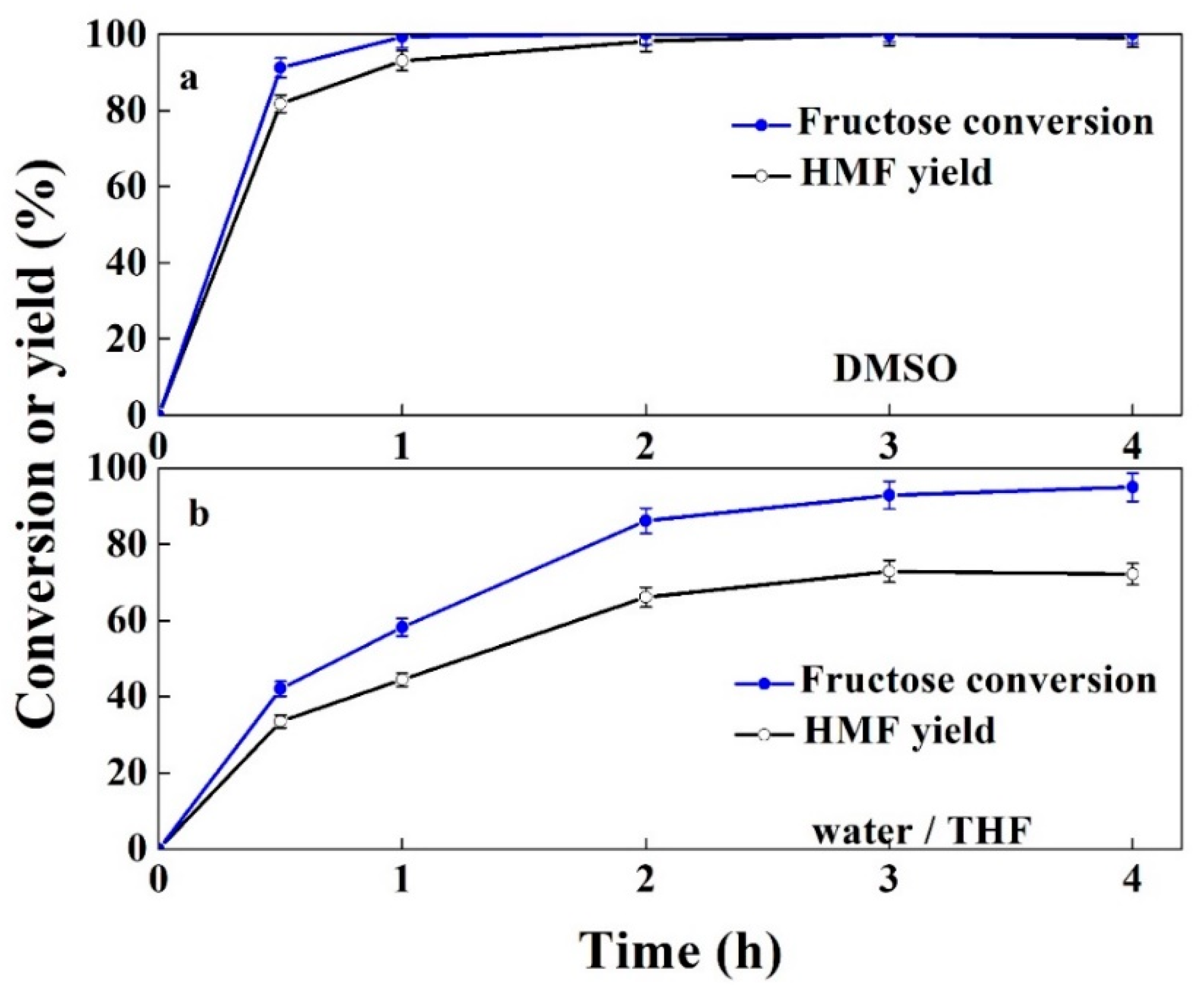

| Catalyst | Glucose Conversion (%) | Fructose Yield (%) | HMF Yield (%) |
|---|---|---|---|
| Imidazole | 63.2 0.6 | 21.1 0.3 | 1.9 0.1 |
| SPA-TinPCP | 70.3 0.7 | 1.1 0.1 | 37.3 0.7 |
| SPA-Imd-TinPCP | 86.2 0.5 | 1.0 0.1 | 49.8 0.5 |
| Solvent | Catalyst | Yield (%) | Selec. (%) | T (°C) | Time (min) | Ref. |
|---|---|---|---|---|---|---|
| DMSO | SnCl4 | 10.8 | 32.7 | 80 | 180 | [14] |
| DMSO | SnPO | 24.2 | 120 | 300 | [22] | |
| DMSO | Sn-Mont | 43.6 | 44.0 | 160 | 180 | [43] |
| DMSO | SPA-Imd-TinPCP | 59.5 | 66.5 | 160 | 240 | this work |
| H2O/1-butanol | Sn-Beta | 13.5 | 18.0 | 160 | 90 | [5] |
| H2O/THF/HCl | Sn-Beta/HCl | 56.9 | 72.0 | 180 | 70 | [5] |
| H2O/THF | SPA-Imd-TinPCP | 49.8 | 57.8 | 160 | 300 | this work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, L.; Sun, S.; Meng, X.; Ji, P. Sn-Based Porous Coordination Polymer Synthesized with Two Ligands for Tandem Catalysis Producing 5-Hydroxymethylfurfural. Catalysts 2019, 9, 739. https://doi.org/10.3390/catal9090739
Jiao L, Sun S, Meng X, Ji P. Sn-Based Porous Coordination Polymer Synthesized with Two Ligands for Tandem Catalysis Producing 5-Hydroxymethylfurfural. Catalysts. 2019; 9(9):739. https://doi.org/10.3390/catal9090739
Chicago/Turabian StyleJiao, Lutong, Siyu Sun, Xianling Meng, and Peijun Ji. 2019. "Sn-Based Porous Coordination Polymer Synthesized with Two Ligands for Tandem Catalysis Producing 5-Hydroxymethylfurfural" Catalysts 9, no. 9: 739. https://doi.org/10.3390/catal9090739
APA StyleJiao, L., Sun, S., Meng, X., & Ji, P. (2019). Sn-Based Porous Coordination Polymer Synthesized with Two Ligands for Tandem Catalysis Producing 5-Hydroxymethylfurfural. Catalysts, 9(9), 739. https://doi.org/10.3390/catal9090739




