One-Pot Aqueous and Template-Free Synthesis of Mesoporous Polymeric Resins
Abstract
:1. Introduction
2. Results
2.1. Nitrogen Adsorption-Desorption Analysis
2.2. Thermogravimetric Analysis (TGA)
2.3. Scanning Electron Microscopy (SEM)
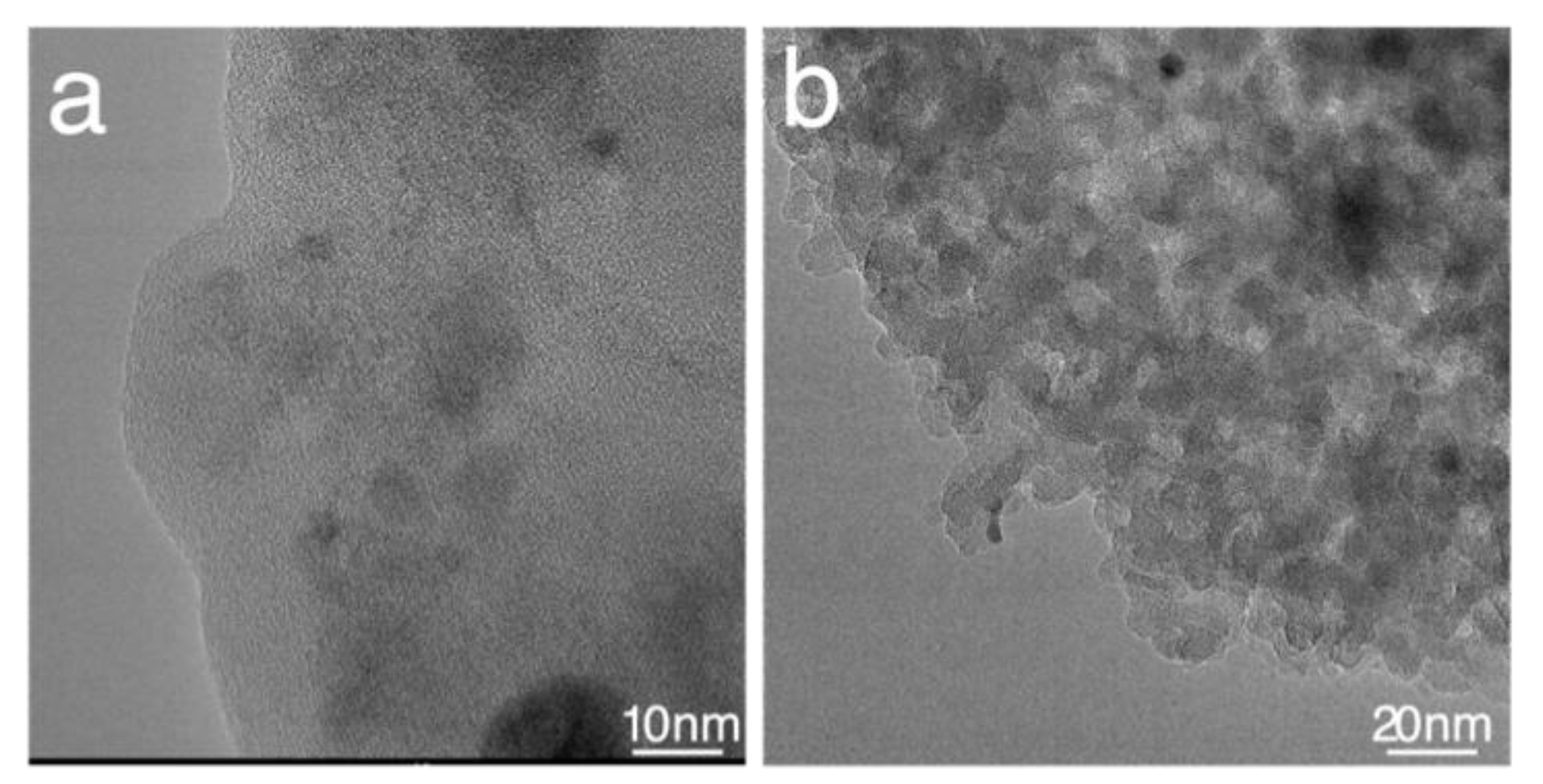
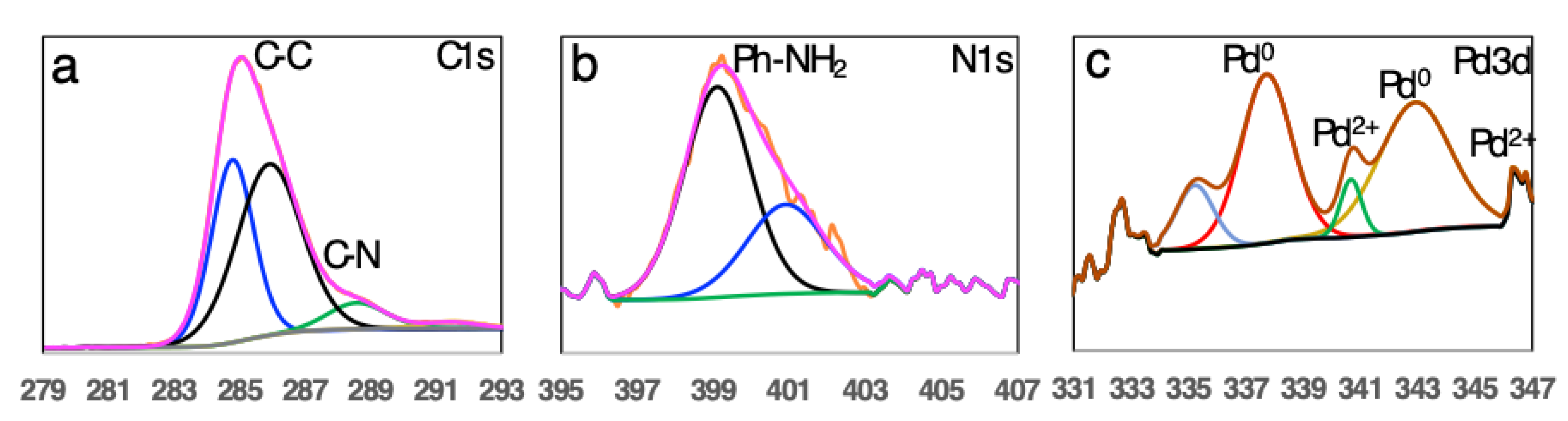
2.4. TEM and XPS and Elemental Analysis
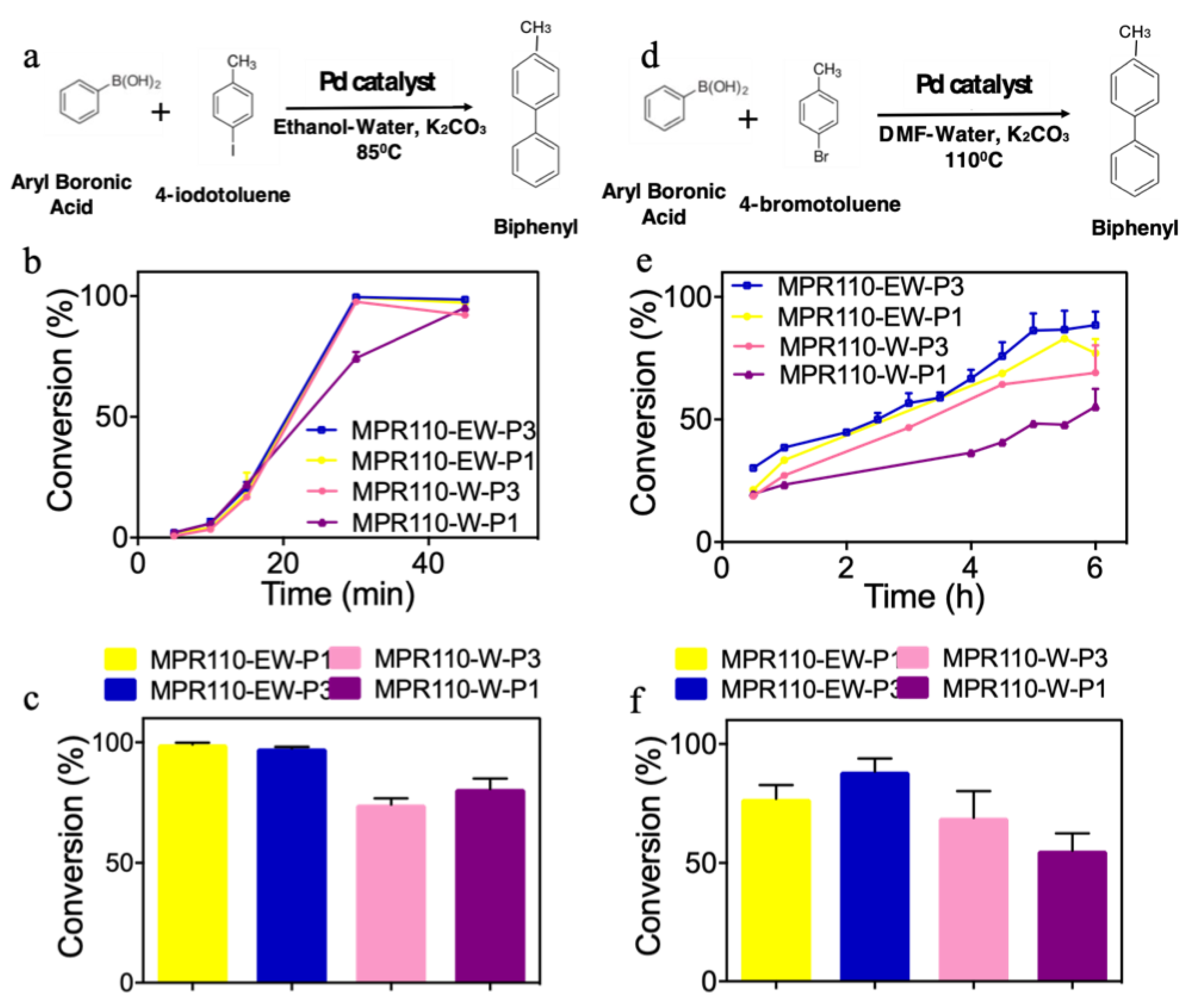
2.5. Catalytic Application
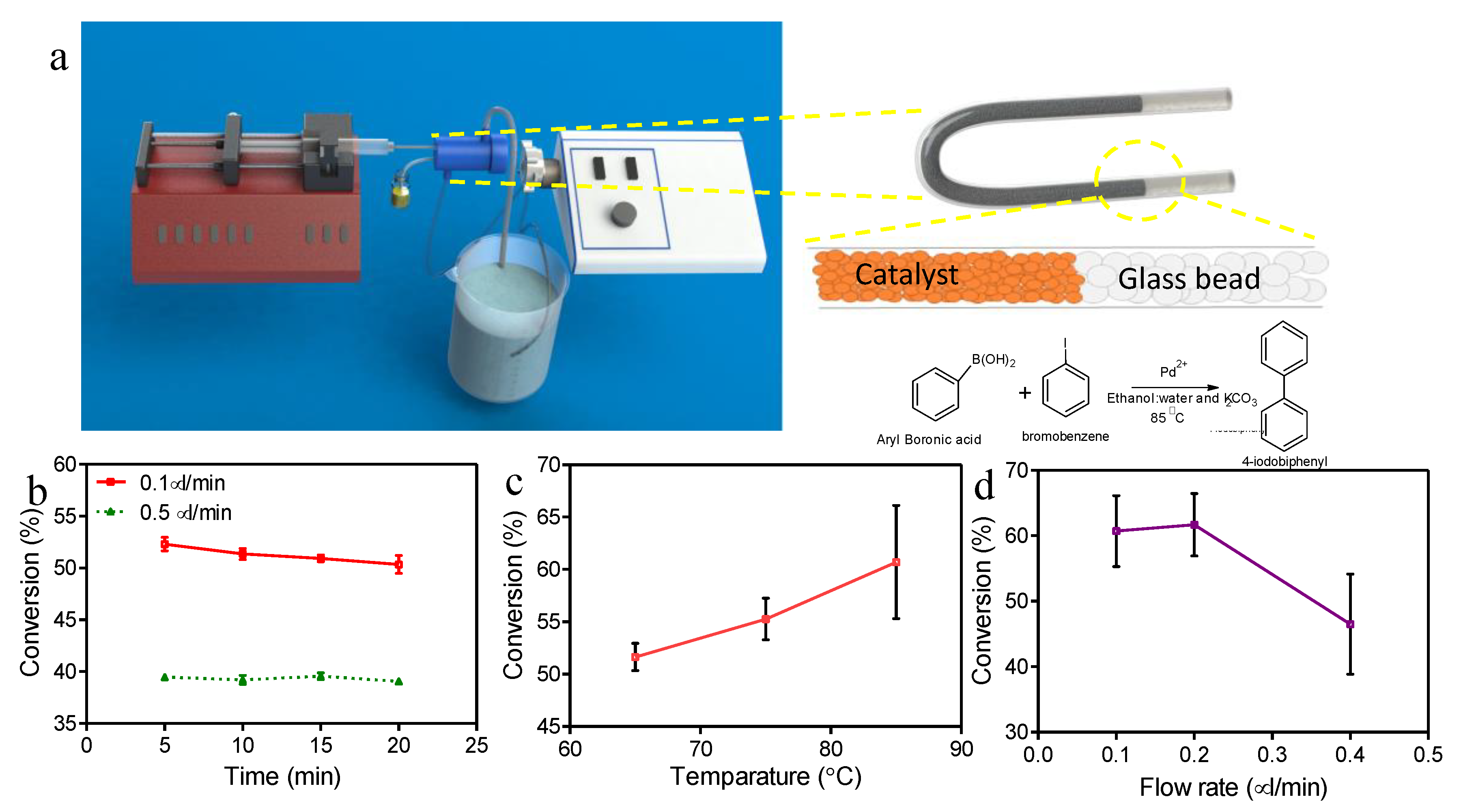
2.6. Continuous Suzuki-Miyaura Reaction in a Microreactor
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthetic Procedures
4.3. Characterizations
4.4. Catalytic Activity
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kung, S.C.; Chang, C.C.; Fan, W.; Snyder, M.A. Template-free ordered mesoporous silicas by binary nanoparticle assembly. Langmuir 2014, 30, 11802–11811. [Google Scholar] [CrossRef] [PubMed]
- Muylaert, I.; Verberckmoes, A.; De Decker, J.; Van Der Voort, P. Ordered mesoporous phenolic resins: Highly versatile and ultra-stable support materials. Adv. Colloid Interface Sci. 2012, 175, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.; Buchwald, S.L. palladium-catalyzed suzuki−miyaura cross-coupling reactions employing dialkylbiaryl phosphine ligands. Acc. Chem. Res. 2008, 41, 1461–1473. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, J.P.; Singer, R.A.; Yang, B.H.; Buchwald, S.L. Highly active palladium catalysts for suzuki coupling reactions. J. Am. Chem. Soc. 1999, 121, 9550–9561. [Google Scholar] [CrossRef]
- Choma, J.; Jedynak, K.; Fahrenholz, W.; Ludwinowicz, J.; Jaroniec, M. Microporosity development in phenolic resin-based mesoporous carbons for enhancing CO2 adsorption at ambient conditions. Appl. Surf. Sci. 2014, 289, 592–600. [Google Scholar] [CrossRef]
- Liu, F.; Wu, Q.; Liu, C.; Qi, C.; Huang, K.; Zheng, A.; Dai, S. Ordered mesoporous polymers for biomass conversions and cross-coupling reactions. ChemSusChem 2016, 9, 2496–2504. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Bulger, P.G.; Sarlah, D. Palladium-catalyzed cross-coupling reactions in total synthesis. Angew. Chem. Int. Ed. 2005, 44, 4442–4489. [Google Scholar] [CrossRef]
- Fang, Y.; Huang, X.J.; Chen, P.C.; Xu, Z.K. Polymer materials for enzyme immobilization and their application in bioreactors. BMB Rep. 2011, 44, 87–95. [Google Scholar] [CrossRef]
- Khan, M.K.; Giese, M.; Yu, M.; Kelly, J.A.; Hamad, W.Y.; MacLachlan, M.J. Flexible mesoporous photonic resins with tunable chiral nematic structures. Angew. Chem. Int. Ed. 2013, 52, 8921–8924. [Google Scholar] [CrossRef]
- Inagaki, M.; Toyoda, M.; Soneda, Y.; Tsujimura, S.; Morishita, T. Templated mesoporous carbons: Synthesis and applications. Carbon 2016, 107, 448–473. [Google Scholar] [CrossRef]
- Jiao, Y.; Han, D.; Ding, Y.; Zhang, X.; Guo, G.; Hu, J.; Yang, D.; Dong, A. Fabrication of three-dimensionally interconnected nanoparticle superlattices and their lithium-ion storage properties. Nat. Commun. 2015, 6, 6420. [Google Scholar] [CrossRef] [PubMed]
- Kailasam, K.; Jun, Y.-S.; Katekomol, P.; Epping, J.D.; Hong, W.H.; Thomas, A. Mesoporous melamine resins by soft templating of block-co-polymer mesophases. Chem. Mater. 2010, 22, 428–434. [Google Scholar] [CrossRef]
- Tanaka, S.; Doi, A.; Nakatani, N.; Katayama, Y.; Miyake, Y. Synthesis of ordered mesoporous carbon films, powders, and fibers by direct triblock-copolymer-templating method using an ethanol/water system. Carbon 2009, 47, 2688–2698. [Google Scholar] [CrossRef]
- Lu, H.; Shi, Q.; Huang, Z. pH-responsive anionic wormlike micelle based on sodium oleate induced by NaCl. J. Phys. Chem. B 2014, 118, 12511–12517. [Google Scholar] [CrossRef] [PubMed]
- Indra, A.; Gopinath, C.S.; Bhaduri, S.; Lahiri, G.K. Hydroxyapatite supported palladium catalysts for Suzuki–Miyaura cross-coupling reaction in aqueous medium. Catal. Sci. Technol. 2013, 3, 1625–1633. [Google Scholar] [CrossRef]
- Biffis, A.; Centomo, P.; Del Zotto, A.; Zecca, M. Pd metal catalysts for cross-couplings and related reactions in the 21st century: A critical review. Chem. Rev. 2018, 118, 2249–2295. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Kamat, R.K.; Noshadi, I.; Peck, D.; Parnas, R.S.; Zheng, A.; Qi, C.; Lin, Y. Depolymerization of crystalline cellulose catalyzed by acidic ionic liquids grafted onto sponge-like nanoporous polymers. Chem. Commun. 2013, 49, 8456. [Google Scholar] [CrossRef]
- Liu, F.J.; Zheng, A.M.; Noshadi, I.; Xiao, F.-S. Design and synthesis of hydrophobic and stable mesoporous polymeric solid acid with ultra strong acid strength and excellent catalytic activities for biomass transformation. Appl. Catal. B 2013, 193, 136–137. [Google Scholar] [CrossRef]
- Noshadi, I.; Kanjilal, B.; Du, S.; Bollas, G.M.; Suib, S.L.; Provatas, A.; Liu, F.; Parnas, R.S. Catalyzed production of biodiesel and bio-chemicals from brown grease using Ionic Liquid functionalized ordered mesoporous polymer. Appl. Energy 2014, 129, 112–122. [Google Scholar] [CrossRef]
- Noshadi, I.; Jafari, T.; Kanjilal, B.; Moharreri, E.; Khakpash, N.; Masoumi, A.; Liu, F.; Suib, S.L. Amine/Thiol functionalized mesoporous polydivinylbenzene for CO2 adsorption. Mater. Today Energy 2017, 4, 81–88. [Google Scholar] [CrossRef]
- Jafari, T.; Moharreri, E.; Toloueinia, P.; Amin, A.S.; Sahoo, S.; Khakpash, N.; Noshadi, I.; Alpay, A.P.; Suib, S.L. Microwave-assisted synthesis of amine functionalized mesoporous polydivinylbenzene for CO2 adsorption. J. CO2 Util. 2017, 19, 79–90. [Google Scholar] [CrossRef]
- Wang, H.; Jiao, F.; Gao, F.; Huang, J.; Zhao, Y.; Shen, Y.; Zhang, Y.; Qian, X. Facile synthesis of magnetic covalent organic frameworks for the hydrophilic enrichment of N-glycopeptides. ACS Macro Lett. 2015, 4, 570–574. [Google Scholar] [CrossRef]
- Dupont, J.; Pfeffer, M. Palladacycles: Synthesis, Characterization, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2014; p. 4. [Google Scholar]
- Wan, Y.; Wang, H.; Zhao, Q.; Klingstedt, M.; Terasaki, O.; Zhao, D. Ordered mesoporous Pd/Silica−Carbon as a highly active heterogeneous catalyst for coupling reaction of chlorobenzene in aqueous media. J. Am. Chem. Soc. 2009, 131, 4541–4550. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Yu, C.; Wang, X.; Wan, Y.; Li, D.; Lu, G. Liquid phase hydrodechlorination of chlorophenols at lower temperature on a novel Pd catalyst. J. Hazard. Mater. 2011, 186, 1726–1732. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xue, H.; Ju, F.; Yang, H. Acceleration of batch-type heterogeneous ligand-free Suzuki-Miyaura reactions with polymer composite supported Pd catalyst. Sci. Rep. 2017, 7, 7006. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Xiong, Z.; Zhu, X.; Mehio, N.; Chen, Y.; Hu, J.; Zhang, W.; Zou, H.; Liu, H.; Dai, S. Template-free synthesis of mesoporous polymers for highly selective enrichment of glycopeptides. ACS Macro Lett. 2015, 4, 570–574. [Google Scholar] [CrossRef]
- Wan, Y.; Qian, X.; Jia, N.; Wang, Z.; Li, H.; Zhao, D. Direct triblock-copolymer-templating synthesis of highly ordered fluorinated mesoporous carbon. Chem. Mater. 2008, 20, 1012–1018. [Google Scholar] [CrossRef]
- Yemul, O.; Imae, T. Synthesis and characterization of poly(ethyleneimine) dendrimers. Colloid Polym. Sci. 2008, 286, 747–752. [Google Scholar] [CrossRef]
- Zhu, L.; Dasgupta, T.; Huang, Q. A D-optimal design for estimation of parameters of an exponential-linear growth curve of nanostructures. Technometrics 2014, 56, 432–442. [Google Scholar] [CrossRef]
- Dasgupta, T.; Weintraub, B.; Joseph, V.R. A physical–statistical model for density control of nanowires. IIE Trans. 2011, 43, 233–241. [Google Scholar] [CrossRef]
- Dasgupta, T.; Weintraub, B.; Joseph, V.R. Palladium PEPPSI complexes: Synthesis and catalytic activity on the Suzuki-Miyaura coupling reactions for aryl bromides at room temperature in aqueous media. Inorg. Chim. Acta 2018, 478, 187–194. [Google Scholar]
- Touj, N.; Gürbüz, N.; Hamdi, N.; Yaşar, S.; Özdemir, İ. The palladium(II) complex of N,N-diethyl-1-ferrocenyl-3-thiabutanamine: Synthesis, solution and solid state structure and catalytic activity in Suzuki–Miyaura reaction. RSC Adv. 2014, 4, 43792–43799. [Google Scholar]
- Opanasenko, M.; Stepnicka, P.; Cejka, J. Heterogeneous Pd catalysts supported on silica matrices. RSC Adv. 2014, 4, 65137. [Google Scholar] [CrossRef]
- Gao, S.; Zhao, N.; Shu, M.; Che, S. Palladium nanoparticles supported on MOF-5: A highly active catalyst for a ligand-and copper-free Sonogashira coupling reaction. Appl. Catal. A 2010, 388, 196–201. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Luque, R.; Fihri, A.; Zhu, H.; Bouhrara, M.; Bassett, J.-M. Magnetically recoverable nanocatalysts. Chem. Rev. 2011, 111, 3036–3075. [Google Scholar] [CrossRef]
- Zhang, L.; Wen, G.; Liu, H.; Wang, N.; Su, D.S. Preparation of palladium catalysts supported on carbon nanotubes by an electrostatic adsorption method. ChemCatChem 2014, 6, 2600–2606. [Google Scholar] [CrossRef]
- Farina, V. High-turnover palladium catalysts in cross-coupling and heck chemistry: A critical overview. Adv. Synth. Catal. 2004, 346, 1553–1582. [Google Scholar] [CrossRef]
- Groppo, E.; Agostini, G.; Borfecchia, E.; Wei, L.; Giannici, F.; Portale, G.; Longo, A.; Lamberti, C. Formation and growth of Pd nanoparticles inside a highly cross-linked polystyrene support: Role of the reducing agent. J. Phys. Chem. C 2014, 118, 8406–8415. [Google Scholar] [CrossRef]
- Capka, M.; Hetflejs, J. Hydrosilylation catalysed by transition metal complexes coordinately bound to inorganic supports. Collect. Czech. Chem. Commun. 1974, 39, 154–166. [Google Scholar] [CrossRef]
- Sharf, V.Z.; Gurovets, A.S.; Finn, L.P.; Slinyakova, I.B.; Krutii, V.N.; Freidlin, L.K. Catalytic activity of metal complexes anchored to a solid support. 2. Synthesis of palladium complexes on amino-containing silica gels and their properties in the hydrogenation of C=C, C≡C, and C=N bonds. Acad. Sci. USSR Div. Chem. Sci. 1979, 28, 93–96. [Google Scholar]
- Alan, R.K. Advances in Heterocyclic Chemistry; Acad. Press: Cambridge, MA, USA, 2013; p. 326. [Google Scholar]
- Tamami, B.; Farjadian, F.; Ghasemi, S.; Allahyari, H. Synthesis and applications of polymeric N-heterocyclic carbene palladium complex-grafted silica as a novel recyclable nano-catalyst for Heck and Sonogashira coupling reactions. New J. Chem. 2013, 37, 2011–2018. [Google Scholar] [CrossRef]
- Paul, S.; Clark, J.H. Structure-activity relationship between some novel silica supported palladium catalysts: A study of the Suzuki reaction. J. Mol. Catal. A Chem. 2004, 215, 107–111. [Google Scholar] [CrossRef]
- Vassylyev, O.; Chen, J.; Panarello, A.P.; Khinast, J.G. Catalytic properties of several supported Pd(II) complexes for Suzuki coupling reactions. Tetrahedron Lett. 2005, 46, 6865–6869. [Google Scholar] [CrossRef]





| Surface Area (m2/g) | Pore Volume (cc/g) | |||||
|---|---|---|---|---|---|---|
| BET (Meso) | BET (Micro) | BJH | NLDFT | BJH | NLDFT | |
| RF | 14 | 20 | 8 | 13 | 0.033 | 0.030 |
| MPR110-EW | 207 | 326 | 315 | 390 | 0.350 | 1.522 |
| MPR220-EW | 80 | 212 | 80 | 167 | 0.340 | 0.274 |
| MPR110-EW-P1 | 108 | 257 | 109 | 177 | 0.240 | 0.175 |
| MPR110-W-P1 | 74 | 149 | 74 | 114 | 0.850 | 0.493 |
| MPR110-W-P3 | 129 | 205 | 129 | 139 | 0.170 | 0.802 |
| MPR110-EW-P3 | 226 | 432 | 226 | 384 | 0.350 | 0.447 |
| MPR220-EW-P3 | 123 | 342 | 123 | 236 | 0.140 | 0.241 |
| Sample | Carbon | Oxygen | Nitrogen | Palladium |
|---|---|---|---|---|
| MR110-EW | 50.14 | 41.55 | 8.31 | 0 |
| MR110-EW-P1 | 53.32 | 37.62 | 7.25 | 1.55 |
| MR110-W-P1 | 51.87 | 35.42 | 11.08 | 1.63 |
| MR110-W-P3 | 52.67 | 10.34 | 36.75 | 0.25 |
| MR110-EW-P3 | 55.58 | 36.96 | 7.31 | 0.16 |
| MR220-EW-P3 | 54.67 | 37.12 | 7.42 | 0.79 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nabavinia, M.; Kanjilal, B.; Hesketh, A.; Wall, P.; Shirazi Amin, A.; Kerns, P.; Stanzione, J.F., III; Suib, S.; Liu, F.; Noshadi, I. One-Pot Aqueous and Template-Free Synthesis of Mesoporous Polymeric Resins. Catalysts 2019, 9, 782. https://doi.org/10.3390/catal9090782
Nabavinia M, Kanjilal B, Hesketh A, Wall P, Shirazi Amin A, Kerns P, Stanzione JF III, Suib S, Liu F, Noshadi I. One-Pot Aqueous and Template-Free Synthesis of Mesoporous Polymeric Resins. Catalysts. 2019; 9(9):782. https://doi.org/10.3390/catal9090782
Chicago/Turabian StyleNabavinia, Mahboubeh, Baishali Kanjilal, Alexander Hesketh, Philip Wall, Alireza Shirazi Amin, Peter Kerns, Joseph F. Stanzione, III, Steven Suib, Fujian Liu, and Iman Noshadi. 2019. "One-Pot Aqueous and Template-Free Synthesis of Mesoporous Polymeric Resins" Catalysts 9, no. 9: 782. https://doi.org/10.3390/catal9090782
APA StyleNabavinia, M., Kanjilal, B., Hesketh, A., Wall, P., Shirazi Amin, A., Kerns, P., Stanzione, J. F., III, Suib, S., Liu, F., & Noshadi, I. (2019). One-Pot Aqueous and Template-Free Synthesis of Mesoporous Polymeric Resins. Catalysts, 9(9), 782. https://doi.org/10.3390/catal9090782






