Abstract
An efficient and useful method for the incorporation of colloidal quantum dots (QDs) into ionic matrices is demonstrated. We prepared three different synthesis methods, which are traditional saturated-salt water, methanol-assisted, and ethanol-assisted methods. The continuous thermal and photonic stress tests indicate that the high temperature, instead of photonic excitation stress, is more detrimental to the illumination capability of the quantum dots. While the traditional saturated-salt water synthesis and methanol-assisted method are quite effective in low temperature and low photon excitation intensity, the quantum dots sealed by the ethanol-assisted method cannot hold under all conditions. An over-1000-h aging test can provide crucial information for the longevity of these quantum dots, and more than 10,000 h of lifetime can be expected.
1. Introduction
Quantum dots (QDs) or colloidal quantum dots (CQDs) are nano-meter scale particles made of semiconductors. The strong spatial confinement leading to energy level quantization can provide excellent photonic transitions (absorption or emission). Many materials have been demonstrated to have very good photonic properties (such as quantum efficiencies). Among them, the cadmium-based quantum dot is the most studied [1], and also has the best results in terms of illumination and photon-absorption [2,3,4]. Many special traits associated with QDs, like narrow linewidth emission, wide color gamut coverage, and size-dependent emission wavelengths, are very crucial for the next generation of photonic devices [5,6,7,8]. Various research results aimed at optically and electrically pumped CQD light emitting diodes (LEDs) have been published [9,10,11,12,13,14,15]. For electrically pumped CQD devices, the electrons and holes are injected directly into the CQD layer from the electron-transport layer (ETL) and hole-transport layer (HTL). The chemistry and band-alignment between these layers, including the work function of the contact metal, are quite important for the device to succeed. On the other hand, the optically pumped CQD LEDs have the external light source (usually in ultraviolet (UV) or blue) to pump the CQD layer, and the luminescent down-shifting effect (LDS) can help to convert the photons with high energy into the ones with low energy (visible bands).
However, both types of devices suffered from the continuous degradation in luminous intensities caused by the environmental erosion, such as humidity, oxygen, and photonic excitation [16]. In the past, several methods have been proposed to prevent this phenomenon from happening. People used extra shell materials [17], atomic layer deposited (ALD) dielectric layers [18], a liquid-type sealed compartment [19], a silica-sealed matrix [20], and an embedded ionic crystal [4,21,22,23,24]. Among them, the use of ionic crystals like sodium chloride (NaCl) and potassium chloride (KCl) was a very attractive way to shield out those environmental factors [24]. The solid formation encapsulates the nanocrystals and the solution-based precipitation can be accommodated for with the available storage format of the colloidal quantum dots (solvent or water). With proper chemical mixtures, the precipitation can be quick and good CQD powders can be provided. Previously, we achieved a prolonged lifetime for CQD embedded in NaCl by recrystallization in the saturated salt water [4,23]. However, this method usually takes tens of h to completely extract the CQD substances. Faster methods are possible, and many precipitation methods have been applied to realize this scheme, but there is no comparative study on which method is most effective. In this article, we will reveal our results based on different synthesis methods used to generate CQD + NaCl powders, and then we will put all these CQDs into the independent heat and photonic excitation experiments to test the durability of the CQDs. The result shall be useful for the future commercialization of the CQD-related light emitting devices [4,23].
2. Sample Preparation and Experiment
In this work, we used water-soluble CdTe colloidal quantum dots, which emitted 610 nm photons and were capped with carboxylic acid. In these synthesis methods, we tried three different methods in order to seal the CQDs in a core-shell structure, including traditional (or saturated-salt), methanol-assisted, and ethanol-assisted methods [21,25]. We also attempted to explain the influence of outside impact on these three synthesis methods, which provided high quality QD-salt crystals. The CdTe QDs were purchased from Sigma-Aldrich® (3050 Spruce St. St. Louis, MO 63103).
For the traditional method, we prepared a supersaturated solution at 30 °C. The solution, which contained 6.88 g of NaCl in 20 mL deionized (DI) water, was warmed to 60 °C, where it was still saturated, and then it was stirred before cooling to 30 °C. The CdTe QDs powders were then added into the water and were poured into the saturated NaCl solution slowly. After 20 h, the CQDs were sealed inside the NaCl crystals and they could be dried for later use.
For the methanol- and ethanol-assisted methods, we used the characteristic of the different solubility between methanol and ethanol for NaCl salt. In methanol-assisted synthesis, we prepared the DI water and the saturated NaCl + CQD solution. Then we injected it into the bottom of methanol solution by dropping pipet. After 15 h, there were crystallization on the surface of the methanol solution. We took them out and dried them.
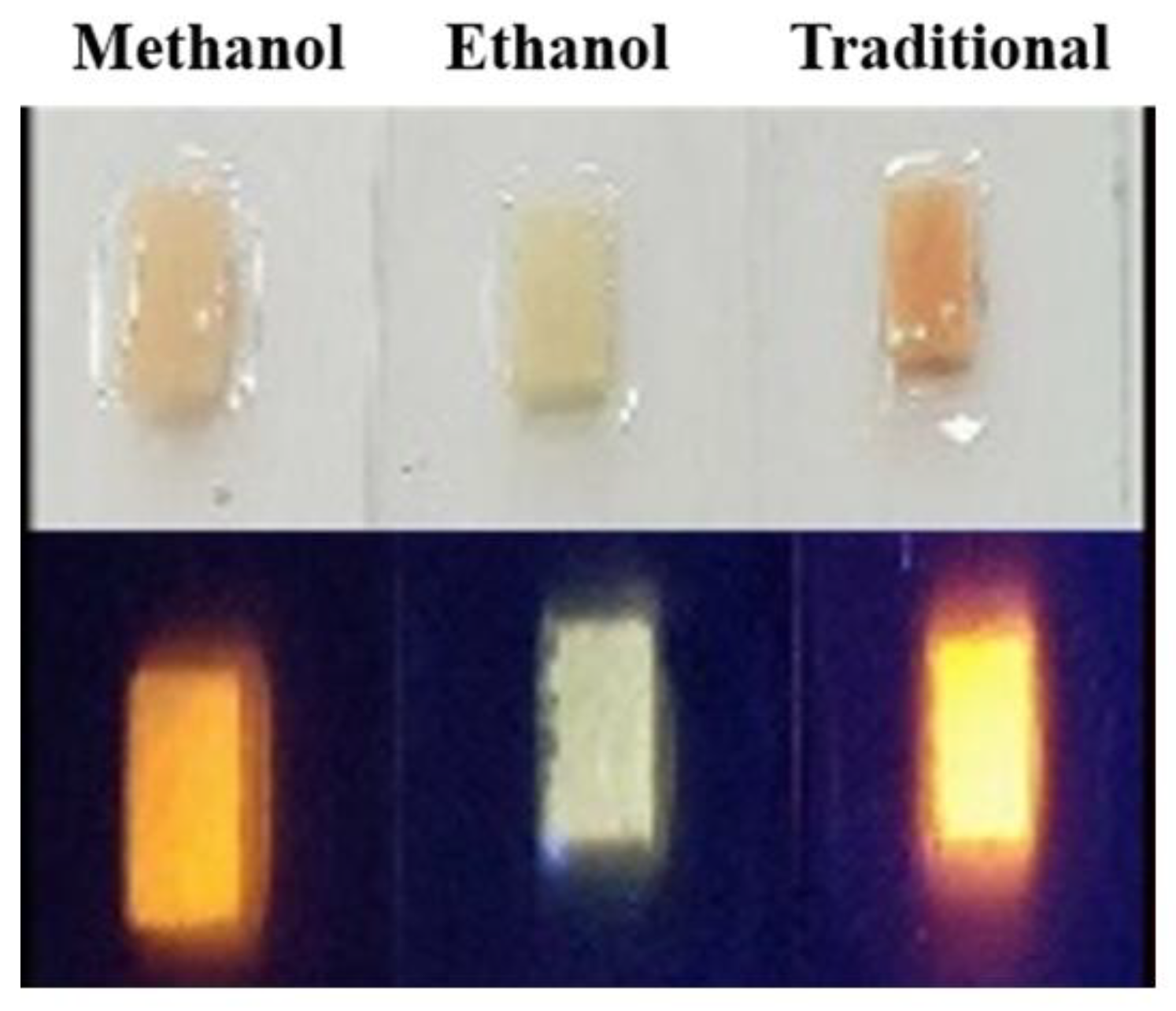
For the ethanol-assisted method, because of the lower solubility than the methanol solution, the ethanol solvent was poured directly into the NaCl + CQD solution and was followed by fast stirring. The re-crystallization process of the CQD in NaCl took about 2.5 min in the ethanol method. After the synthesis process, we filtered the CQD capped NaCl crystals and then dried them in order use them in our experiment. After the CQD powders were properly manufactured, they were ground into finer grains and mixed with polydimethylsiloxane (PDMS) by the method we described previously [4,23]. Figure 1 shows the powders under the UV light, which are methanol-assisted, ethanol-assisted, and the traditional method.
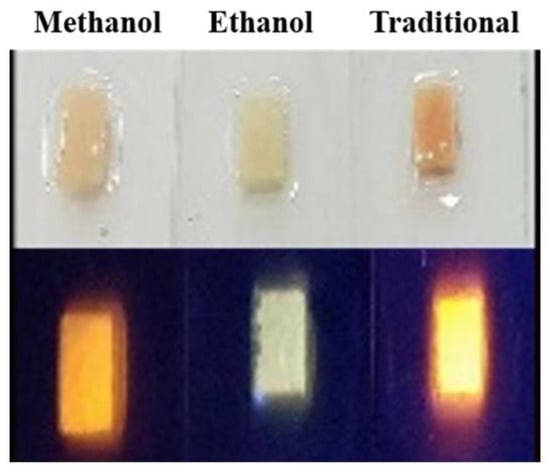
Figure 1.
The CQD + NaCl for three synthesis methods under ultra-violet (UV) light.
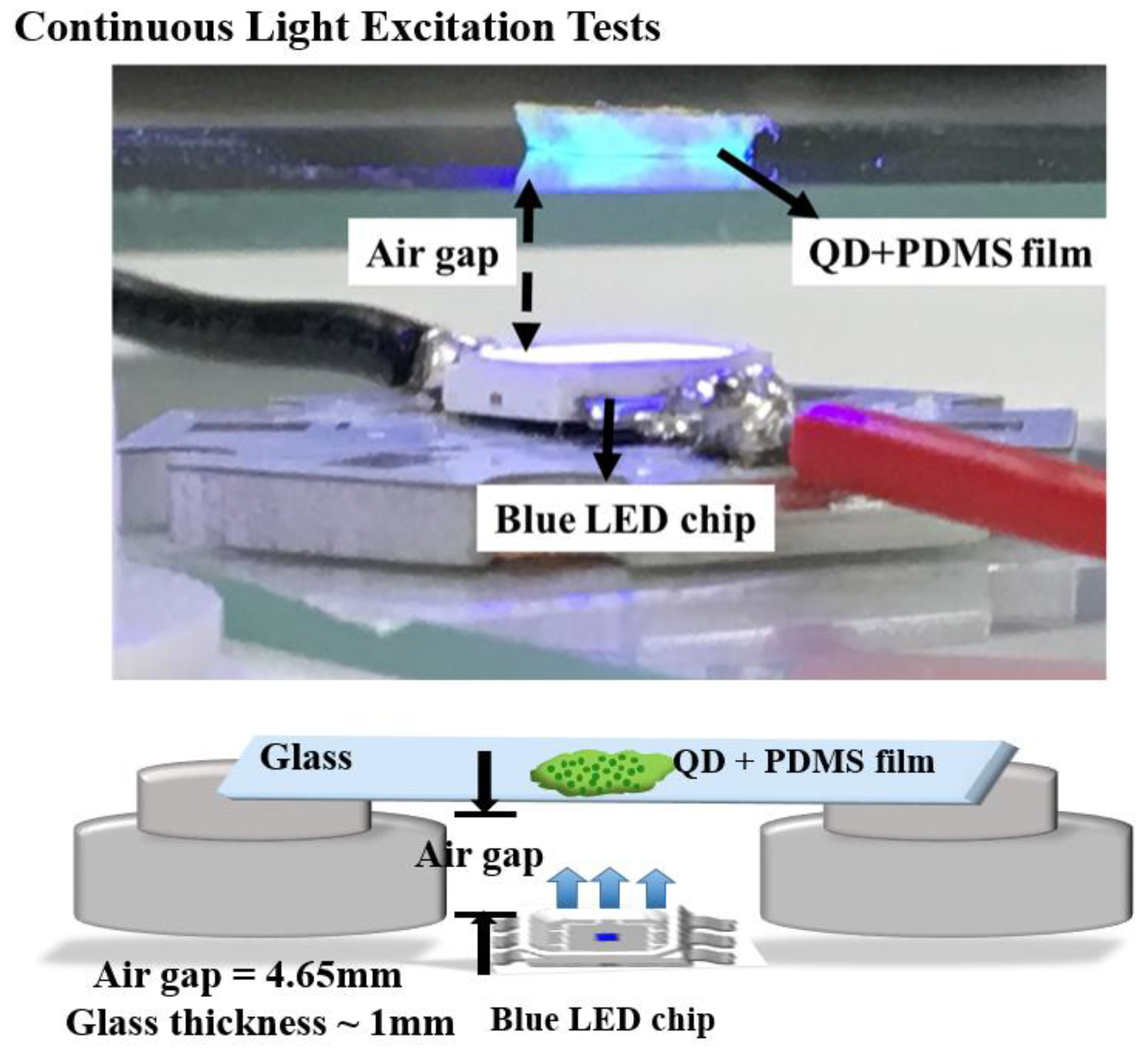
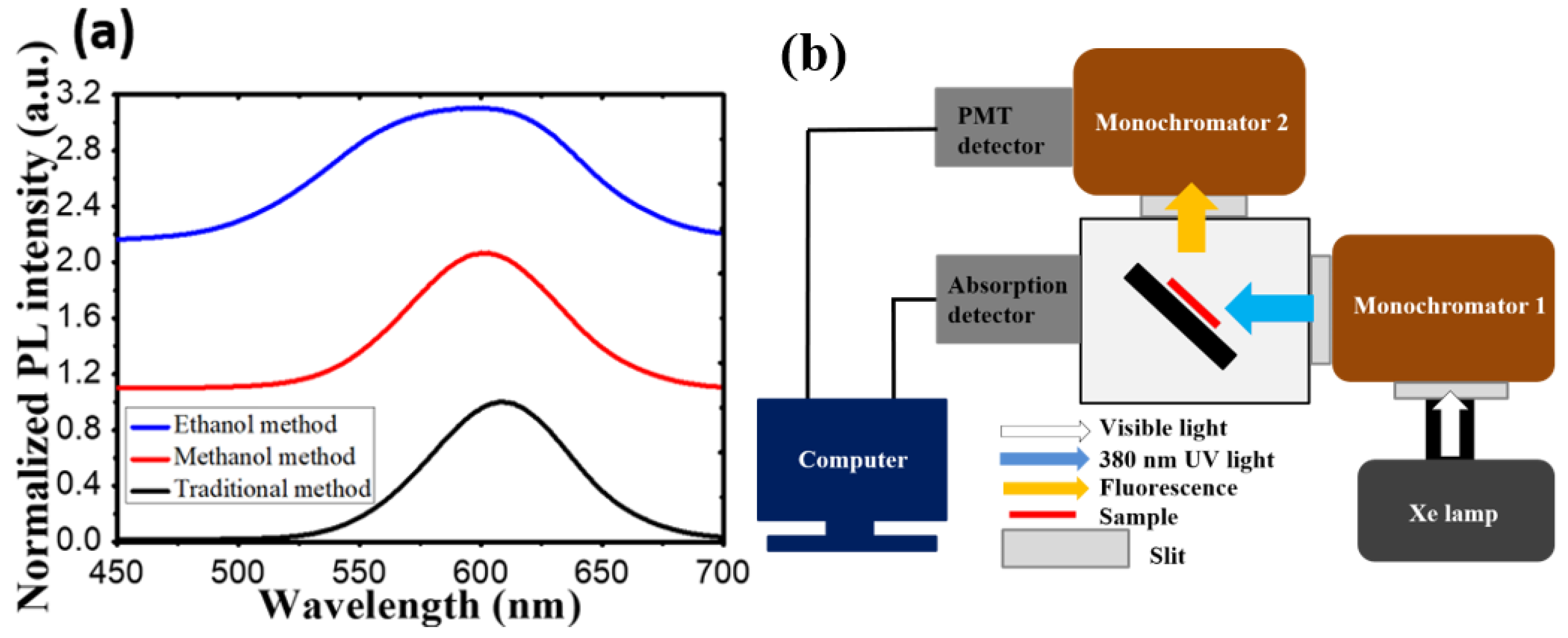
In this experiment, we will discuss the effect toward the degradation among these three samples by setting up a continuous heat source, and photonic excitation stress, as shown in Figure 2. In the temperature-dependent aging test, samples were placed on hotplates under a low-temperature (60 °C) condition and a high-temperature (120 °C) condition. In the photonic excitation intensity test, in the beginning, we placed the CdTe@NaCl with PDMS into the square template and cured it at room temperature. In order to isolate the heat source, the samples were fixed on the glass that was at the distance of 4.65 mm from the blue LED, as shown in Figure 2. The injection currents of the LED were set to be 20 mA (7.65 mW) and 200 mA (69.6 mW) for low and high intensities. The corresponding photonic power intensities are 62 mW/cm2 and 568 mW/cm2, respectively. In the photonic excitation experiments, the optically pumped CQD LEDs had an external light source (usually in ultraviolet (UV) or blue light) to pump the CQD layer. The luminescent down-shifting effect helped convert the photons with high energy into ones with low energy (visible bands). This CQD layer acted like a phosphor layer in the modern white LED, and thus, no electrical current needed to pass through these nanocrystals. Without current conduction, the design rule of CQD layer became much easier and a proper insulating and protective layer could be developed for the CQD reliability. Figure 3a shows the initial photoluminescence (PL) spectra of the samples by the three synthesis methods. The characteristics of the samples were regularly measured in the computer-controlled PL measurement system, whose schematic diagram is shown in Figure 3b, and the PL system is composed of a Xe lamp, a monochromator, a photomultiplier (PMT), and optical components in order to collect the emitted photons. It was purchased from Princeton Instruments (3660 Quakerbridge Road Trenton, NJ 08619 USA) and the model number is Acton2150. The measurement was calibrated every time with the standard sample with a known PL intensity to make sure the results were consistent over a long period of time.
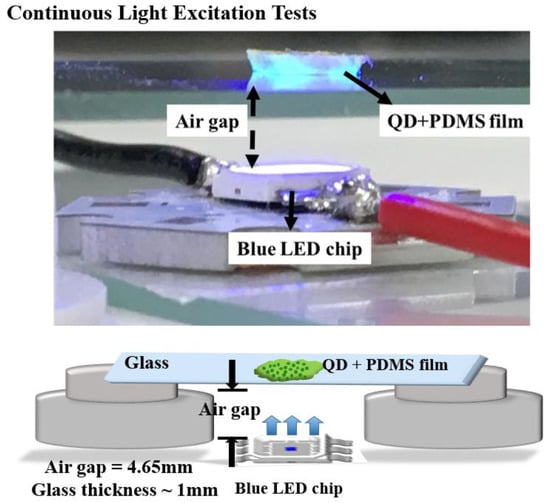
Figure 2.
The picture (up) schematic diagram (bottom) of the continuous light excitation setup.
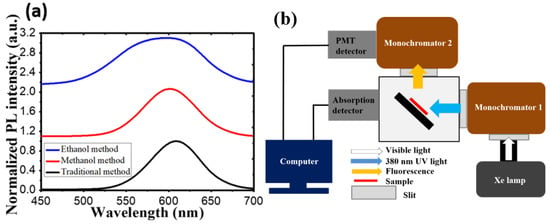
Figure 3.
(a) The PL spectra for samples synthesized by three different co-precipitation methods. (b) Computer-controlled photoluminescence measurement system.
3. Results and Discussion
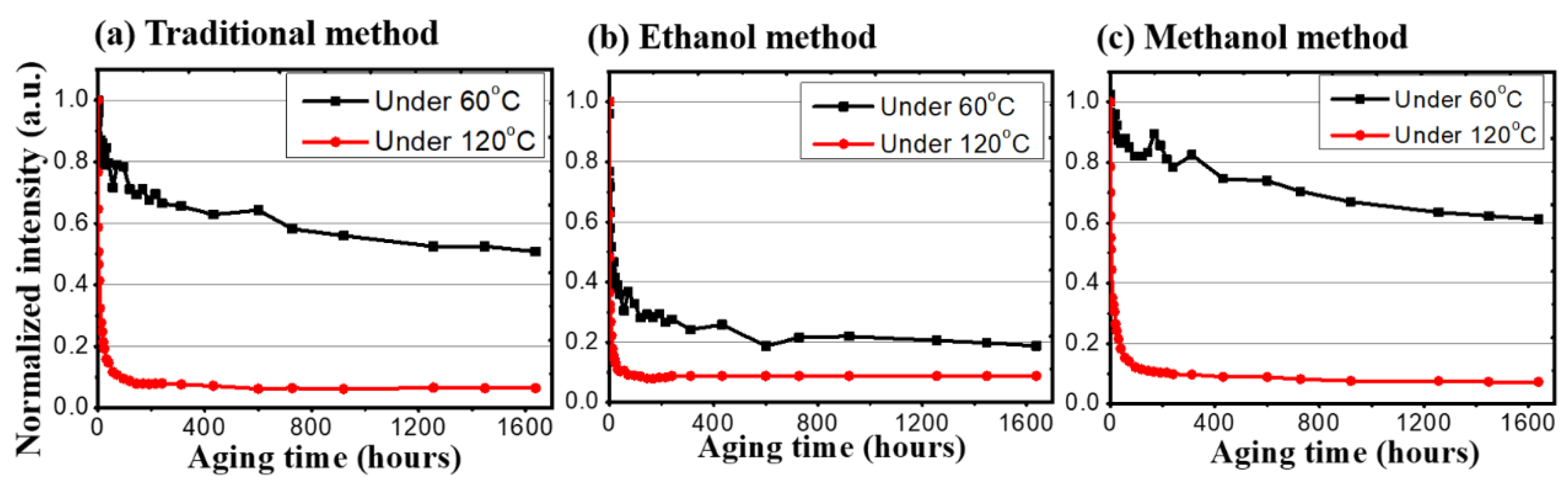
After all the preparations were finished, the thermal stress and photonic excitation stress tests began. Figure 4 shows the heat effect between different samples. In the thermal stress test, each of the synthesis methods would be under a low temperature (60 °C) and a high temperature (120 °C). For the traditional synthesis method, the low temperature sample remained at 50% after 1948 h, while the high temperature one dropped to 50% in the first 24 h. For the methanol-assisted synthesis method and the ethanol-assisted synthesis method under 60 °C, their extrapolated lifetimes, that are determined as the time spans to degrade to 50% of the initial value (LT50), are 3287 h and 8 h, respectively. For the 120 °C high temperature stress tests, while all three samples dropped below 20% in the first 24 h, the stability of the emission intensities of the CQDs was established and was maintained up to 1638 h.
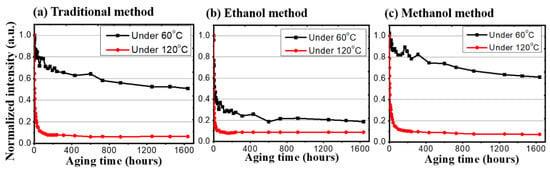
Figure 4.
The normalized intensity of three different synthesis methods under low (60 °C) and high (120 °C) temperatures.
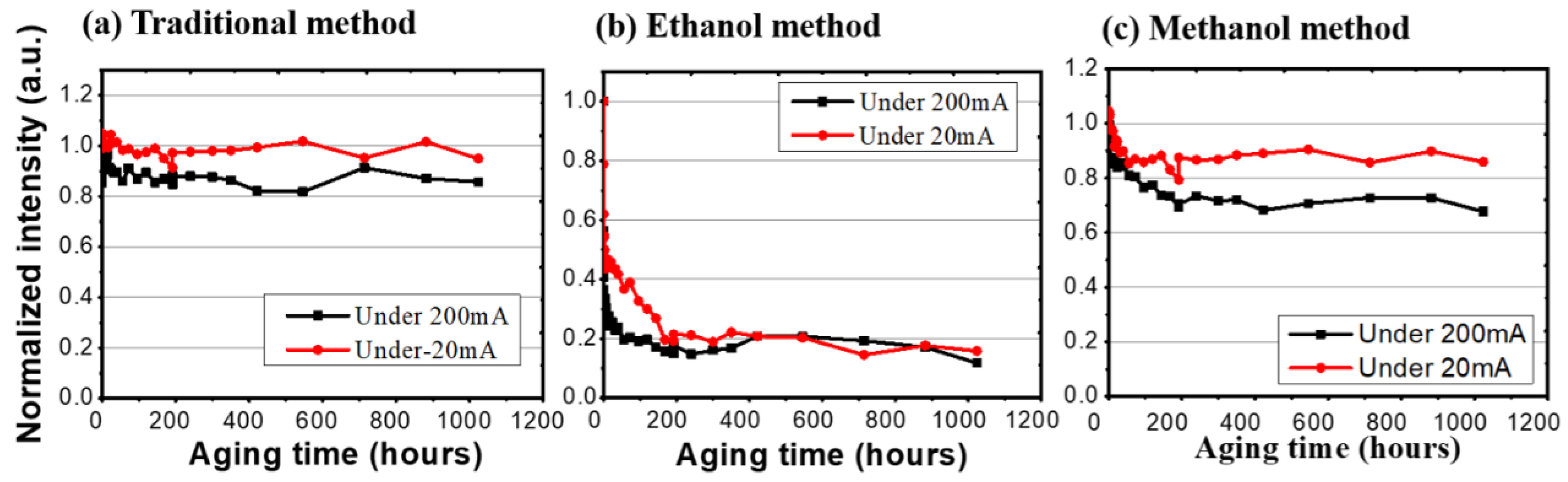
In photonic excitation endurance tests, as mentioned in the previous section, there are two currents (200 mA and 20 mA) to pump the blue LEDs for the different light intensities. We compared these three synthesis methods under a 20 mA test. The traditional synthesis method and the methanol-assisted samples degraded to 94.98% and 85.89% after 1024 h; however, the ethanol-assisted sample dropped rapidly to 15.82%. On the other hand, for the high excitation (200 mA), the traditional salt synthesis and the methanol-assisted samples stayed at 85.73% and 67.74%, and the ethanol-assisted sample decreased to 11.75%, as shown in Figure 5. For the ethanol samples, both of the current settings caused an immediate reduction in luminous intensities, which showed as 15.8% and 11.7% of the initial values after 200 h of burn-in. The difference between them is that it took a longer time for the low current sample to reach this stabilized but degraded level. Highly stable light emissions can be expected from both the traditional and methanol-assisted methods. By using the same 50% criterion, long lifetimes (23,310 h for the traditional method and 14,103 h for the methanol-assisted method) can be expected from both methods at a 62 mW/cm2 pumping condition, while the samples prepared by the ethanol method failed within 3 h. Table 1 shows the summarized LT50 values for all samples based on over 1000 h of continuous tests.
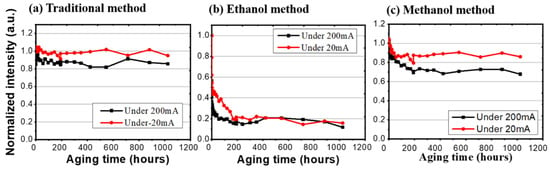
Figure 5.
The normalized intensity of three different synthesis methods under high (200 mA) and low (20 mA) injected currents.

Table 1.
The projected lifetime (LT50) for all samples under different aging conditions.
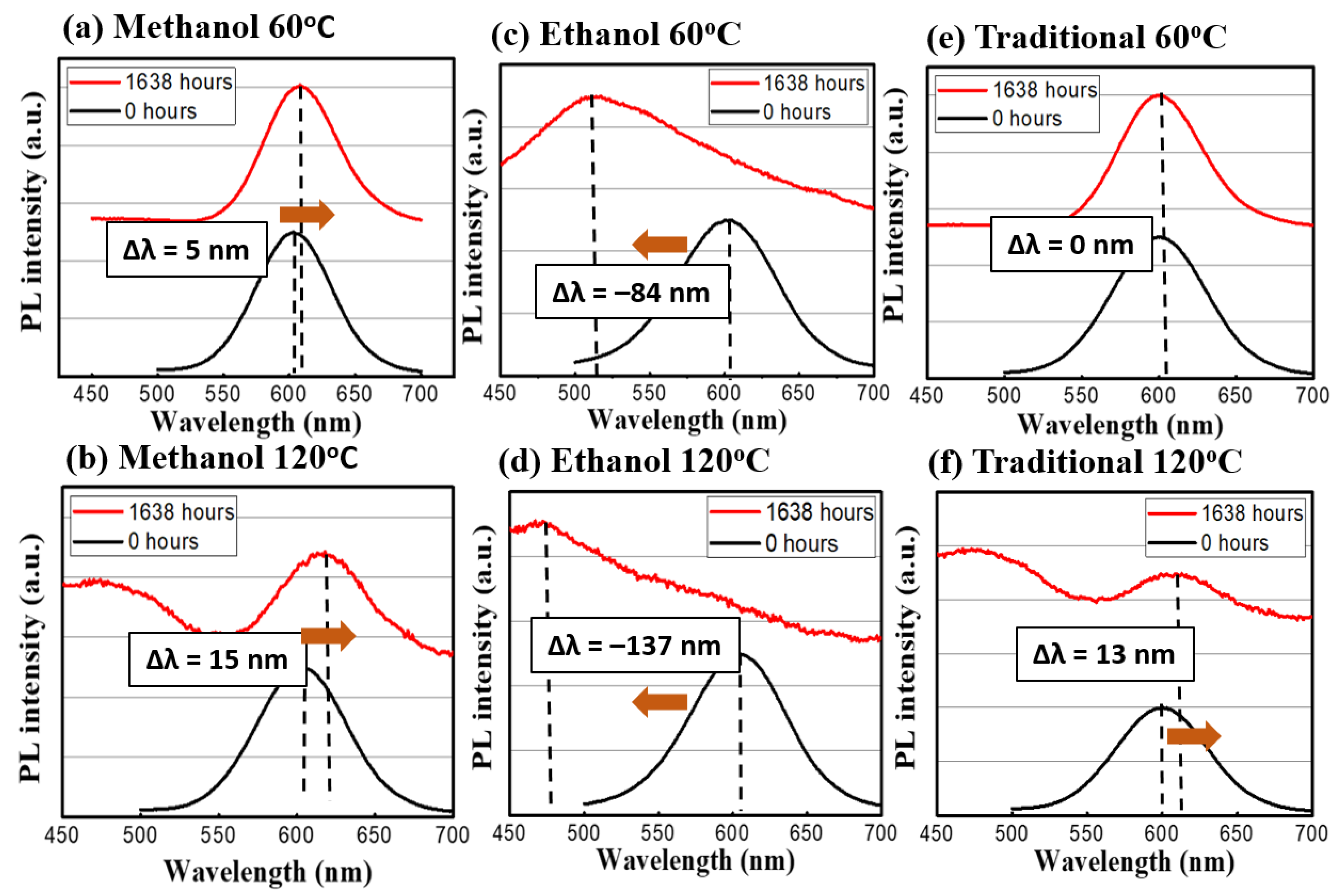
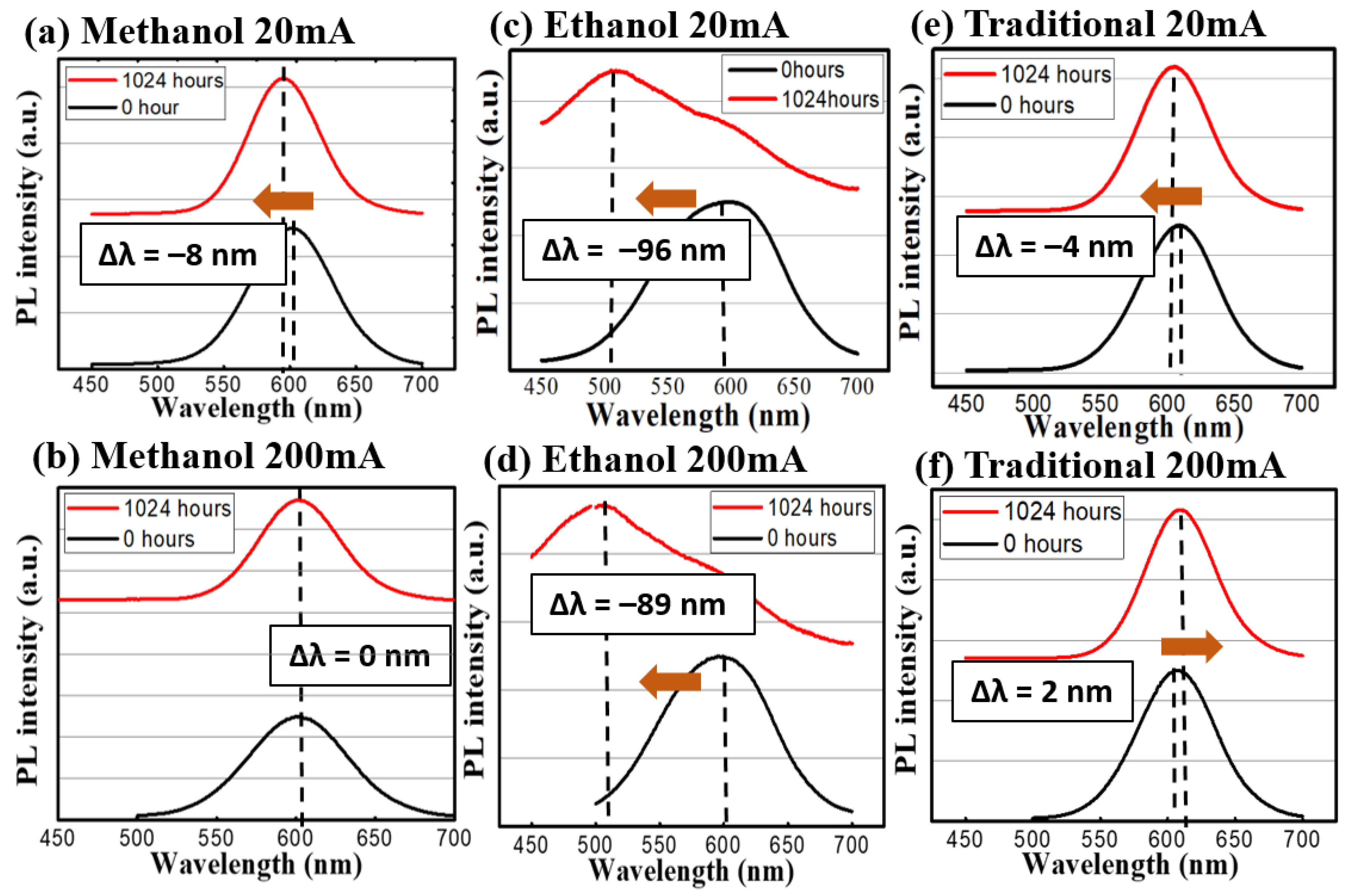
In terms of spectral evolution during the stress test, the change of the emission spectra can be seen in Figure 6 and Figure 7. The methanol-assisted and traditional methods could maintain the shapes and intensities of the peaks at both low levels of thermal and optical excitations. When a nanocrystal (or a quantum dot) is under external influences, its peak of emission can move towards two directions: red-shift (become longer in wavelength) or blue-shift (shorter). Because the emission of the photon in a quantum dot is the direct result of quantum confinement, its size can affect the wavelength directly [12]. External influences, such as oxygen, temperature, or high-energy photons, can alter the active volume of the dots via physical or chemical reactions. For example, the oxygen can oxidize the dot outer shell and make the volume smaller. According to quantum mechanics, the emission wavelength becomes shorter [18]. Meanwhile, the high temperature or high photon excitation under the no-oxygen condition can lead to the sintering of or re-shaping of the dots [18], which leads to a red shift of the emission peaks. In our case, it is interesting to observe the strong blue shifts in ethanol-assisted QDs, which can be interpreted as the indicator of incomplete encapsulation due to fast solidifying process. The exposed QDs can easily become oxidized during the continuous heating or photon-excitation tests in our study [25]. Whereas, in the traditional and methanol cases, the QDs are entirely covered by sodium chloride crystal and thus, most of the shift comes from the ripening and sintering of the dots under thermal or photonic stress. The CQDs prepared by the ethanol-assisted method did not hold well under low and high test conditions and even developed multiple peaks and a broadened spectrum. Besides, the evolution of the spectral full-width-of-half-maximum (FWHM) was recorded and analyzed. For the traditional method sample, the variations were small for both 60 °C and 120 °C conditions. In the methanol-assisted samples, the FWHM variations were even smaller than those of the traditional ones were. On the other hand, the samples made by the ethanol-assisted method broadened quite a lot. The broadening of FWHM in the ethanol-assisted QDs was also a direct result of this oxidation. Since the exposure and the incomplete coverage were random, the size change became random as well. The distribution of the dot size could certainly become wider, causing the FWHM to be broader [26]. When an external force (like thermal stress or photonic irradiation in this study), the non-uniform distribution of this force within the CQD layer could generate local hot spots, which would cause larger damage. This is the fundamental reason why the size distribution of CQDs usually became larger when they were degraded. A properly managed heat environment, light environment, or a CQD protective layer is, thus, necessary to keep the FWHM constant for the operation. In the link of the FWHM to the dots, the quantum-confined effect lead to the size-dependent photonic transition in the dots, whose size distribution was observed in the PL spectrum in turns [27]. Table 2 summarizes the peak shifts and FWHM variations of three different synthesis methods for the thermal and light stress experiment.
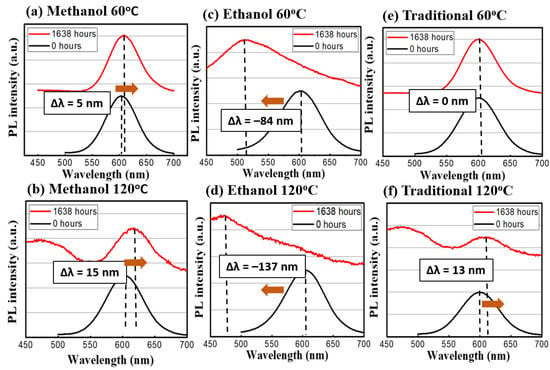
Figure 6.
The emission spectrum before and after the temperature stress test. The red profiles are at the 1638th h and the blue ones are at the beginning. (a) Methanol at 60 °C and (b) at 120 °C; (c) Ethanol at 60 °C and (d) at 120 °C; (e) Traditional at 60 °C and (f) at 120 °C.
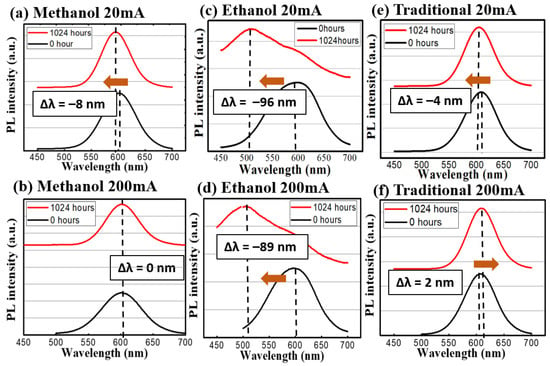
Figure 7.
The emission spectrum before and after the photon excitation stress test. The red profiles are at the 1024th h and the blue ones are at the beginning. (a) Methanol 20 mA and (b) 200 mA; (c) Ethanol 20 mA and (d) 200 mA; (e) Traditional 20 mA and (f) 200 mA.

Table 2.
Summary of peak wavelength shift (Δλ) and full-width-of-half-maximum (FWHM) change of the tested samples (unit:nm).
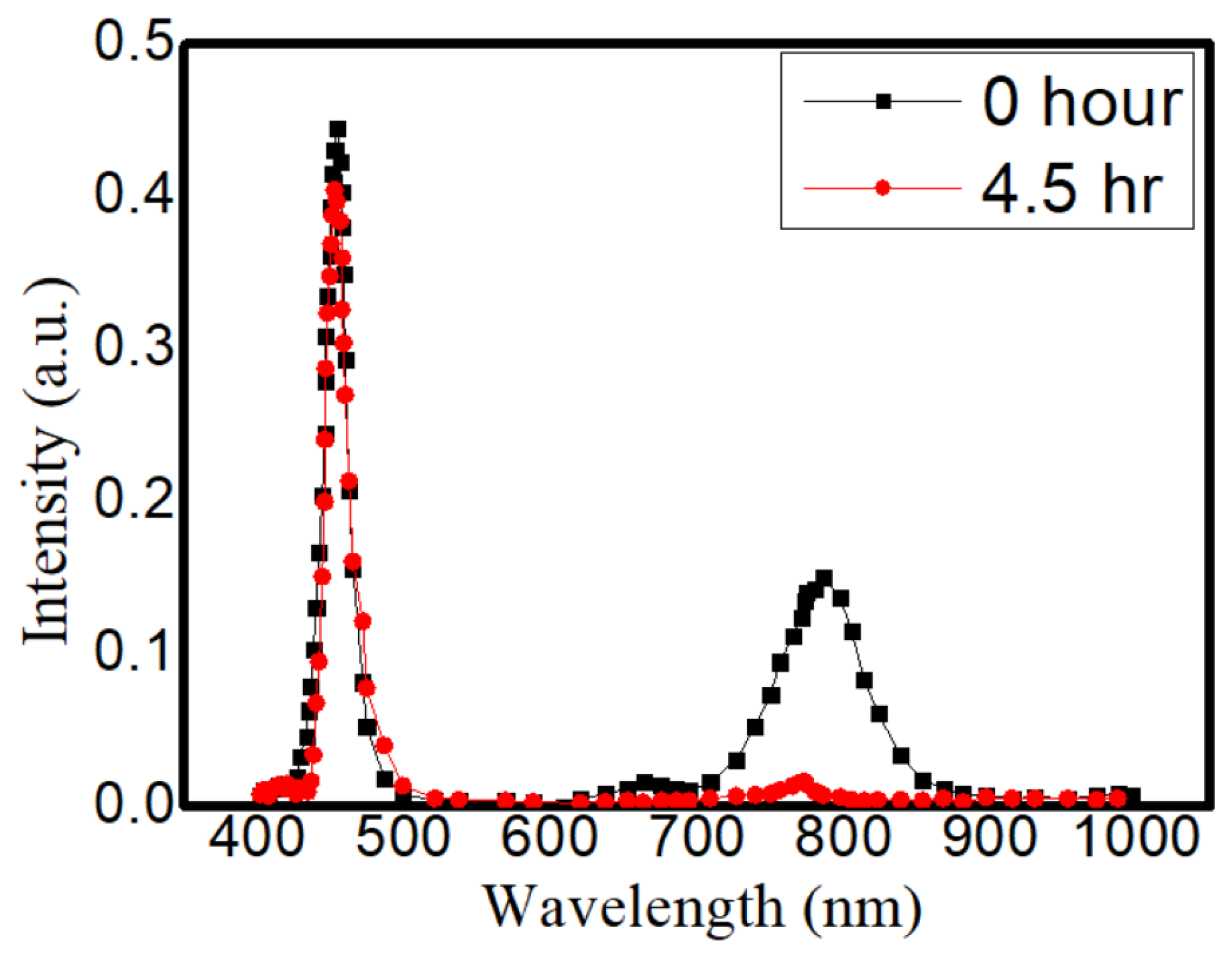
From these aging tests, several things could be found out: (A) the high-temperature condition is more detrimental towards CQDs than light excitation is. This difference can be seen especially in the medium level of heat. (B) The traditional method to embed CQD in NaCl is still the best way to prevent outside erosion. It took much longer (20 h vs. 15 h, vs. a few min) to recrystallize for the traditional method, but the sealing could be more complete in the traditional way. (C) Between the methanol- and ethanol-assisted methods, the methanol-assisted method is better in terms of preventing CQDs from FWHM widening or intensity degradation. A possible cause could also be the better sealing of the CQDs to the NaCl crystal. Meanwhile, compared to those QDs without any NaCl encapsulation, the continuous life test was more precarious towards their illuminative efficiencies. As shown in Figure 8, the CdTe QDs without the NaCl protection were aged by a blue LED; the intensity could be reduced by 88.2% and the emission peak wavelength was shifted by 19.4 nm. From previous thermal images, these QDs were bathed in a temperature around 70 °C, and the degradation under this unprotected condition was usually fast (dropped in intensity within first 24 h of tests). The necessity of the NaCl protection in QDs for sustainable operation can be clearly demonstrated.
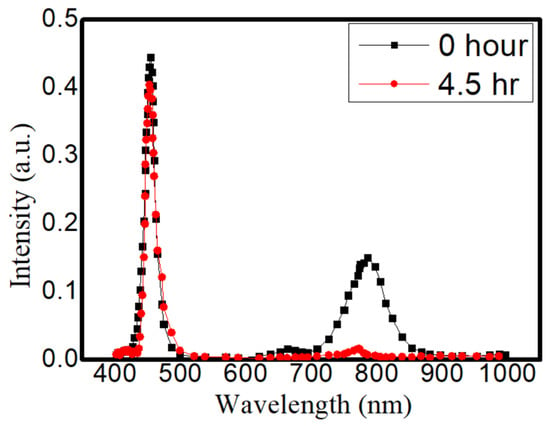
Figure 8.
The emission spectrum before and after 4.5 h for Cadmium telluride (CdTe) QDs without the NaCl.
4. Micro-Scale Crystal Analysis
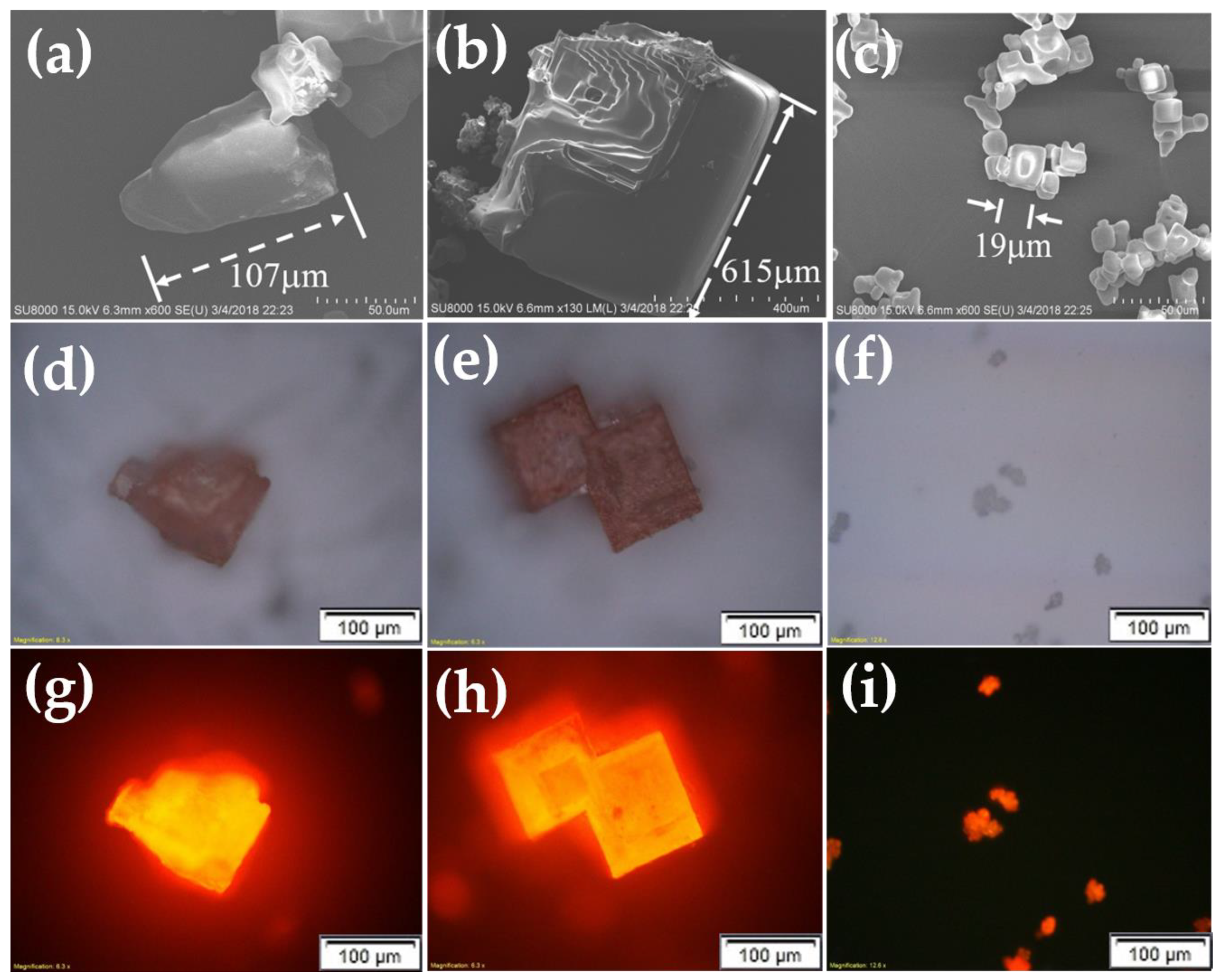
After the CQD@NaCl re-crystallization was done and the drying process was finished, we saw that the powders formed in the ethanol-assisted method tended to be finer. The images from Figure 9a–i show the crystals under Scanning Electron Microscope (SEM) and optical microscopes. The grain sizes of these three methods are very different. The traditional and methanol-assisted methods had larger, square crystal shapes, usually in the hundreds of microns. However, for the ethanol-assisted method, the grain size was limited to 10 or below 50 microns. The sheer size of the crystal and the analysis of the X-ray lead us to believe that the coverage between the ethanol method and others can be quite different. If the same amount of CQDs was dispersed into the saltwater solution, the number of the grains in ethanol solution would be much larger and would cause much more incomplete coverage on the surface of CQDs, thus leading to their eventual degradation.
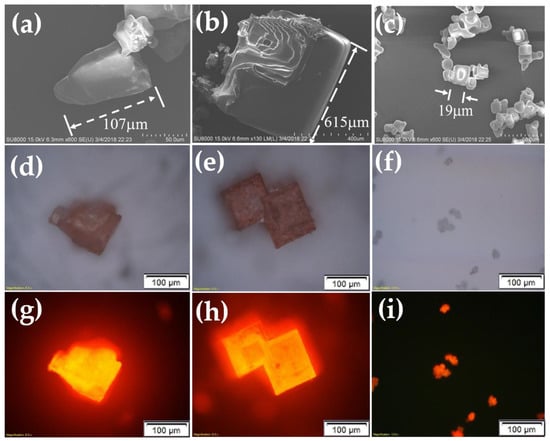
Figure 9.
(a–i) The crystals under SEM and optical microscopes.
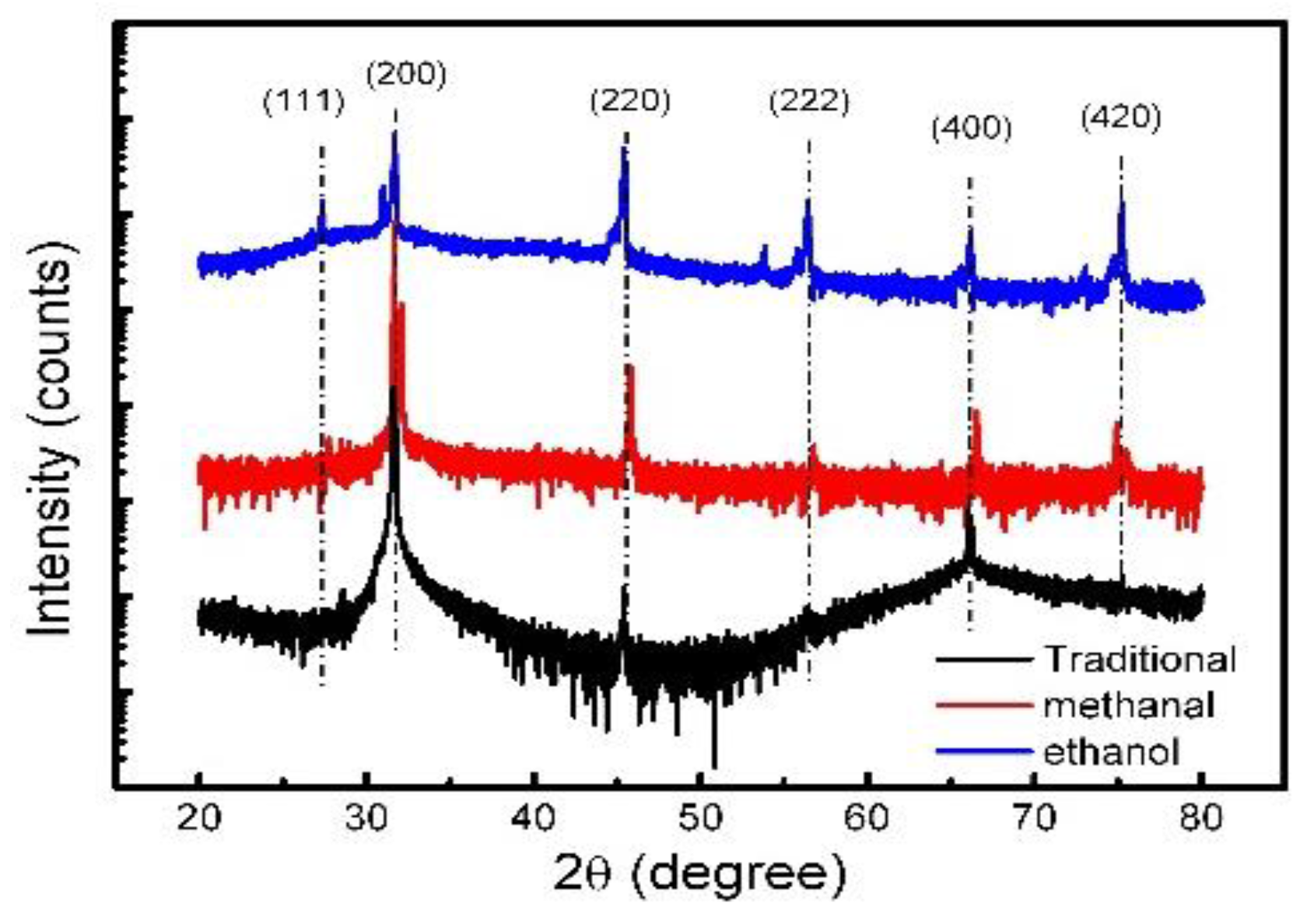
To look for the micro scale grain size of our NaCl-sealed CQDs, an X-ray diffraction test was carried out. The results could be used as an indicator to judge the crystallization of NaCl in the micro-scale. The pure NaCl crystals prepared by the same methods were collected for the XRD measurement, and the actual microscopic formation of these NaCl crystals could be obtained the diffraction intensity vs. 2θ plots, which are shown in Figure 10. For the traditional assisted method, we can see that the peaks are sharper than the other two. In addition, we can get the micro-scale crystal size by the Scherrer Equation:
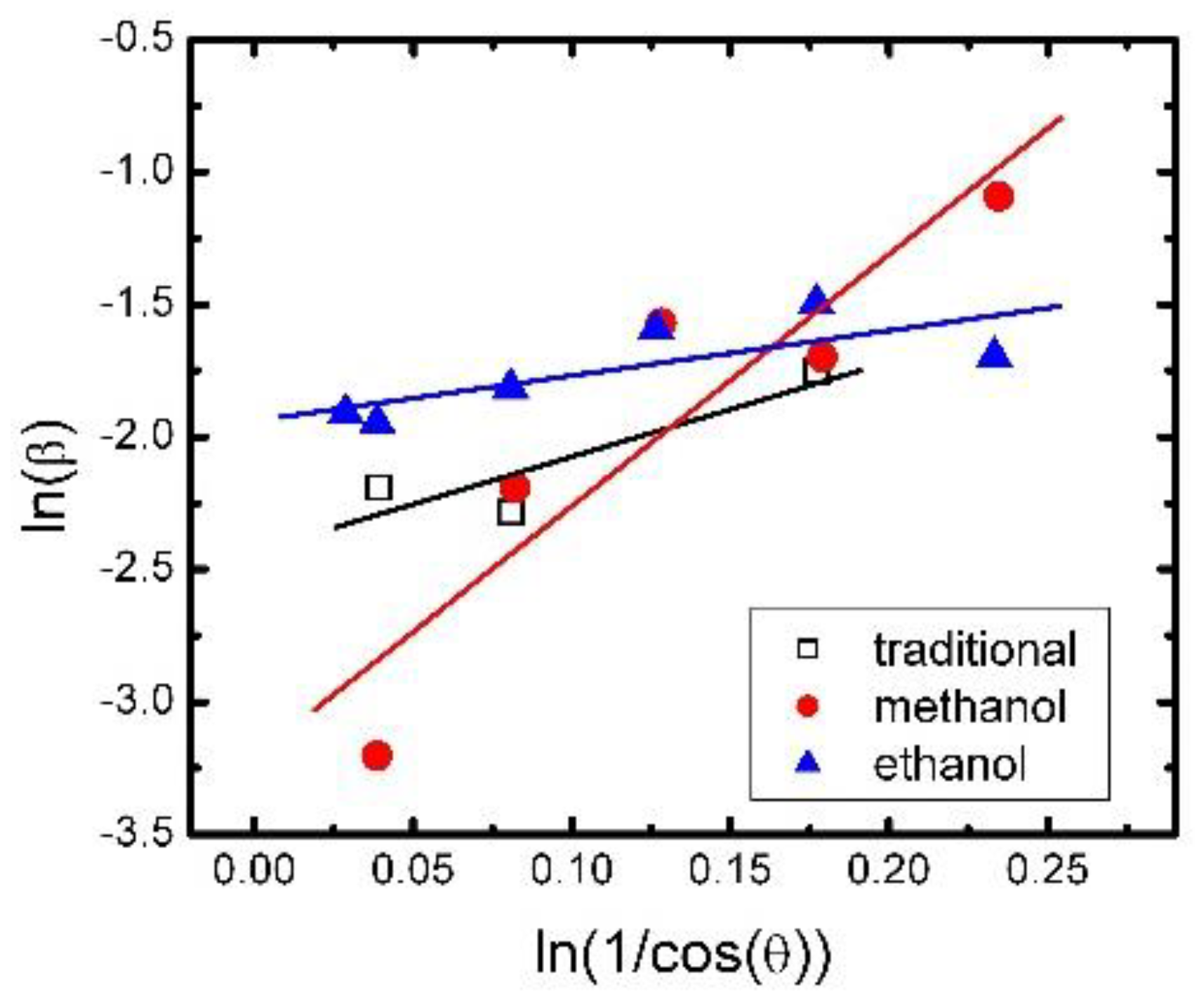
where L is crystallite size, λ is the X-ray wavelength in nanometer, β is full width at half maximum of peak of diffraction peak profile, and K is constant, which equal 0.9. We also modified the Scherrer equation plots lnβ against ln(1/cos θ) in Figure 11, and got the intercept of a least squares line regression. We can obtain a single value of L through all of the available peaks [28]. For the traditional synthesis method, the methanol synthesis method, and the ethanol synthesis method, the crystallite sizes were about 1.4 μm, 3.1 μm, and 0.9 μm, respectively. The direct interpretation of this outcome can be that the micro grain size seems to be correlated positively to the aging performance of the samples by three methods.
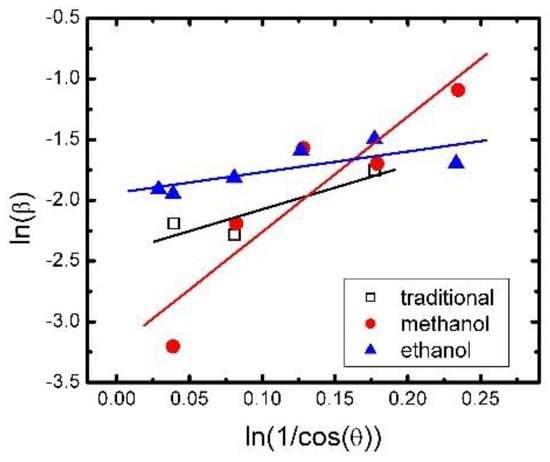
Figure 10.
Modified Scherrer equation fitting for different synthesis methods.
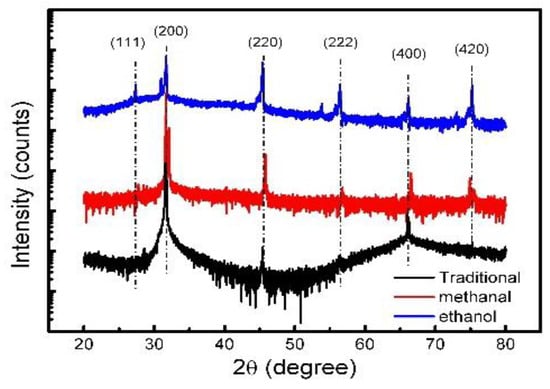
Figure 11.
The X-ray diffraction (XRD) diffraction of three synthesis methods
On the other hand, slower re-crystallization can make the crystal more complete and create a better seal for the CQDs. and the testing results from the traditional and methanol-assisted samples demonstrated this trend. Smaller salt particles can mean a larger surface, which increases the chance of exposure of CQD to the outside environment. Similarly, in the micro scale, a smaller grain size also leads to more grain boundaries and fractures in the CQD@NaCl composites.
5. Conclusions
In conclusion, the continuous thermal and photonic stress tests were set up and executed for more than 1000 h to examine the longevity of the CQD luminescent efficiencies under different precipitation methods. The results showed that the temperature factor had a more pronounced impact on CQDs than the high-energy photon exposure did. Among three methods, the traditional saturated-salt method and the methanol-assisted method are preferred, and they preserved the light emission characteristics much better than the ethanol-assisted one. This study can clarify the major failure mechanism when CQDs are integrated into the next generation of a solid-state light source, and this should be useful for process integration in the future.
Author Contributions
Conceptualization, S.-C.H. and C.-C.L.; methodology, Y.-M.H., S.-H.C., and C.-P.Y.; validation, Y.-M.H. and C.-C.L.; Data analysis, Y.-M.H., C.-P.H., N.L., and Y.-L.C.; investigation, Y.-M.H., L.-A.K., and C.-P.Y.; resources, C.-C.L.; data curation, Y.-M.H. and C.-C.L.; writing—original draft preparation, Y.-M.H. and S.-C.H.; writing—review and editing, C.-C.L.; supervision, H.-C.K., C.-C.L.; project administration, C.-C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research is supported by the Ministry of Science and Technology in Taiwan under the project No. MOST 107-2221-E-009-114-MY3.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pal, B.N.; Ghosh, Y.; Brovelli, S.; Laocharoensuk, R.; Klimov, V.; Hollingsworth, J.A.; Htoon, H. ‘Giant’CdSe/CdS core/shell nanocrystal quantum dots as efficient electroluminescent materials: Strong influence of shell thickness on light-emitting diode performance. Nano Lett. 2011, 12, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhang, Y.; Ruan, C.; Yin, C.; Wang, X.; Wang, Y.; Yu, W.W. Efficient and Stable White LEDs with Silica-Coated Inorganic Perovskite Quantum Dots. Adv. Mater. 2016, 28, 10088–10094. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-H.; Han, C.-Y.; Kang, H.-D.; Ko, H.; Lee, C.; Lee, J.; Myoung, N.; Yim, S.-Y.; Yang, H. Highly efficient, color-reproducible full-color electroluminescent devices based on red/green/blue quantum dot-mixed multilayer. ACS nano 2015, 9, 10941–10949. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.-C.; Chen, Y.-H.; Tu, Z.-Y.; Han, H.-V.; Lin, S.-L.; Chen, T.-M.; Kuo, H.-C.; Lin, C.-C. Highly stable and efficient hybrid quantum dot light-emitting diodes. IEEE Photonics J. 2015, 7, 1–10. [Google Scholar] [CrossRef]
- Guzelturk, B.; Kelestemur, Y.; Olutas, M.; Delikanli, S.; Demir, H.V. Amplified spontaneous emission and lasing in colloidal nanoplatelets. Acs Nano 2014, 8, 6599–6605. [Google Scholar] [CrossRef] [PubMed]
- Demir, H.V.; Nizamoglu, S.; Erdem, T.; Mutlugun, E.; Gaponik, N.; Eychmüller, A. Quantum dot integrated LEDs using photonic and excitonic color conversion. Nano Today 2011, 6, 632–647. [Google Scholar] [CrossRef]
- Klimov, V.I.; Ivanov, S.A.; Nanda, J.; Achermann, M.; Bezel, I.; McGuire, J.A.; Piryatinski, A. Single-exciton optical gain in semiconductor nanocrystals. Nature 2007, 447, 441. [Google Scholar] [CrossRef]
- Klimov, V.; Mikhailovsky, A.; Xu, S.; Malko, A.; Hollingsworth, J.; Leatherdale, C.; Eisler, H.-J.; Bawendi, M. Optical gain and stimulated emission in nanocrystal quantum dots. Science 2000, 290, 314–317. [Google Scholar] [CrossRef]
- Chen, B.; Zhong, H.; Zhang, W.; Tan, Z.; Li, Y.; Yu, C.; Zhai, T.; Bando, Y.; Yang, S.; Zou, B. Highly emissive and color-tunable CuInS2-based colloidal semiconductor nanocrystals: Off-stoichiometry effects and improved electroluminescence performance. Adv. Funct. Mater. 2012, 22, 2081–2088. [Google Scholar] [CrossRef]
- Dai, Q.; Duty, C.E.; Hu, M.Z. Semiconductor-Nanocrystals-Based White Light-Emitting Diodes. small 2010, 6, 1577–1588. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, C.; Su, H.; Liu, J.; Pickering, S.; Wang, J.; Wang, Y.; Hahm, J.-I. Employing heavy metal-free colloidal quantum dots in solution-processed white light-emitting diodes. Nano Lett. 2010, 11, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Alivisatos, A.P. Semiconductor clusters, nanocrystals, and quantum dots. Science 1996, 271, 933–937. [Google Scholar] [CrossRef]
- Rogach, A.L.; Gaponik, N.; Lupton, J.M.; Bertoni, C.; Gallardo, D.E.; Dunn, S.; Li Pira, N.; Paderi, M.; Repetto, P.; Romanov, S.G. Light-emitting diodes with semiconductor nanocrystals. Angew. Chem. Int. Ed. 2008, 47, 6538–6549. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Zhang, X.; Zhang, Y.; Rogach, A.L. Recent progress in quantum dot based white light-emitting devices. In Photoactive Semiconductor Nanocrystal Quantum Dots; Springer: Cham, Switzerland, 2017; pp. 123–147. [Google Scholar]
- Shirasaki, Y.; Supran, G.J.; Bawendi, M.G.; Bulović, V. Emergence of colloidal quantum-dot light-emitting technologies. Nat. Photonics 2013, 7, 13. [Google Scholar] [CrossRef]
- Nazzal, A.Y.; Wang, X.; Qu, L.; Yu, W.; Wang, Y.; Peng, X.; Xiao, M. Environmental effects on photoluminescence of highly luminescent CdSe and CdSe/ZnS core/shell nanocrystals in polymer thin films. J. Phys. Chem. B 2004, 108, 5507–5515. [Google Scholar] [CrossRef]
- Jang, E.; Jun, S.; Jang, H.; Lim, J.; Kim, B.; Kim, Y. White-light-emitting diodes with quantum dot color converters for display backlights. Adv. Mater. 2010, 22, 3076–3080. [Google Scholar] [CrossRef]
- Ihly, R.; Tolentino, J.; Liu, Y.; Gibbs, M.; Law, M. The photothermal stability of PbS quantum dot solids. ACS Nano 2011, 5, 8175–8186. [Google Scholar] [CrossRef]
- Sher, C.-W.; Lin, C.-H.; Lin, H.-Y.; Lin, C.-C.; Huang, C.-H.; Chen, K.-J.; Li, J.-R.; Wang, K.-Y.; Tu, H.-H.; Fu, C.-C. A high quality liquid-type quantum dot white light-emitting diode. Nanoscale 2015, 8, 1117–1122. [Google Scholar] [CrossRef]
- Ziegler, J.; Xu, S.; Kucur, E.; Meister, F.; Batentschuk, M.; Gindele, F.; Nann, T. Silica-coated InP/ZnS nanocrystals as converter material in white LEDs. Adv. Mater. 2008, 20, 4068–4073. [Google Scholar] [CrossRef]
- Adam, M.; Wang, Z.; Dubavik, A.; Stachowski, G.M.; Meerbach, C.; Soran-Erdem, Z.; Rengers, C.; Demir, H.V.; Gaponik, N.; Eychmüller, A. Liquid–Liquid Diffusion-Assisted Crystallization: A Fast and Versatile Approach Toward High Quality Mixed Quantum Dot-Salt Crystals. Adv. Funct. Mater. 2015, 25, 2638–2645. [Google Scholar] [CrossRef]
- Kalytchuk, S.; Zhovtiuk, O.; Rogach, A.L. Sodium chloride protected CdTe quantum dot based solid-state luminophores with high color quality and fluorescence efficiency. Appl. Phys. Lett. 2013, 103, 103105. [Google Scholar] [CrossRef]
- Hsu, S.-C.; Ke, L.-A.; Lin, H.-C.; Chen, T.-M.; Lin, H.-Y.; Chen, Y.-Z.; Chueh, Y.-L.; Kuo, H.-C.; Lin, C.-C. Fabrication of a highly stable white light-emitting diode with multiple-layer colloidal quantum dots. IEEE J. Sel. Top. in Quantum Electron. 2017, 23, 1–9. [Google Scholar] [CrossRef]
- Otto, T.; Müller, M.; Mundra, P.; Lesnyak, V.; Demir, H.V.; Gaponik, N.; Eychmüller, A. Colloidal nanocrystals embedded in macrocrystals: Robustness, photostability, and color purity. Nano Lett. 2012, 12, 5348–5354. [Google Scholar] [CrossRef]
- Chang, Y.; Yao, X.; Mi, L.; Li, G.; Wang, S.; Wang, H.; Zhang, Z.; Jiang, Y. A water–ethanol phase assisted co-precipitation approach toward high quality quantum dot–inorganic salt composites and their application for WLEDs. Green Chem. 2015, 17, 4439–4445. [Google Scholar] [CrossRef]
- O’Donnell, K.; Chen, X. Temperature dependence of semiconductor band gaps. Appl. Phys. Lett. 1991, 58, 2924–2926. [Google Scholar] [CrossRef]
- Crooker, S.; Hollingsworth, J.; Tretiak, S.; Klimov, V.I. Spectrally resolved dynamics of energy transfer in quantum-dot assemblies: Towards engineered energy flows in artificial materials. Phys. Rev. Lett. 2002, 89, 186802. [Google Scholar] [CrossRef]
- Monshi, A.; Foroughi, M.R.; Monshi, M.R. Modified Scherrer equation to estimate more accurately nano-crystallite size using XRD. World J. Nano Sci. Eng. 2012, 2, 154–160. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).