Graphene to Advanced MoS2: A Review of Structure, Synthesis, and Optoelectronic Device Application
Abstract
:1. Introduction
2. Transition Metal Dichalcogenides (TMDCs)
Crystalline Structure of MoS2
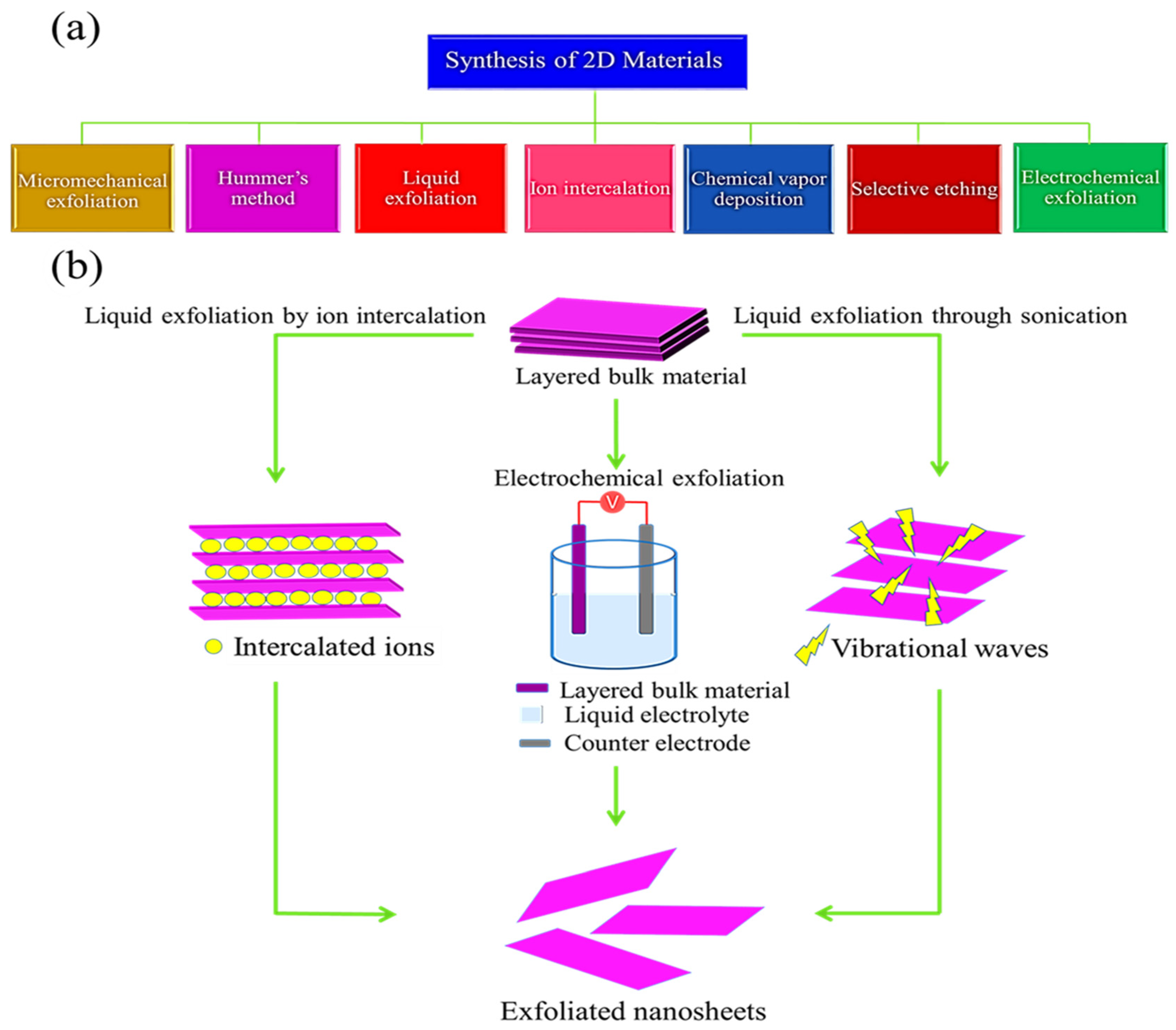
3. Synthesis of Two-Dimensional Materials
3.1. Synthetic Strategies for Graphene: Graphene as a Beginner
3.1.1. Liquid Exfoliation of Graphene
3.1.2. Electrochemical Exfoliation of Graphene
3.2. Synthetic Strategies for TMDCs
3.2.1. Liquid Phase Exfoliation of TMDCs
3.2.2. Electrochemical Exfoliation of MoS2/WS2
4. Applications of MoS2 in Opto-Electric Devices
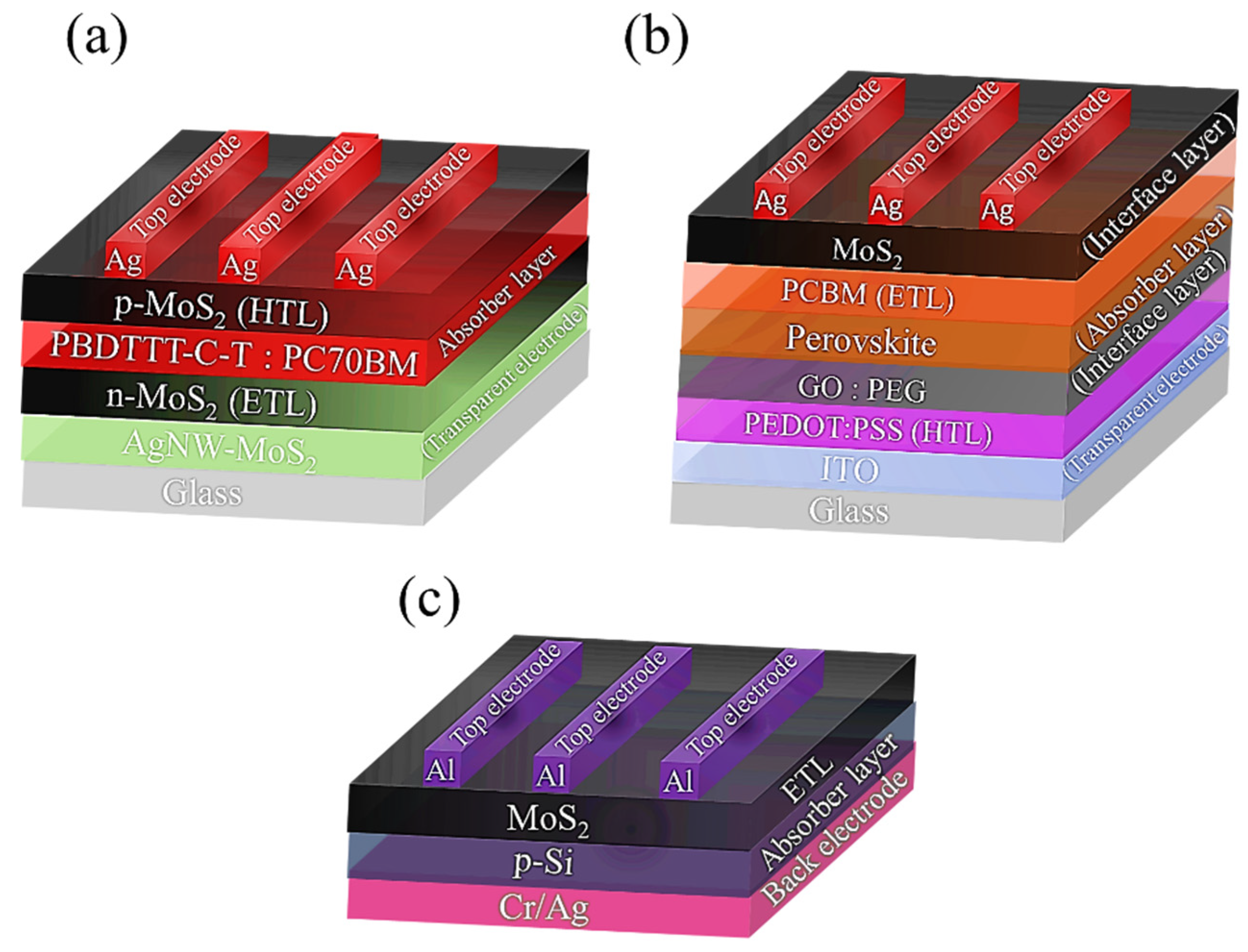
4.1. MoS2 as Absorber Material in Solar Cells
4.2. MoS2 as a Counter Electrode in Dye-Sensitized Solar Cells
4.3. MoS2 as a Diverse Role Material in Organic Solar Cells
4.4. MoS2 Efficient Role in Perovskite Solar Cells
4.5. MoS2 in Silicon-Based Heterojunction Solar Cells
5. Summary and Outlook
Funding
Conflicts of Interest
References
- Geim, A.K.; Novoselov, K.S.; Jiang, D.; Schedin, F.; Booth, T.J.; Khotkevich, V.V.; Morozov, S.V. Two-dimensional atomic crystals. Proc. Natl. Acad. Sci. USA 2005, 102, 10451–10453. [Google Scholar]
- Novoselov, K.S.; Mishchenko, A.; Carvalho, A.; Castro Neto, A.H. 2D materials and van der Waals heterostructures. Science 2016, 353, 6298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butler, S.Z.; Hollen, S.M.; Cao, L.; Cui, Y.; Gupta, J.A.; Gutiérrez, H.R.; Heinz, T.F.; Hong, S.S.; Huang, J.; Ismach, A.F.; et al. Progress, challenges, and opportunities in two-dimensional materials beyond graphene. ACS Nano 2013, 7, 2898–2926. [Google Scholar] [CrossRef] [PubMed]
- Lauhon, L.J.; Marks, T.J.; Hersam, M.C.; Sangwan, V.K.; Jariwala, D. Emerging Device Applications for Semiconducting Two-Dimensional Transition Metal Dichalcogenides. ACS Nano 2014, 8, 1102–1120. [Google Scholar]
- Duan, X.; Wang, C.; Pan, A.; Yu, R.; Duan, X. Two-dimensional transition metal dichalcogenides as atomically thin semiconductors: Opportunities and challenges. Chem. Soc. Rev. 2015, 44, 8859–8876. [Google Scholar] [CrossRef]
- Zhang, H. Introduction: 2D Materials Chemistry. Chem. Rev. 2018, 118, 6089–6090. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Cheng, H.M.; Ye, P. 2D nanomaterials: Beyond graphene and transition metal dichalcogenides. Chem. Soc. Rev. 2018, 47, 6009–6012. [Google Scholar] [CrossRef]
- Li, X.; Wu, X. Low-dimensional boron nitride nanomaterials. J. Univ. Sci. Technol. China 2014, 44, 389–402. [Google Scholar]
- Wang, Z.F.; Liu, F.; Wu, D.; Jin, C.; Li, Y.; Storr, K.; Srivastava, A.; Balicas, L.; Ajayan, P.M.; Ci, L.; et al. Atomic layers of hybridized boron nitride and graphene domains. Nat. Mater. 2010, 9, 430–435. [Google Scholar]
- Vogt, P.; De Padova, P.; Quaresima, C.; Avila, J.; Frantzeskakis, E.; Asensio, M.C.; Resta, A.; Ealet, B.; Le Lay, G. Silicene: Compelling experimental evidence for graphenelike two-dimensional silicon. Phys. Rev. Lett. 2012, 108, 1–5. [Google Scholar] [CrossRef]
- Tan, C.; Cao, X.; Wu, X.J.; He, Q.; Yang, J.; Zhang, X.; Chen, J.; Zhao, W.; Han, S.; Nam, G.H.; et al. Recent Advances in Ultrathin Two-Dimensional Nanomaterials. Chem. Rev. 2017, 117, 6225–6331. [Google Scholar] [CrossRef]
- Choi, W.; Choudhary, N.; Han, G.H.; Park, J.; Akinwande, D.; Lee, Y.H. Recent development of two-dimensional transition metal dichalcogenides and their applications. Mater. Today. 2017, 20, 116–130. [Google Scholar] [CrossRef]
- Colombo, L.; Kim, K.; Gellert, P.R.; Novoselov, K.S.; Fal’ko, V.I.; Schwab, M.G. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar]
- Fivaz, R.; Mooser, E. Mobility of charge carriers in semiconducting layer structures. Phys. Rev. 1967, 163, 743–755. [Google Scholar] [CrossRef]
- Tributsch, H.; Bennett, J.C. Electrochemistry and photochemistry of MoS2 layer crystals. I. J. Electroanal. Chem. 1977, 81, 97–111. [Google Scholar] [CrossRef]
- Wang, H.; Liu, F.; Fu, W.; Fang, Z.; Zhou, W.; Liu, Z. Two-dimensional heterostructures: Fabrication, characterization, and application. Nanoscale 2014, 6, 12250–12272. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Chrisey, D.B.; Mattoussi, H.; Gilmore, C.M.; Horwitz, J.S.; Kim, H.; Kafafi, Z.H.; Piqué, A.; Murata, H. Electrical, optical, and structural properties of indium–tin–oxide thin films for organic light-emitting devices. J. Appl. Phys. 2002, 86, 6451–6461. [Google Scholar]
- Gusakova, J.; Wang, X.; Shiau, L.L.; Krivosheeva, A.; Shaposhnikov, V.; Borisenko, V.; Gusakov, V.; Tay, B.K. Electronic Properties of Bulk and Monolayer TMDs: Theoretical Study Within DFT Framework (GVJ-2e Method). Phys. Status Solidi Appl. Mater. Sci. 2017, 214, 1–7. [Google Scholar] [CrossRef]
- Toth, P.S. From two-dimensional materials to their heterostructures: An electrochemist’s perspective. Appl. Mater. Today 2017, 8, 68–103. [Google Scholar]
- Pumera, M.; Sofer, Z.; Ambrosi, A. Layered transition metal dichalcogenides for electrochemical energy generation and storage. J. Mater. Chem. A 2014, 2, 8981–8987. [Google Scholar] [CrossRef]
- Xiao, J.; Long, M.; Li, X.; Zhang, Q.; Xu, H.; Chan, K.S. Effects of van der Waals interaction and electric field on the electronic structure of bilayer MoS2. J. Phys. Condens. Matter 2014, 26, 405302. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar]
- Podberezskaya, N.V.; Magarill, S.A.; Pervukhina, N.V.; Borisov, S.V. Crystal chemistry of dichalcogenides MX. J. Struct. Chem. 2001, 42, 654–681. [Google Scholar] [CrossRef]
- Li, X.; Zhu, H. Two-dimensional MoS2: Properties, preparation, and applications. J. Mater. 2015, 1, 33–44. [Google Scholar] [CrossRef] [Green Version]
- Mak, K.F.; Lee, C.; Hone, J.; Shan, J.; Heinz, T.F. Atomically thin MoS2: A new direct-gap semiconductor. Phys. Rev. Lett. 2010, 105, 136805. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H. Ultrathin Two-Dimensional Nanomaterials. ACS Nano 2015, 9, 9451–9469. [Google Scholar] [CrossRef]
- Cheng, R.; Jiang, S.; Chen, Y.; Liu, Y.; Weiss, N.; Cheng, H.C.; Wu, H.; Huang, Y.; Duan, X. Few-layer molybdenum disulfide transistors and circuits for high-speed flexible electronics. Nat. Commun. 2014, 5, 1–9. [Google Scholar] [CrossRef]
- Akinwande, D.; Petrone, N.; Hone, J. Two-dimensional flexible nanoelectronics. Nat. Commun. 2014, 5, 1–12. [Google Scholar] [CrossRef]
- Samadi, M.; Sarikhani, N.; Zirak, M.; Zhang, H.; Zhang, H.L.; Moshfegh, A.Z. Group 6 transition metal dichalcogenide nanomaterials: Synthesis, applications and future perspectives. Nanoscale Horiz. 2018, 3, 90–204. [Google Scholar] [CrossRef]
- Fleischauer, P.D.; Lince, J.R.; Bertrand, P.A.; Bauer, R. Electronic Structure and Lubrication Properties of MoS2: A Qualitative Molecular Orbital Approach. Langmuir 1989, 5, 1009–1015. [Google Scholar] [CrossRef]
- Kumara, A.; Ahluwalia, P.K. Electronic structure of transition metal dichalcogenides monolayers 1H-MX2 (M = Mo, W; X = S, Se, Te) from ab-initio theory: New direct band gap semiconductors. Eur. Phys. J. B 2012, 85, 18–22. [Google Scholar] [CrossRef]
- Thanh, T.D.; Chuong, N.D.; Van Hien, H.; Kshetri, T.; Tuan, L.H.; Kim, N.H.; Lee, J.H. Recent advances in two-dimensional transition metal dichalcogenides-graphene heterostructured materials for electrochemical applications. Prog. Mater. Sci. 2018, 96, 51–85. [Google Scholar] [CrossRef]
- Ni, S.Z.; Chen, W. Two-dimensional transition metal dichalcogenides: Interface and defect engineering. Chem Soc Rev Rev. 2018, 47, 3100–3128. [Google Scholar]
- Py, M.A.; Haering, R.R. Structural destabilization induced by lithium intercalation in MoS2 and related compounds. Can. J. Phys. 1983, 61, 76–84. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Dumcenco, D.O.; Huang, Y.-S.; Suenaga, K. Atomic mechanism of the semiconducting-to-metallic phase transition in single-layered MoS2. Nat. Nanotechnol. 2014, 9, 391–396. [Google Scholar]
- Voiry, D.; Salehi, M.; Silva, R.; Fujita, T.; Chen, M.; Asefa, T.; Shenoy, V.B.; Eda, G.; Chhowalla, M. Conducting MoS2; nanosheets as catalysts for hydrogen evolution reaction. Nano Lett. 2013, 13, 6222–6227. [Google Scholar] [CrossRef]
- Al, R.S.; Tatsumi, Y.; Huang, S.; Limg, X.; Dresselhaus, M.S. Raman spectroscopy of transition metal dichalcogenides. J. Phys. Condens. Matter 2016, 28, 353002. [Google Scholar]
- Zhang, S.; Zhang, N.; Zhao, Y.; Cheng, T.; Li, X.; Feng, R.; Xu, H.; Liu, Z.; Zhang, J.; Tong, L. Spotting the differences in two-dimensional materials-the Raman scattering perspective. Chem. Soc. Rev. 2018, 47, 3217–3240. [Google Scholar] [CrossRef]
- Jung, J.; Bark, H.; Byun, D.; Lee, C.; Cho, D.H. Mechanical characterization of phase-changed single-layer MoS 2 sheets. 2D Mater. 2019, 6, 025024. [Google Scholar] [CrossRef]
- Berkdemir, A.; Gutiérrez, H.R.; Botello-Méndez, A.R.; Perea-López, N.; Elías, A.L.; Chia, C.I.; Wang, B.; Crespi, V.H.; López-Urías, F.; Charlier, J.C.; et al. Identification of individual and few layers of WS2 using Raman Spectroscopy. Sci. Rep. 2013, 3, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zhang, Q.; Yap, C.C.R.; Tay, B.K.; Edwin, T.H.T.; Olivier, A.; Baillargeat, D. From bulk to monolayer MoS2: Evolution of Raman scattering. Adv. Funct. Mater. 2012, 22, 1385–1390. [Google Scholar] [CrossRef]
- Han, S.A.; Bhatia, R.; Kim, S.-W. Synthesis, properties and potential applications of two-dimensional transition metal dichalcogenides. Nano Converg. 2015, 2, 17. [Google Scholar] [CrossRef] [Green Version]
- Li, L.-J.; Eda, G.; Zhang, H.; Loh, K.P.; Shin, H.S.; Chhowalla, M. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar]
- Park, S.; Ruoff, R.S. Synthesis and characterization of chemically modified graphenes. Curr. Opin. Colloid Interface Sci. 2015, 20, 322–328. [Google Scholar] [CrossRef]
- Nicolosi, V.; Chhowalla, M.; Kanatzidis, M.G.; Strano, M.S.; Coleman, J.N. Liquid exfoliation of layered materials. Science. 2013, 340, 72–75. [Google Scholar] [CrossRef] [Green Version]
- Cai, Z.; Liu, B.; Zou, X.; Cheng, H. Chemical Vapor Deposition Growth and Applications of Two- Dimensional Materials and Their Heterostructures. Chem. Rev. 2018, 118, 6091–6133. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Li, H.; Wu, J.; Yin, Z.; Zhang, H. Preparation and applications of mechanically exfoliated single-layer and multilayer MoS2 and WSe2 nanosheets. Acc. Chem. Res. 2014, 47, 1067–1075. [Google Scholar] [CrossRef]
- Min Yi, Z.S. A review on mechanical exfoliation for the scalable production of graphene. R. Soc. Chem. 2015, 11700–11715. [Google Scholar]
- Narayan, R.; Kim, S.O. Surfactant mediated liquid phase exfoliation of graphene. Nano Converg. 2015, 2, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dines, M.B. Lithium intercalation via n-Butyllithium of the layered transition metal dichalcogenides. Mater. Res. Bull. 1975, 10, 287–291. [Google Scholar] [CrossRef]
- Parvez, K.; Wu, Z.S.; Li, R.; Liu, X.; Graf, R.; Feng, X.; Müllen, K. Exfoliation of Graphite into Graphene in Aqueous Solutions of Inorganic Salts. J. Am. Chem. Soc. 2014, 136, 6083–6091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, J.; Zhang, H.; Dong, S.; Liu, Y.; Nai, C.T.; Shin, H.S.; Jeong, H.Y. High yield exfoliation of two-dimensional chalcogenides using sodium naphthalenide. Nat. Commun. 2014, 5, 1–7. [Google Scholar] [CrossRef]
- Zeng, Z.; Sun, T.; Zhu, J.; Huang, X.; Yin, Z.; Lu, G.; Fan, Z.; Yan, Q.; Hng, H.H.; Zhang, H. An Effective Method for the Fabrication of Few-Layer-Thick Inorganic Nanosheets. Angew. Chem. Int. Ed. 2012, 51, 9052–9056. [Google Scholar] [CrossRef] [PubMed]
- Khan, U.; May, P.; Neill, A.O.; Bell, A.P.; Boussac, E.; Martin, A.; Coleman, J.N. Polymer reinforcement using liquid-exfoliated boron nitride nanosheets. Nanoscale 2013, 5, 581–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’ko, Y.K.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar]
- Niu, L.; Coleman, J.N.; Zhang, H.; Shin, H.; Chhowalla, M.; Zheng, Z. Production of Two-Dimensional Nanomaterials via Liquid-Based Direct Exfoliation. Small 2016, 12, 272–293. [Google Scholar] [CrossRef]
- Halim, U.; Zheng, C.R.; Chen, Y.; Lin, Z.; Jiang, S.; Cheng, R.; Huang, Y.; Duan, X. A rational design of cosolvent exfoliation of layered materials by directly probing liquid-solid interaction. Nat. Commun. 2013, 4, 1–7. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.B.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon N. Y. 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Lee, Y.; Zhang, X.; Zhang, W.; Chang, M.; Lin, C.; Chang, K.; Yu, Y.; Wang, J.T.; Chang, C.; Li, L.; et al. Synthesis of Large-Area MoS2 Atomic Layers with Chemical Vapor Deposition. Adv. Mater. 2012, 24, 2320–2325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reina, A.; Jia, X.; Ho, J.; Nezich, D.; Son, H.; Bulovic, V.; Dresselhaus, M.S.; Kong, J. Large Area, Few-Layer Graphene Films on Arbitrary Substrates by Chemical Vapor Deposition. Nano Lett. 2009, 9, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Li, J.; Zhang, W.; Chang, H. Synthesis of high quality two-dimensional materials via chemical vapor deposition. Chem. Sci. 2015, 6, 6705–6716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, Q.; Zheng, Y.; Zhang, Y.; Liu, Z. Chemical vapour deposition of group-VIB metal dichalcogenide monolayers: Engineered substrates from amorphous to single crystalline. Chem. Soc. Rev. 2015, 44, 2587–2602. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; et al. Large-area synthesis of high-quality and uniform graphene films on copper foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Yuan, C.; Shen, S.; Yi, X.; Gong, H.; Yang, K.; Liu, Z. Bottom-up Synthesis of Metal-Ion-Doped WS2 Nanoflakes for Cancer Theranostics. ACS Nano 2015, 9, 11090–11101. [Google Scholar] [CrossRef]
- Lhuillier, E.; Pedetti, S.; Ithurria, S.; Nadal, B.; Heuclin, H.; Dubertret, B. Two-Dimensional colloidal metal chalcogenides semiconductors: Synthesis, spectroscopy, and applications. Acc. Chem. Res. 2015, 48, 22–30. [Google Scholar] [CrossRef]
- Gebreegziabher, G.G.; Asemahegne, A.S.; Ayele, D.W.; Dhakshnamoorthy, M.; Kumar, A. One-step synthesis and characterization of reduced graphene oxide using chemical exfoliation method. Mater. Today Chem. 2019, 12, 233–239. [Google Scholar] [CrossRef]
- Son, J.S.; Yu, J.H.; Kwon, S.G.; Lee, J.; Joo, J.; Hyeon, T. Colloidal synthesis of ultrathin two-dimensional semiconductor nanocrystals. Adv. Mater. 2011, 23, 3214–3219. [Google Scholar] [CrossRef]
- Yang, Y.; Hou, H.; Zou, G.; Shi, W.; Shuai, H.; Li, J.; Ji, X. Electrochemical exfoliation of graphene-like two-dimensional nanomaterials. Nanoscale 2019, 11, 16–33. [Google Scholar] [CrossRef]
- Ambrosi, A.; Pumera, M. Electrochemical Exfoliation of MoS2 Crystal for Hydrogen Electrogeneration. Chem. Eur. J. 2018, 24, 18551–18555. [Google Scholar] [CrossRef] [PubMed]
- Schedy, A.; Quarthal, D.; Oetken, M. Graphene—Exciting Insights into the Synthesis and Chemistry of the Miracle Material of the 21st Century and Its Implementation in Chemistry Lessons for the First Time. World J. Chem. Educ. 2018, 6, 43–53. [Google Scholar] [CrossRef]
- Chakrabarti, M.H.; Low, C.T.J.; Brandon, N.P.; Yufit, V.; Hashim, M.A.; Irfan, M.F.; Akhtar, J.; Ruiz-trejo, E.; Hussain, M.A. Electrochimica Acta Progress in the electrochemical modification of graphene-based materials and their applications. Electrochim. Acta 2013, 107, 425–440. [Google Scholar] [CrossRef]
- Schulman, D.S.; Sebastian, A.; Buzzell, D.; Huang, Y.T.; Arnold, A.J.; Das, S. Facile Electrochemical Synthesis of 2D Monolayers for High-Performance Thin-Film Transistors. ACS Appl. Mater. Interfaces 2017, 9, 44617–44624. [Google Scholar] [CrossRef]
- Buzaglo, M.; Bar, I.P.; Varenik, M.; Shunak, L.; Pevzner, S.; Regev, O. Graphite-to-Graphene: Total Conversion. Adv. Mater. 2017, 29, 1–5. [Google Scholar] [CrossRef]
- Naguib, M.; Halim, J.; Lu, J.; Cook, K.M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. New two-dimensional niobium and vanadium carbides as promising materials for li-ion batteries. J. Am. Chem. Soc. 2013, 135, 15966–15969. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [Green Version]
- Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional transition metal carbides. ACS Nano 2012, 6, 1322–1331. [Google Scholar] [CrossRef]
- Thakare, S.R.; Fendarkar, D.A.; Bidkar, C.; Yadav, J.; Gedam, S.D. Chemical Functionalization of Graphene and Graphene Oxide—A Review. Issn 2017, 5, 1–23. [Google Scholar]
- Singh, N.; Kalotra, P.; Srivastava, S.; Sharma, S.S. Comparative Study for the Synthesis of Graphene Oxide from Different Chemical Routes. Adv. Sci. Eng. Med. 2019, 11, 92–94. [Google Scholar] [CrossRef]
- Roberts, E.P.L.; Sharif, F.; Holmes, S.; Perez-page, M. Synthesis of Graphene by Electrochemical Exfoliation of Graphite in Aqueous Solution. ECS Meet. Abstr. 2018, MA2018-02, 546. [Google Scholar]
- Lin, L. Graphene Synthesis via Electrochemical Exfoliation of Graphite Nanoplatelets in Aqueous Sulfuric Acid. In Proceedings of the CARBON 2016, Graphene Characterisation Protocol, State College, PA, USA, 10–15 July 2016. [Google Scholar]
- Gu, X.; Zhao, Y.; Sun, K.; Vieira, C.L.Z.; Jia, Z.; Cui, C.; Wang, Z.; Walsh, A.; Huang, S. Method of ultrasound-assisted liquid-phase exfoliation to prepare graphene. Ultrason. Sonochem. 2019, 58, 104630. [Google Scholar] [CrossRef] [PubMed]
- Spanu, L.; Sorella, S.; Galli, G. Nature and Strength of Interlayer Binding in Graphite. Phys. Rev. Lett. 2009, 103, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alba, A. Enfermedad De Vogt-Koyanagi-Harada. Seminarios de la Fundación Española de Reumatología 1999, 1906, 666–670. [Google Scholar]
- Li, H.; Lu, G.; Wang, Y.; Yin, Z.; Cong, C.; He, Q.; Wang, L. Mechanical Exfoliation and Characterization of Single- and Few-Layer Nanosheets of WSe 2, TaS 2, and TaSe 2. Small 2013, 1974–1981. [Google Scholar] [CrossRef]
- Geim, A.K. Nobel Lecture: Random walk to graphene. Rev. Mod. Phys. 2011, 83, 851–862. [Google Scholar] [CrossRef] [Green Version]
- Dresselhaus, M.S.; Araujo, P.T. Perspectives on the 2010 Nobel Prize in physics for graphene. ACS Nano 2010, 4, 6297–6302. [Google Scholar] [CrossRef]
- Alem, N.; Erni, R.; Kisielowski, C.; Rossell, M.D.; Gannett, W.; Zettl, A. Atomically thin hexagonal boron nitride probed by ultrahigh-resolution transmission electron microscopy. Phys. Rev. B 2009, 80, 155425. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Z.; Wang, Q.; Wang, F.; Yin, L.; Xu, K.; Huang, Y.; He, J. Synthesis, properties and applications of 2D non-graphene materials. Nanotechnology 2015, 26, 292001. [Google Scholar] [CrossRef]
- Huang, Y.; Sutter, E.; Shi, N.N.; Zheng, J.; Yang, T.; Englund, D.; Gao, H.J.; Sutter, P. Reliable Exfoliation of Large-Area High-Quality Flakes of Graphene and Other Two-Dimensional Materials. ACS Nano 2015, 9, 10612–10620. [Google Scholar] [CrossRef]
- Brodie, B.C., XIII. On the atomic weight of graphite. R. Soc. 1859, 149, 249–259. [Google Scholar] [CrossRef] [Green Version]
- Staudenmaier, L. Verfahren zur Darstellung der Graphitsäure. Berichte der Dtsch. Chem. Gesellschaft 1898, 31, 1481–1487. [Google Scholar] [CrossRef] [Green Version]
- Gao, W. Graphite Oxide: Structure, Reduction and Applications; RICE University: Houston, TX, USA, 2012. [Google Scholar]
- Chua, C.K.; Pumera, M. Chemical reduction of graphene oxide: A synthetic chemistry viewpoint. Chem. Soc. Rev. 2014, 43, 291–312. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, U.; König, E. Untersuchungen über Graphitoxyd. Zeitschrift für Anorg. und Allg. Chem. 1937, 234, 311–336. [Google Scholar] [CrossRef]
- Peng, L.; Xu, Z.; Liu, Z.; Wei, Y.; Sun, H.; Li, Z.; Zhao, X.; Gao, C. An iron-based green approach to 1-h production of single-layer graphene oxide. Nat. Commun. 2015, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Blanco, P.; Granda, M.; Blanco, C.; Botas, C.; Patricia, A.; Romasanta, L.J.; Verdejo, R.; Mene, R.; Lo, M.A. Graphene materials with different structures prepared from the same graphite by the Hummers and Brodie methods. Carbon N. Y. 2013, 65, 156–164. [Google Scholar]
- Chen, J.; Yao, B.; Li, C.; Shi, G. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon N. Y. 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Alam, S.N.; Sharma, N.; Kumar, L. Synthesis of Graphene Oxide (GO) by Modified Hummers Method and Its Thermal Reduction to Obtain Reduced Graphene Oxide (rGO)*. Graphene 2017, 6, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Zaaba, N.I.; Foo, K.L.; Hashim, U.; Tan, S.J.; Liu, W.W.; Voon, C.H. Synthesis of Graphene Oxide using Modified Hummers Method: Solvent Influence. Procedia Eng. 2017, 184, 469–477. [Google Scholar] [CrossRef]
- Sohail, M.; Saleem, M.; Ullah, S.; Saeed, N.; Afridi, A.; Khan, M.; Arif, M. Modified and improved Hummer’s synthesis of graphene oxide for capacitors applications. Mod. Electron. Mater. 2017, 3, 110–116. [Google Scholar] [CrossRef]
- Romero, A.; Lavin-Lopez, M.P.; Sanchez-Silva, L.; Valverde, J.L.; Paton-Carrero, A. Comparative study of different scalable routes to synthesize graphene oxide and reduced graphene oxide. Mater. Chem. Phys. 2018, 203, 284–292. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Zhang, B.; Bulin, C.; Li, R.; Xing, R. High-efficient Synthesis of Graphene Oxide Based on Improved Hummers Method. Sci. Rep. 2016, 6, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerrero-Contreras, J.; Caballero-Briones, F. Graphene oxide powders with different oxidation degree, prepared by synthesis variations of the Hummers method. Mater. Chem. Phys. 2015, 153, 209–220. [Google Scholar] [CrossRef]
- Gao, W. The chemistry of graphene oxide. In Graphene Oxide; Springer: Berlin/Heidelberg, Germany, 2015; pp. 61–95. [Google Scholar]
- Fan, X.; Peng, W.; Li, Y.; Li, X.; Wang, S.; Zhang, G.; Zhang, F. Deoxygenation of Exfoliated Graphite Oxide under Alkaline Conditions: A Green Route to Graphene Preparation. Adv. Mater. 2008, 20, 4490–4493. [Google Scholar] [CrossRef]
- Williams, G.; Seger, B.; Kamat, P.V. TiO2-Graphene Nanocomposites. UV-Assisted Photocatalytic Reduction of Graphene Oxide. ACS Nano 2008, 2, 1487–1491. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhou, X.; Zhang, J.; Boey, F.; Zhang, H. Direct electrochemical reduction of single-layer graphene oxide and subsequent functionalization with glucose oxidase. J. Phys. Chem. C 2009, 113, 14071–14075. [Google Scholar] [CrossRef]
- Smith, A.T.; LaChance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Emiru, T.F.; Ayele, D.W. Controlled synthesis, characterization and reduction of graphene oxide: A convenient method for large scale production. Egypt. J. Basic Appl. Sci. 2017, 4, 74–79. [Google Scholar] [CrossRef] [Green Version]
- Ghorbani, M.; Abdizadeh, H.; Golobostanfard, M.R. Reduction of Graphene Oxide via Modified Hydrothermal Method. Procedia Mater. Sci. 2015, 11, 326–330. [Google Scholar] [CrossRef] [Green Version]
- Jung, I.; Dikin, D.A.; Piner, R.D.; Ruoff, R.S. Tunable Electrical Conductivity of Individual Graphene Oxide Sheets Reduced at Low Temperatures. Nano Lett. 2008, 8, 1–5. [Google Scholar] [CrossRef]
- Kim, F.; Cote, L.J.; Huang, J. Graphene oxide: Surface activity and two-dimensional assembly. Adv. Mater. 2010, 22, 1954–1958. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Xie, X.; Yin, K.; Zhou, Y.; Wan, S.; He, L.; Xu, F.; Banhart, F.; Sun, L.; Ruoff, R.S. Spongy graphene as a highly efficient and recyclable sorbent for oils and organic solvents. Adv. Funct. Mater. 2012, 22, 4421–4425. [Google Scholar] [CrossRef]
- Liu, N.; Luo, F.; Wu, H.; Liu, Y.; Zhang, C.; Chen, J. One-Step Ionic-Liquid-Assisted Electrochemical Synthesis of Ionic-Liquid-Functionalized Graphene Sheets Directly from Graphite**. Adv. Funct. Mater. 2008, 18, 1518–1525. [Google Scholar] [CrossRef]
- Lu, J.; Yang, J.; Wang, J.; Lim, A.; Wang, S.; Loh, K.P. One-Pot Synthesis of Fluorescent Carbon Nanoribbons, Nanoparticles, and Graphene by the Exfoliation of Graphite in Ionic Liquids. ACS Nano 2009, 3, 2367–2375. [Google Scholar] [CrossRef]
- You, X.; Chang, J.H.; Ju, B.K.; Pak, J.J. An electrochemical route to graphene oxide. J. Nanosci. Nanotechnol. 2011, 11, 5965–5968. [Google Scholar] [CrossRef]
- Su, C.Y.; Lu, A.Y.; Xu, Y.; Chen, F.R.; Khlobystov, A.N.; Li, L.J. High-quality thin graphene films from fast electrochemical exfoliation. ACS Nano 2011, 5, 2332–2339. [Google Scholar] [CrossRef]
- Tripathi, P.; Patel, C.R.P.; Shaz, M.A.; Srivastava, O.N. Synthesis of High-Quality Graphene through Electrochemical Exfoliation of Graphite in Alkaline Electrolyte. arXiv 2013, arXiv:1310.7371. [Google Scholar]
- Parvez, K.; Li, R.; Puniredd, S.R.; Hernandez, Y.; Hinkel, F.; Wang, S.; Feng, X.; Mu, K.; Engineering, C.; Road, D. Electrochemically Exfoliated Graphene as Solution-Processable, Highly Conductive Electrodes for Organic Electronics. ACS Nano 2013, 7, 3598–3606. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Tang, J.; Cheng, Q.; Xu, G.; Cui, P.; Qin, L.C. Few-layer graphene obtained by electrochemical exfoliation of graphite cathode. Chem. Phys. Lett. 2013, 572, 61–65. [Google Scholar] [CrossRef]
- Kakaei, K.; Hasanpour, K. Synthesis of graphene oxide nanosheets by electrochemical exfoliation of graphite in cetyltrimethylammonium bromide and its application for oxygen reduction. J. Mater. Chem. A 2014, 2, 15428–15436. [Google Scholar] [CrossRef]
- Rao, K.S.; Senthilnathan, J.; Liu, Y.F.; Yoshimura, M. Role of peroxide ions in formation of graphene nanosheets by electrochemical exfoliation of graphite. Sci. Rep. 2014, 4, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wei, C.; Zhu, K.; Zhang, Y.; Gong, C.; Guo, J.; Zhang, J.; Yu, L.; Zhang, J. Preparation of Graphene Sheets by Electrochemical Exfoliation of Graphite in Confined Space and Their Application in Transparent Conductive Films. ACS Appl. Mater. Interfaces 2017, 9, 34456–34466. [Google Scholar] [CrossRef] [PubMed]
- Essig, S.; Allebé, C.; Remo, T.; Geisz, J.F.; Steiner, M.A.; Horowitz, K.; Barraud, L.; Ward, J.S.; Schnabel, M.; Descoeudres, A.; et al. Raising the one-sun conversion efficiency of III-V/Si solar cells to 32.8% for two junctions and 35.9% for three junctions. Nat. Energy 2017, 2, 17144. [Google Scholar] [CrossRef]
- Singh, R.; Charu Tripathi, C. Electrochemical Exfoliation of Graphite into Graphene for Flexible Supercapacitor Application. Mater. Today Proc. 2018, 5, 1125–1130. [Google Scholar] [CrossRef]
- Vartak, R.; Rag, A.; De, S.; Bhat, S. A Facile Synthesis of Graphene Oxide (GO) and Reduced Graphene Oxide (RGO) by Electrochemical Exfoliation of Battery Electrode; Springer: Singapore, 2019; ISBN 9789811316425. [Google Scholar]
- Komba, N.; Wei, Q.; Zhang, G.; Rosei, F.; Sun, S. Controlled synthesis of graphene via electrochemical route and its use as efficient metal-free catalyst for oxygen reduction. Appl. Catal. B Environ. 2019, 243, 373–380. [Google Scholar] [CrossRef]
- He, D.; Marsden, A.J.; Li, Z.; Zhao, R.; Xue, W.; Bissett, M.A. A single step strategy to fabricate graphene fibres via electrochemical exfoliation for micro-supercapacitor applications. Electrochim. Acta 2019, 299, 645–653. [Google Scholar] [CrossRef] [Green Version]
- Altavilla, C.; Sarno, M.; Ciambelli, P. A Novel Wet Chemistry Approach for the Synthesis of Hybrid 2D Free-Floating Single or Multilayer Nanosheets of MS2@oleylamine (M = Mo, W). Chem. Mater. 2011, 23, 3879–3885. [Google Scholar] [CrossRef]
- Benavente, E.; Ana, M.A.; Mendizabal, F.; Gonzalez, G. Intercalation chemistry of molybdenum disulfide. Coord. Chem. Rev. 2002, 224, 87–109. [Google Scholar] [CrossRef]
- Tao, H.; Zhang, Y.; Gao, Y.; Sun, Z.; Yan, C.; Texter, J. Scalable exfoliation and dispersion of two-dimensional materials-an update. Phys. Chem. Chem. Phys. 2017, 19, 921–960. [Google Scholar] [CrossRef]
- Brent, J.R.; Savjani, N.; Brien, P.O. Synthetic approaches to two-dimensional transition metal dichalcogenide nanosheets. Prog. Mater. Sci. 2017, 89, 411–478. [Google Scholar] [CrossRef]
- Li, H.; Yin, Z.; He, Q.; Li, H.; Huang, X.; Lu, G.; Fam, D.W.H.; Tok, A.I.Y.; Zhang, Q.; Zhang, H. Fabrication of single- and multilayer MoS2 film-based field-effect transistors for sensing NO at room temperature. Small 2012, 8, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Bernal, M.M.; Milano, D. Two-Dimensional Nanomaterials via Liquid-Phase Exfoliation: Synthesis, Properties and Applications; One Central Press (OCP): Cheshire, UK, 2014; pp. 159–185. [Google Scholar]
- Sreekumar, K.; Bindhu, B. Aqueous Exfoliation of Molybdenum Disulfide Using Ultrasonication. Mater. Today Proc. 2018, 5, 13152–13156. [Google Scholar] [CrossRef]
- Cai, X.; Luo, Y.; Liu, B.; Cheng, H.M. Preparation of 2D material dispersions and their applications. Chem. Soc. Rev. 2018, 47, 6224–6266. [Google Scholar] [CrossRef]
- De-Mello, G.B.; Smith, L.; Rowley-Neale, S.J.; Gruber, J.; Hutton, S.J.; Banks, C.E. Surfactant-exfoliated 2D molybdenum disulphide (2D-MoS2): The role of surfactant upon the hydrogen evolution reaction. RSC Adv. 2017, 7, 36208–36213. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Ma, Q.; Xuan, Z.; Du, F.; Zhong, Y. Facile surfactant-assisted synthesis of CTAB-incorporated MoS2 ultrathin nanosheets for efficient hydrogen evolution. RSC Adv. 2016, 6, 16730–16735. [Google Scholar] [CrossRef]
- Ciesielski, A.; Samorì, P. Graphene via sonication assisted liquid-phase exfoliation. Chem. Soc. Rev. 2014, 43, 381–398. [Google Scholar] [CrossRef]
- Amiri, A.; Naraghi, M.; Ahmadi, G.; Soleymaniha, M.; Shanbedi, M. A review on liquid-phase exfoliation for scalable production of pure graphene, wrinkled, crumpled and functionalized graphene and challenges. FlatChem 2018, 8, 40–71. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, L.; Zhou, B.; Zhu, Y.; Jiang, X. The pristine graphene produced by liquid exfoliation of graphite in mixed solvent and its application to determination of dopamine. J. Colloid Interface Sci. 2018, 513, 279–286. [Google Scholar] [CrossRef]
- Wu, J.Y.; Lai, Y.C.; Chang, C.L.; Hung, W.C.; Wu, H.M.; Liao, Y.C.; Huang, C.H.; Liu, W.R. Facile and green synthesis of graphene-based conductive adhesives via liquid exfoliation process. Nanomaterials 2019, 9, 38. [Google Scholar] [CrossRef] [Green Version]
- Ye, H.; Lu, T.; Xu, C.; Han, B.; Meng, N.; Xu, L. Liquid-Phase Exfoliation of Hexagonal Boron Nitride into Boron Nitride Nanosheets in Common Organic Solvents with Hyperbranched Polyethylene as Stabilizer. Macromol. Chem. Phys. 2018, 219, 1700482. [Google Scholar] [CrossRef]
- Tian, Z.; Chen, K.; Sun, S.; Zhang, J.; Cui, W.; Xie, Z.; Liu, G. Crystalline boron nitride nanosheets by sonication-assisted hydrothermal exfoliation. J. Adv. Ceram. 2019, 8, 72–78. [Google Scholar] [CrossRef] [Green Version]
- Del Rio Castillo, A.E.; Pellegrini, V.; Sun, H.; Buha, J.; Dinh, D.A.; Lago, E.; Ansaldo, A.; Capasso, A.; Manna, L.; Bonaccorso, F. Exfoliation of Few-Layer Black Phosphorus in Low-Boiling-Point Solvents and Its Application in Li-Ion Batteries. Chem. Mater. 2018, 30, 506–516. [Google Scholar] [CrossRef] [Green Version]
- Jia, C.; Zhao, L.; Cui, M.; Yang, F.; Cheng, G.; Yang, G.; Zeng, Z. Surface coordination modification and electrical properties of few-layer black phosphorus exfoliated by the liquid-phase method. J. Alloys Compd. 2019, 799, 99–107. [Google Scholar] [CrossRef]
- Zhao, G.; Wu, Y.; Shao, Y.; Hao, X. Large-quantity and continuous preparation of two-dimensional nanosheets. Nanoscale 2016, 8, 5407–5411. [Google Scholar] [CrossRef]
- Shen, J.; He, Y.; Wu, J.; Gao, C.; Keyshar, K.; Zhang, X.; Yang, Y.; Ye, M.; Vajtai, R.; Lou, J.; et al. Liquid Phase Exfoliation of Two-Dimensional Materials by Directly Probing and Matching Surface Tension Components. Nano Lett. 2015, 15, 5449–5454. [Google Scholar] [CrossRef]
- Gerchman, D.; Alves, A.K. Solution-processable exfoliation and suspension of atomically thin WSe. J. Colloid Interface Sci. 2016, 468, 247–252. [Google Scholar] [CrossRef]
- Hennrich, F.; Krupke, R.; Arnold, K.; Stu, J.A.R.; Lebedkin, S.; Koch, T.; Schimmel, T.; Kappes, M.M. The Mechanism of Cavitation-Induced Scission of Single-Walled Carbon Nanotubes. J. Phys. Chem 2007, 111, 1932–1937. [Google Scholar] [CrossRef]
- Suslick, K.S.; Hammerton, D.A.; Cline, R.E. The Sonochemical Hot Spot. J. Am. Chem. Soc. 1986, 108, 5641–5642. [Google Scholar] [CrossRef]
- Duclaux, L.; Alvarez, L.; Hawełek, Ł. Cleavage and size reduction of graphite crystal using ultrasound radiation. Carbon N. Y. 2013, 55, 53–61. [Google Scholar]
- Han, J.T.; Jang, J.I.; Kim, H.; Hwang, J.Y.; Yoo, H.K.; Woo, J.S.; Choi, S.; Kim, H.Y.; Jeong, H.J.; Jeong, S.Y.; et al. Extremely Efficient Liquid Exfoliation and Dispersion of Layered Materials by Unusual Acoustic Cavitation. Sci. Rep. 2014, 4, 5133. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.N.; Lotya, M.; O’Neill, A.; Bergin, S.D.; King, P.J.; Khan, U.; Young, K.; Gaucher, A.; De, S.; Smith, R.J. Two-Dimensional Nanosheets Produced by Liquid Exfoliation of Layered Materials. Science 2011, 331, 568–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonaccorso, F.; Bartolotta, A.; Coleman, J.N.; Backes, C.; Vi, I.I.I. 2D-Crystal-Based Functional Inks. Adv. Mater. 2016, 28, 6136–6166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lai, Z.; Tan, C.; Zhang, H. Solution-Processed Two-Dimensional MoS2 Nanosheets: Preparation, Hybridization, and Applications. Angew. Chem. Int. Ed. 2016, 55, 8816–8838. [Google Scholar] [CrossRef]
- Backes, C.; Higgins, T.M.; Kelly, A.; Boland, C.; Harvey, A.; Hanlon, D.; Coleman, J.N. Guidelines for exfoliation, characterization and processing of layered materials produced by liquid exfoliation. Chem. Mater. 2017, 29, 243–255. [Google Scholar] [CrossRef]
- Backes, C.; Hanlon, D.; Szydlowska, B.M.; Harvey, A.; Smith, R.J.; Higgins, T.M.; Coleman, J.N. Preparation of Liquid-exfoliated Transition Metal Dichalcogenide Nanosheets with Controlled Size and Thickness: A State of the Art Protocol. J. Vis. Exp. 2016, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, G.; Lotya, M.; Cucinotta, C.S.; Sanvito, S.; Bergin, S.D.; Menzel, R.; Shaffer, M.S.P.; Coleman, J.N. Solvent exfoliation of transition metal dichalcogenides: Dispersibility of exfoliated nanosheets varies only weakly between compounds. ACS Nano 2012, 6, 3468–3480. [Google Scholar] [CrossRef]
- Huang, J.; Deng, X.; Wan, H.; Chen, F.; Lin, Y.; Xu, X.; Ma, R.; Sasaki, T. Liquid Phase Exfoliation of MoS2 Assisted by Formamide Solvothermal Treatment and Enhanced Electrocatalytic Activity Based on (H3Mo12O40P/MoS2)n Multilayer Structure. ACS Sustain. Chem. Eng. 2018, 6, 5227–5237. [Google Scholar] [CrossRef]
- Rathod, N.; Hatzikiriakos, S.G. The effect of surface energy of boron nitride on polymer processability. Polym. Eng. Sci. 2004, 44, 1543–1550. [Google Scholar] [CrossRef]
- O’Neill, A.; Khan, U.; Nirmalraj, P.N.; Boland, J.; Coleman, J.N. Graphene dispersion and exfoliation in low boiling point solvents. J. Phys. Chem. C 2011, 115, 5422–5428. [Google Scholar] [CrossRef]
- Kim, J.; Kwon, S.; Cho, D.-H.; Kang, B.; Kwon, H.; Kim, Y.; Park, S.O.; Jung, G.Y.; Shin, E.; Kim, W.-G.; et al. Direct exfoliation and dispersion of two-dimensional materials in pure water via temperature control. Nat. Commun. 2015, 6, 8294. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Wei, Q.; Guo, B. A generic solvent exchange method to disperse MoS2 in organic solvents to ease the solution process. Chem. Commun. 2014, 50, 3934–3937. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, R.X.; Quhe, R.; Zhong, H.; Cong, L.; Ye, M.; Ni, Z.; Song, Z.; Yang, J.; Shi, J.; et al. Does p-type ohmic contact exist in WSe2-metal interfaces? Nanoscale 2016, 8, 1179–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Zeng, Z.; Cao, X.; Lu, G.; Wang, L.-H.; Fan, Q.-L.; Huang, W.; Zhang, H. Preparation of MoS2;-polyvinylpyrrolidone nanocomposites for flexible nonvolatile rewritable memory devices with reduced graphene oxide electrodes. Small 2012, 8, 3517–3522. [Google Scholar] [CrossRef]
- May, P.; Khan, U.; Hughes, J.M.; Coleman, J.N. Role of solubility parameters in understanding the steric stabilization of exfoliated two-dimensional nanosheets by adsorbed polymers. J. Phys. Chem. C 2012, 116, 11393–11400. [Google Scholar] [CrossRef]
- Bari, R.; Parviz, D.; Khabaz, F.; Klaassen, C.D.; Metzler, S.D.; Hansen, M.J.; Khare, R.; Green, M.J. Liquid phase exfoliation and crumpling of inorganic nanosheets. Phys. Chem. Chem. Phys. 2015, 17, 9383–9393. [Google Scholar] [CrossRef]
- Smith, R.J.; King, P.J.; Lotya, M.; Wirtz, C.; Khan, U.; De, S.; O’Neill, A.; Duesberg, G.S.; Grunlan, J.C.; Moriarty, G.; et al. Large-scale exfoliation of inorganic layered compounds in aqueous surfactant solutions. Adv. Mater. 2011, 23, 3944–3948. [Google Scholar] [CrossRef]
- Štengl, V.; Tolasz, J.; Popelková, D. Ultrasonic preparation of tungsten disulfide single-layers and quantum dots. RSC Adv. 2015, 5, 89612–89620. [Google Scholar] [CrossRef]
- Biccai, S.; Barwich, S.; Boland, D.; Harvey, A.; Hanlon, D.; McEvoy, N.; Coleman, J.N. Exfoliation of 2D materials by high shear mixing. 2D Mater. 2019, 6, 015008. [Google Scholar] [CrossRef]
- Tian, J.; Guo, L.; Yin, X.; Wu, W. The liquid-phase preparation of graphene by shear exfoliation with graphite oxide as a dispersant. Mater. Chem. Phys. 2019, 223, 1–8. [Google Scholar] [CrossRef]
- Paton, K.R.; Varrla, E.; Backes, C.; Smith, R.J.; Khan, U.; O’Neill, A.; Boland, C.; Lotya, M.; Istrate, O.M.; King, P.; et al. Scalable production of large quantities of defect-free few-layer graphene by shear exfoliation in liquids. Nat. Mater. 2014, 13, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Varrla, E.; Backes, C.; Paton, K.R.; Harvey, A.; Gholamvand, Z.; McCauley, J.; Coleman, J.N. Large-Scale Production of Size-Controlled MoS2 Nanosheets by Shear Exfoliation. Chem. Mater. 2015, 27, 1129–1139. [Google Scholar] [CrossRef]
- Xu, F.; Ge, B.; Chen, J.; Nathan, A.; Xin, L.L.; Ma, H.; Min, H.; Zhu, C.; Xia, W.; Li, Z.; et al. Scalable shear-exfoliation of high-quality phosphorene nanoflakes with reliable electrochemical cycleability in nano batteries. 2D Mater. 2016, 3, 1–12. [Google Scholar] [CrossRef]
- Heising, J.; Kanatzidis, M.G. Exfoliated and Restacked MoS2 and WS2: Ionic or Neutral Species? Encapsulation and Ordering of Hard Electropositive Cations. J. Am. Chem. Soc. 1999, 121, 11720–11732. [Google Scholar] [CrossRef]
- Liang, W.Y. Electronic Properties of Transition Metal Dichalcogenides and Their Intercalation Complexes. In Intercalation in Layered Materials; Springer: Berlin/Heidelberg, Germany, 1986; pp. 31–73. [Google Scholar]
- Koike, Y.; Tanuma, S.I.; Suematsu, H.; Higuchi, K. Superconductivity in the graphite-potassium intercalation compound C8K. Solid State Commun. 1978, 27, 623–627. [Google Scholar] [CrossRef]
- Joensen, P.; Frindt, R.F.; Morrison, S.R. Single-layer MoS. Mater. Res. Bull. 1986, 21, 457–461. [Google Scholar] [CrossRef]
- Eng, A.Y.S.; Ambrosi, A.; Sofer, Z.; Šimek, P.; Pumera, M. Electrochemistry of transition metal dichalcogenides: Strong dependence on the metal-to-chalcogen composition and exfoliation method. ACS Nano 2014, 8, 12185–12198. [Google Scholar] [CrossRef]
- Ambrosi, A.; Sofer, Z.; Pumera, M. Lithium intercalation compound dramatically influences the electrochemical properties of exfoliated MoS. Small 2015, 11, 605–612. [Google Scholar] [CrossRef]
- Murphy, D.W.; Di Salvo, F.J.; Hull, G.W.; Waszczak, J. V Convenient preparation and physical properties of lithium intercalation compounds of Group 4B and 5B layered transition metal dichalcogenides. Inorg. Chem. 1976, 15, 17–21. [Google Scholar] [CrossRef]
- Eda, G.; Fujita, T.; Yamaguchi, H.; Voiry, D.; Chen, M.; Chhowalla, M. Coherent Atomic and Electronic Heterostructures of Single-Layer MoS2. ACS Nano 2012, 6, 7311–7317. [Google Scholar] [CrossRef]
- Fujita, T.; Ito, Y.; Tan, Y.; Yamaguchi, H.; Hojo, D.; Hirata, A.; Voiry, D.; Chhowalla, M.; Chen, M. Chemically exfoliated ReS2 nanosheets. Nanoscale 2014, 6, 12458–12462. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, A.; Sofer, Z.; Pumera, M. 2H-1T phase transition and hydrogen evolution activity of MoS2, MoSe2, WS2 and WSe2 strongly depends on the MX2 composition. Chem. Commun. 2015, 51, 8450–8453. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Xu, P.; Zhou, D.; Sun, Y.; Li, Y.C.; Nguyen, M.A.T.; Terrones, M.; Mallouk, T.E. Fast and Efficient Preparation of Exfoliated 2H MoS2 Nanosheets by Sonication-Assisted Lithium Intercalation and Infrared Laser-Induced 1T to 2H Phase Reversion. Nano Lett. 2015, 15, 5956–5960. [Google Scholar] [CrossRef] [PubMed]
- Lukowski, M.A.; Daniel, A.S.; English, C.R.; Meng, F.; Forticaux, A.; Hamers, R.J.; Jin, S. Highly active hydrogen evolution catalysis from metallic WS2 nanosheets. Energy Environ. Sci. 2014, 7, 2608–2613. [Google Scholar] [CrossRef]
- Ramakrishna Matte, H.S.S.; Gomathi, A.; Manna, A.K.; Late, D.J.; Datta, R.; Pati, S.K.; Rao, C.N.R. MoS2 and WS2 analogues of graphene. Angew. Chem. Int. Ed. 2010, 49, 4059–4062. [Google Scholar] [CrossRef]
- Yuwen, L.; Yu, H.; Yang, X.; Zhou, J.; Zhang, Q.; Zhang, Y.; Luo, Z.; Su, S.; Wang, L. Rapid preparation of single-layer transition metal dichalcogenide nanosheets via ultrasonication enhanced lithium intercalation. Chem. Commun. 2016, 52, 529–532. [Google Scholar] [CrossRef]
- Giacometti, V.; Radisavljevic, B.; Radenovic, A.; Brivio, J.; Kis, A.; Giacometti, V.; Kis, A. Single-layer MoS2 transistors. Nat. Nanotechnol. 2011, 6, 147–150. [Google Scholar]
- Jeong, S.; Yoo, D.; Ahn, M.; Miro, P.; Heine, T.; Cheon, J. Tandem intercalation strategy for single-layer nanosheets as an effective alternative to conventional exfoliation processes. Nat. Commun. 2015, 6, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Cullen, P.L.; Cox, K.M.; Bin Subhan, M.K.; Picco, L.; Payton, O.D.; Buckley, D.J.; Miller, T.S.; Hodge, S.A.; Skipper, N.T.; Tileli, V.; et al. Ionic solutions of two-dimensional materials. Nat. Chem. 2017, 9, 244–249. [Google Scholar] [CrossRef] [Green Version]
- Anto Jeffery, A.; Nethravathi, C.; Rajamathi, M. Two-dimensional nanosheets and layered hybrids of MoS2 and WS2 through exfoliation of ammoniated MS2 (M = Mo, W). J. Phys. Chem. C 2014, 118, 1386–1396. [Google Scholar] [CrossRef]
- Liu, G.; Ma, H.; Teixeira, I.; Sun, Z.; Xia, Q.; Hong, X.; Tsang, S.C.E. Hydrazine-Assisted Liquid Exfoliation of MoS2 for Catalytic Hydrodeoxygenation of 4-Methylphenol. Chem. Eur. J. 2016, 22, 2910–2914. [Google Scholar] [CrossRef]
- Bang, G.S.; Nam, K.W.; Kim, J.Y.; Shin, J.; Choi, J.W.; Choi, S.-Y. Effective liquid-phase exfoliation and sodium ion battery application of MoS2 nanosheets. ACS Appl. Mater. Interfaces 2014, 6, 7084–7089. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Yin, Z.; Huang, X.; Li, H.; He, Q.; Lu, G.; Boey, F.; Zhang, H. Single-layer semiconducting nanosheets: High-yield preparation and device fabrication. Angew. Chem. Int. Ed. 2011, 50, 11093–11097. [Google Scholar] [CrossRef] [PubMed]
- You, X.; Liu, N.; Lee, C.J.; Jungho, J. An electrochemical route to MoS 2 nanosheets for device applications. Mater. Lett. 2014, 121, 31–35. [Google Scholar] [CrossRef]
- Liu, N.; Kim, P.; Kim, J.H.; Ye, J.H.; Kim, S.; Lee, C.J. Large-area atomically thin MoS2 nanosheets prepared using electrochemical exfoliation. ACS Nano 2014, 8, 6902–6910. [Google Scholar] [CrossRef]
- Ambrosi, A.; Pumera, M. Exfoliation of layered materials using electrochemistry. Chem. Soc. Rev. 2018, 47, 7213–7224. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Liu, Y.; Halim, U.; Ding, M.; Liu, Y.; Wang, Y.; Jia, C.; Chen, P.; Duan, X.; Wang, C.; et al. Solution-processable 2D semiconductors for high-performance large-area electronics. Nature 2018, 562, 254–258. [Google Scholar] [CrossRef]
- Li, F.; Xue, M.; Zhang, X.; Chen, L.; Knowles, G.P.; MacFarlane, D.R.; Zhang, J. Advanced Composite 2D Energy Materials by Simultaneous Anodic and Cathodic Exfoliation. Adv. Energy Mater. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Das, S.; Pandey, D.; Thomas, J.; Roy, T. The Role of Graphene and Other 2D Materials in Solar Photovoltaics. Adv. Mater. 2019, 31, 1–35. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Sivula, K. Toward Large-Area Solar Energy Conversion with Semiconducting 2D Transition Metal Dichalcogenides. ACS Energy Lett. 2016, 1, 315–322. [Google Scholar] [CrossRef]
- Khan, M.A.; Leuenberger, M.N. Optoelectronics with single layer group-VIB transition metal dichalcogenides. Nanophotonics 2018, 7, 1589–1600. [Google Scholar] [CrossRef]
- Akama, T.; Okita, W.; Nagai, R.; Li, C.; Kaneko, T.; Kato, T. Schottky solar cell using few-layered transition metal dichalcogenides toward large-scale fabrication of semitransparent and flexible power generator. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Singh, E.; Kim, K.S.; Yeom, G.Y.; Nalwa, H.S. Atomically thin-layered molybdenum disulfide (MoS2) for bulk-heterojunction solar cells. ACS Appl. Mater. Interfaces 2017, 9, 3223–3245. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Rassay, S.S.; Ravindra, N.M. Electronic & Optical properties of Transition-Metal Dichalcogenides. Madr. J. Nanotechnol. Nanosci. 2017, 2, 58–64. [Google Scholar]
- Bernardi, M.; Palummo, M.; Grossman, J.C. Extraordinary sunlight absorption and one nanometer thick photovoltaics using two-dimensional monolayer materials. Nano Lett. 2013, 13, 3664–3670. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.Y.; Li, G.R.; Song, J.; Gao, X.P. Nickel phosphide-embedded graphene as counter electrode for dye-sensitized solar cells. Phys. Chem. Chem. Phys. 2012, 14, 1339–1342. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Jae-Yup, K.; Koo, B.; Son, H.; Kim, D.; Ko, M. Rapid sintering of MoS2 counter electrode using near-infrared pulsed laser for use in highly efficient dye-sensitized solar cells. J. Power Sources 2016, 330, 104–110. [Google Scholar] [CrossRef]
- Antonelou, A.; Syrrokostas, G.; Sygellou, L.; Leftheriotis, G.; Dracopoulos, V.; Yannopoulos, S. Facile, substrate-scale growth of mono- and few-layer homogeneous MoS2 films on Mo foils with enhanced catalytic activity as counter electrodes in DSSCs. Nanotechnology 2015, 27, 45404. [Google Scholar] [CrossRef]
- Cheng, C.K.; Hsieh, C.K. Electrochemical deposition of molybdenum sulfide thin films on conductive plastic substrates as platinum-free flexible counter electrodes for dye-sensitized solar cells. Thin Solid Films 2015, 584, 52–60. [Google Scholar] [CrossRef]
- Lei, B.; Li, G.R.; Gao, X.P. Morphology dependence of molybdenum disulfide transparent counter electrode in dye-sensitized solar cells. J. Mater. Chem. A 2014, 2, 3919–3925. [Google Scholar] [CrossRef]
- Lin, J.-Y.; Yue, G.; Tai, S.-Y.; Xiao, Y.; Cheng, H.-M.; Wang, F.-M.; Wu, J. Hydrothermal synthesis of graphene flake embedded nanosheet-like molybdenum sulfide hybrids as counter electrode catalysts for dye-sensitized solar cells. Mater. Chem. Phys. 2013, 143, 53–59. [Google Scholar] [CrossRef]
- Liu, C.-J.; Tai, S.-Y.; Chou, S.-W.; Yu, Y.-C.; Chang, K.-D.; Wang, S.; Chien, F.S.-S.; Lin, J.-Y.; Lin, T.-W. Facile synthesis of MoS2/graphene nanocomposite with high catalytic activity toward triiodide reduction in dye-sensitized solar cells. J. Mater. Chem. 2012, 22, 21057–21064. [Google Scholar] [CrossRef]
- Balis, N.; Stratakis, E.; Kymakis, E. Graphene and transition metal dichalcogenide nanosheets as charge transport layers for solution processed solar cells. Biochem. Pharmacol. 2016, 19, 580–594. [Google Scholar] [CrossRef]
- Kakavelakis, G.; Gouda, L.; Tischler, Y.; Kaliakatsos, I.; Petridis, K. 2D Transition Metal Dichalcogenides for Solution-Processed Organic and Perovskite Solar Cells; Springer: Singapore, 2019; ISBN 9789811390456. [Google Scholar]
- Gu, X.; Cui, W.; Li, H.; Wu, Z.; Zeng, Z.; Lee, S.T.; Zhang, H.; Sun, B. A solution-processed hole extraction layer made from ultrathin MoS2 nanosheets for efficient organic solar cells. Adv. Energy Mater. 2013, 3, 1262–1268. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W.; Wu, Y.; Min, C.; Fang, J. Solution-Processed MoSx as an Efficient Anode Buffer Layer in Organic Solar Cells. ACS Appl. Mater. Interfaces 2013, 5, 8823–8827. [Google Scholar] [CrossRef]
- Ramasamy, M.S.; Ryu, K.Y.; Lim, J.W.; Bibi, A.; Kwon, H.; Lee, J.E.; Kim, D.H.; Kim, K. Solution-processed PEDOT: PSS/MoS2 nanocomposites as efficient hole-transporting layers for organic solar cells. Nanomaterials 2019, 9, 1328. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Fu, W.; Liu, W.; Hong, J.; Cai, Y.; Jin, C.; Xu, M.; Wang, H.; Yang, D.; Chen, H. Engineering crystalline structures of two-dimensional MoS2 sheets for high-performance organic solar cells. J. Mater. Chem. A 2014, 2, 7727–7733. [Google Scholar] [CrossRef]
- Yun, J.-M.; Noh, Y.-J.; Yeo, J.-S.; Go, Y.-J.; Na, S.-I.; Jeong, H.-G.; Kim, J.; Lee, S.; Kim, S.-S.; Koo, H.Y.; et al. Efficient work-function engineering of solution-processed MoS2 thin-films for novel hole and electron transport layers leading to high-performance polymer solar cells. J. Mater. Chem. C 2013, 1, 3777–3783. [Google Scholar] [CrossRef]
- Hu, X.; Chen, L.; Tan, L.; Zhang, Y.; Hu, L.; Xie, B.; Chen, Y. Versatile MoS2 Nanosheets in ITO-Free and Semi-transparent Polymer Power-generating Glass. Sci. Rep. 2015, 5, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Qin, P.; Fang, G.; Ke, W.; Cheng, F.; Zheng, Q.; Wan, J.; Lei, H.; Zhao, X. In situ growth of double-layer MoO3/MoS2 film from MoS2 for hole-transport layers in organic solar cell. J. Mater. Chem. A 2014, 2, 2742–2756. [Google Scholar] [CrossRef]
- Yang, X.; Liu, W.; Xiong, M.; Zhang, Y.; Liang, T.; Yang, J.; Xu, M.; Ye, J.; Chen, H. Au nanoparticles on ultrathin MoS2 sheets for plasmonic organic solar cells. J. Mater. Chem. A 2014, 2, 14798–14806. [Google Scholar] [CrossRef]
- Chuang, M.K.; Yang, S.S.; Chen, F.C. Metal nanoparticle-decorated two-dimensional molybdenum sulfide for plasmonic-enhanced polymer photovoltaic devices. Materials (Basel) 2015, 8, 5414–5425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Wang, S.; Chen, X.; Li, Z.; Wang, J.; Li, T.; Deng, X. Largely enhanced VOC and stability in perovskite solar cells with modified energy match by coupled 2D interlayers. J. Mater. Chem. A 2018, 6, 4860–4867. [Google Scholar] [CrossRef]
- Tsai, M.L.; Su, S.H.; Chang, J.K.; Tsai, D.S.; Chen, C.H.; Wu, C.I.; Li, L.J.; Chen, L.J.; He, J.H. Monolayer MoS2 heterojunction solar cells. ACS Nano 2014, 8, 8317–8322. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Zhou, T.; Tian, Y.; Zhu, X.; Tu, Y. Perovskite-Based Solar Cells: Materials, Methods, and Future Perspectives. J. Nanomater. 2018, 2018, 8148072. [Google Scholar] [CrossRef]
- Zhou, P.; Bu, T.; Shi, S.; Li, L.; Zhang, Y.; Ku, Z.; Peng, Y.; Zhong, J.; Cheng, Y.-B.; Huang, F. Efficient and stable mixed perovskite solar cells using P3HT as a hole transporting layer. J. Mater. Chem. C 2018, 6, 5733–5737. [Google Scholar] [CrossRef]
- Yang, Q.-D.; Li, J.; Cheng, Y.; Li, H.-W.; Guan, Z.; Yu, B.; Tsang, S.-W. Graphene oxide as an efficient hole-transporting material for high-performance perovskite solar cells with enhanced stability. J. Mater. Chem. A 2017, 5, 9852–9858. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, C.; Tong, S.; Xia, H.; Wang, L.; Xie, H.; Gao, Y.; Yang, J. Energy level and thickness control on PEDOT:PSS layer for efficient planar heterojunction perovskite cells. J. Phys. D Appl. Phys. 2018, 51, 025110. [Google Scholar] [CrossRef]
- Sahoo, S.; Tiwari, S.K.; Nayak, G.C. Surface Engineering of Graphene; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Palma, A.L.; Cinà, L.; Pescetelli, S.; Agresti, A.; Raggio, M.; Paolesse, R.; Bonaccorso, F.; Di Carlo, A. Reduced graphene oxide as efficient and stable hole transporting material in mesoscopic perovskite solar cells. Nano Energy 2016, 22, 349–360. [Google Scholar] [CrossRef]
- Uthayaraj, S.; Karunarathne, D.G.B.C.; Kumara, G.R.A. Powder Pressed Cuprous Iodide (CuI) as A Hole Transporting Material for Perovskite Solar Cells. Materials (Basel) 2019, 12, 2037. [Google Scholar] [CrossRef] [Green Version]
- Dasgupta, U.; Chatterjee, S.; Pal, A.J. Thin-film formation of 2D MoS2 and its application as a hole-transport layer in planar perovskite solar cells. Sol. Energy Mater. Sol. Cells 2017, 172, 353–360. [Google Scholar] [CrossRef]
- Kim, Y.G.; Kwon, K.C.; Van Le, Q.; Hong, K.; Jang, H.W.; Kim, S.Y. Atomically thin two-dimensional materials as hole extraction layers in organolead halide perovskite photovoltaic cells. J. Power Sources 2016, 319, 1–8. [Google Scholar] [CrossRef]
- Capasso, A.; Del Rio Castillo, A.E.; Najafi, L.; Pellegrini, V.; Bonaccorso, F.; Matteocci, F.; Cina, L.; Di Carlo, A. Spray deposition of exfoliated MoS2 flakes as hole transport layer in perovskite-based photovoltaics. In Proceedings of the IEEE-NANO 2015—15th International Conference on Nanotechnology; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2015; pp. 1138–1141. [Google Scholar]
- Capasso, A.; Matteocci, F.; Najafi, L.; Prato, M.; Buha, J.; Cinà, L.; Pellegrini, V.; Di Carlo, A.; Bonaccorso, F. Few-Layer MoS2 Flakes as Active Buffer Layer for Stable Perovskite Solar Cells. Adv. Energy Mater. 2016, 6, 1600920. [Google Scholar] [CrossRef]
- Huang, P.; Wang, Z.; Liu, Y.; Zhang, K.; Yuan, L.; Zhou, Y.; Song, B.; Li, Y. Water-Soluble 2D Transition Metal Dichalcogenides as the Hole-Transport Layer for Highly Efficient and Stable p–i–n Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 25323–25331. [Google Scholar] [CrossRef] [PubMed]
- Kakavelakis, G.; Paradisanos, I.; Paci, B.; Generosi, A.; Papachatzakis, M.; Maksudov, T.; Najafi, L.; Del Rio Castillo, A.E.; Kioseoglou, G.; Stratakis, E.; et al. Extending the Continuous Operating Lifetime of Perovskite Solar Cells with a Molybdenum Disulfide Hole Extraction Interlayer. Adv. Energy Mater. 2018, 8, 1702287. [Google Scholar] [CrossRef]
- Kohnehpoushi, S.; Nazari, P.; Nejand, B.A.; Eskandari, M. MoS2: A two-dimensional hole-transporting material for high-efficiency, low-cost perovskite solar cells. Nanotechnology 2018, 29, 205201. [Google Scholar] [CrossRef]
- Narayanasamy Sabari Arul, V.D.N. Two Dimensional Transition Metal Dichalcogenides: Synthesis, Properties and Applications; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Mak, K.F.; Shan, J. Photonics and optoelectronics of 2D semiconductor transition metal dichalcogenides. Nat. Photonics 2016, 10, 216–226. [Google Scholar] [CrossRef]
- Yu, W.J.; Vu, Q.A.; Oh, H.; Nam, H.G.; Zhou, H.; Cha, S.; Kim, J.Y.; Carvalho, A.; Jeong, M.; Choi, H.; et al. Unusually efficient photocurrent extraction in monolayer van der Waals heterostructure by tunnelling through discretized barriers. Nat. Commun. 2016, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Y.; Shin, J.H.; Lee, G.H.; Lee, C.H. Two-dimensional semiconductor optoelectronics based on van der Waals heterostructures. Nanomaterials 2016, 6, 193. [Google Scholar] [CrossRef] [Green Version]
- Pradhan, N.R.; Talapatra, S.; Terrones, M.; Ajayan, P.M.; Balicas, L. Optoelectronic Properties of Heterostructures: The Most Recent Developments Based on Graphene and Transition-Metal Dichalcogenides. IEEE Nanotechnol. Mag. 2017, 11, 18–32. [Google Scholar] [CrossRef]
- Wirth-Lima, A.J.; Alves-Sousa, P.P.; Bezerra-Fraga, W. Graphene/silicon and 2D-MoS2/silicon solar cells: A review. Appl. Phys. A Mater. Sci. Process. 2019, 125, 241. [Google Scholar] [CrossRef]
- Ma, J.; Bai, H.; Zhao, W.; Yuan, Y.; Zhang, K. High efficiency graphene/MoS2/Si Schottky barrier solar cells using layer-controlled MoS2 films. Sol. Energy 2018, 160, 76–84. [Google Scholar] [CrossRef]
- Hao, L.; Liu, Y.; Gao, W.; Han, Z.; Xue, Q.; Zeng, H.; Wu, Z.; Zhu, J.; Zhang, W. Electrical and photovoltaic characteristics of MoS2/Si p-n junctions. J. Appl. Phys. 2015, 117, 114502. [Google Scholar] [CrossRef]
- Hasani, A.; Van Le, Q.; Tekalgne, M.; Choi, M.J.; Lee, T.H.; Kim, S.Y.; Jang, H.W. Direct synthesis of two-dimensional MoS2 on p-type Si and application to solar hydrogen production. NPG Asia Mater. 2019, 11, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Jiao, K.; Duan, C.; Wu, X.; Chen, J.; Wang, Y.; Chen, Y. The role of MoS2 as an interfacial layer in graphene/silicon solar cells. Phys. Chem. Chem. Phys. 2015, 17, 8182–8186. [Google Scholar] [CrossRef]
- Hao, L.Z.; Gao, W.; Liu, Y.J.; Han, Z.D.; Xue, Q.Z.; Guo, W.Y.; Zhu, J.; Li, Y.R. High-performance n-MoS2/i-SiO2/p-Si heterojunction solar cells. Nanoscale 2015, 7, 8304–8308. [Google Scholar] [CrossRef]
- Tsuboi, Y.; Wang, F.; Kozawa, D.; Funahashi, K.; Mouri, S.; Miyauchi, Y.; Takenobu, T.; Matsuda, K. Enhanced photovoltaic performances of graphene/Si solar cells by insertion of a MoS2 thin film. Nanoscale 2015, 7, 14476–14482. [Google Scholar] [CrossRef] [Green Version]
- Furchi, M.M.; Pospischil, A.; Libisch, F.; Burgdörfer, J.; Mueller, T. Photovoltaic Effect in an Electrically Tunable van der Waals Heterojunction. Nano Lett. 2014, 14, 4785–4791. [Google Scholar] [CrossRef]
- Zan, R.; Ramasse, Q.M.; Jalil, R.; Tu, J.S.; Bangert, U.; Novoselov, K.S. Imaging Two Dimensional Materials and their Heterostructures. J. Phys. Conf. Ser. 2017, 902, 012028. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Choi, D.; Woo, W.J.; Lee, J.B.; Ryu, G.H.; Lim, J.H.; Lee, S.; Lee, Z.; Im, S.; Ahn, J.H.; et al. Synthesis of two-dimensional MoS2/graphene heterostructure by atomic layer deposition using MoF6 precursor. Appl. Surf. Sci. 2019, 494, 591–599. [Google Scholar] [CrossRef]
- Lee, J.B.; Lim, Y.R.; Katiyar, A.K.; Song, W.; Lim, J.; Bae, S.; Kim, T.W.; Lee, S.K.; Ahn, J.H. Direct Synthesis of a Self-Assembled WSe2/MoS2 Heterostructure Array and its Optoelectrical Properties. Adv. Mater. 2019, 31, 1–9. [Google Scholar]
- Yang, W.; Kawai, H.; Bosman, M.; Tang, B.; Chai, J.; Le Tay, W.; Yang, J.; Seng, H.L.; Zhu, H.; Gong, H.; et al. Interlayer interactions in 2D WS2/MoS2 heterostructures monolithically grown by: In situ physical vapor deposition. Nanoscale 2018, 10, 22927–22936. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Ma, C.; Huang, Y.; Yang, G. Tunable Control of Interlayer Excitons in WS2/MoS2 Heterostructures via Strong Coupling with Enhanced Mie Resonances. Adv. Sci. 2019, 6, 1802092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehman, A.U.; Khan, M.F.; Shehzad, M.A.; Hussain, S.; Bhopal, M.F.; Lee, S.H.; Eom, J.; Seo, Y.; Jung, J.; Lee, S.H. N-MoS2/p-Si Solar Cells with Al2O3 Passivation for Enhanced Photogeneration. ACS Appl. Mater. Interfaces 2016, 8, 29383–29390. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Xin, L.; Liu, L.; Pang, D.; Jiao, Y.; Cong, R.; Yu, W. Large area MoS2/Si heterojunction-based solar cell through sol-gel method. Mater. Lett. 2019, 238, 13–16. [Google Scholar] [CrossRef]
- bin Mohd Yusoff, A.R.; Kim, D.; Schneider, F.K.; da Silva, W.J.; Jang, J. Au-doped single layer graphene nanoribbons for a record-high efficiency ITO-free tandem polymer solar cell. Energy Environ. Sci. 2015, 8, 1523–1537. [Google Scholar] [CrossRef]
- Saraswat, K.C.; Park, J.H.; Islam, R.; Nazif, K.N.; Elder, E.; Associate, L. Low Cost Silicon (Si)/Transition Metal Dichalcogenides (TMDs) Tandem Solar Cells with goal of >30% Efficiency; Stanford Univ.: Stanford, CA, USA, 2019; Volume S18-187, pp. 1–2. [Google Scholar]


| Synthesis Strategy | Title of Paper | Specifications | Year of Publication | Author + Reference |
|---|---|---|---|---|
| Electrochemical exfoliation | One-step ionic-liquid-assisted electrochemical synthesis of ionic-liquid-functionalized graphene sheets directly from graphite | 1-octyl-3-methyl-imidazolium hexafluoro-phosphate (8mim + PF6) (ionic liquid IL) along with water used as an electrolyte. Two graphite rods were used as electrodes. Obtained graphene nanosheets (GNs) did not disperse in water but polar aprotic solvents. | 2008 | B.N. Liu et al. [117] |
| Electrochemical exfoliation | One-pot synthesis of fluorescent carbon nano-ribbons, nano-particles, and graphene by the ex-foliation of graphite in ionic liquids | Synthesis of graphene sheets in a single vessel using graphite rod and platinum wire as electrodes and 1-methyl-3-butylimidazolium tetrafluoro-borate (or 1-methyl-3-butylimidazolium chloride) as an electrolyte in combination with water in varying ratios. | 2009 | Lu et al. [118] |
| Electrochemical exfoliation | An electro-chemical route to graphene oxide | Expanded graphite used as anode and Pt wire as a counter electrode along with KCl as an electrolyte. 1.9, 2.8, and 3.9 nm were the thicknesses of mono, bi, and tri-layers of graphene, respectively. | 2011 | You et al. [119] |
| Electrochemical exfoliation | High-quality thin graphene films from fast electrochemical exfoliation | Highly oriented pyrolytic graphite (HOPG) used as a graphene source, grounded Pt wire used as a counter electrode, and H2SO4 used as the electrolyte. The lateral size of the exfoliated graphene sheet was 30 µm. The transparent conducting film, containing these exfoliated graphene sheets, was good in conductivity but the yield was low. | 2011 | Su et al. [120] |
| Electrochemical exfoliation | Synthesis of high-quality graphene through electro-chemical exfoliation of graphite in alkaline electrolyte | Graphite rod and platinum wire were used as electrodes along with alkaline electrolyte (KOH) in the electrochemical setup. Graphene is obtained with fewer defects, good quality, 1–4 layers, and with lateral size up to ~80 µm. | 2013 | Tripathi et al. [121] |
| Electrochemical exfoliation | Electrochemically exfoliated graphene as solution-processable, highly conductive electrodes for organic electronics | H2SO4, graphite flakes, and platinum wire were used as electrolyte, anode, and cathode respectively. The thickness of graphene flakes was ~1.5 nm for bilayer and size of exfoliated graphene sheets was up to 5–10 µm. | 2013 | Parvez et al. [122] |
| Electrochemical exfoliation | Few-layer graphene obtained by electrochemical exfoliation of graphite cathode | A system of dimethyl sulfoxide (DMSO), NaCl, water, and thionin acetate was used as an electrolyte for exfoliation of graphite cathode. Acquired graphene sheets contained less degree of oxidation as well as defect sites. | 2013 | Zhou et al. [123] |
| Electrochemical exfoliation | Synthesis of graphene oxide nano-sheets by electrochemical exfoliation of graphite in cetyl-trimethylammonium bromide and its application for oxygen reduction | Cetyltrimethylammonium bromide (CTAB) used as electrolyte where-as graphite rod and Pt wire was used as electrodes. GO/CTAB suspension is tremendously stable in ambient conditions. The single-layer thickness of the obtained graphene is 2.5–4.5 nm. | 2014 | Kakaei and Hasanpour [124] |
| Electrochemical exfoliation | Role of peroxide ions in formation of graphene nanosheets by electrochemical exfoliation of graphite | A network of NaOH/H2O2/H2O was used to exfoliate graphite. High-quality graphene sheets with 3–6 layers are formed exhibiting a thickness of ~1–2 nm with a 95% yield. | 2014 | Rao et al. [125] |
| Electrochemical exfoliation | Exfoliation of graphite into graphene in aqueous solutions of inorganic salts | Inorganic salts such as (NH4)2SO4, K2SO4, and Na2SO4 used as electrolytes for the synthesis of graphene sheets. Graphene sheets showed vast lateral size, low oxidation level, and elevated yield of 85%. | 2014 | Parvez et al. [53] |
| Electrochemical exfoliation | Graphene synthesis via electrochemical exfoliation of graphite nanoplatelets in aqueous sulfuric acid | Utilized pressed-graphene nanoparticles (GNP) used as anode and Pt wire as a cathode with 0.1M sulphuric acid as an electrolyte. Flakes size is more manageable | 2016 | Lin Li et al. [82] |
| Electrochemical exfoliation | Preparation of graphene sheets by electrochemical exfoliation of graphite in confined space and their application in transparent conductive films | In EECS (electrochemical exfoliation in confined space) nickel foam is used as a counter electrode while the working electrode is graphite rod covered with paraffin, with bottom opening, in an alkaline electrolyte (10 mol/L NaOH). Paraffin helps to control the extravagant exfoliation of graphene. Graphene rooted conductive sheets can replace indium tin oxide. | 2017 | Hui Wang et al. [126] |
| Electrochemical exfoliation | Electrochemical exfoliation synthesis of graphene | Graphite rod and Pt wire placed vertically in the electrochemical cell along with protonic acid as an electrolyte. In the vertical configuration, exfoliation is much better organized and graphene synthesized with better quality as well as quantity. | 2017 | J. Liu and Theses [127] |
| Electrochemical exfoliation | Electrochemical exfoliation of graphite into graphene for flexible supercapacitor application | Graphite rod and Pt used as electrodes, 0.1M potassium sulfate used as the electrolyte. The size of graphene nanosheets is in between of 433 nm to 5.07 µm. | 2018 | Singh and Tripathi. [128] |
| Electrochemical Exfoliation | A facile synthesis of graphene oxide (GO) and reduced graphene oxide (rGO) by electro-chemical exfoliation of battery electrode | GO being synthesized by using ionic specie as an electrolyte, graphite rods as electrodes, and regulated DC power supply. GO being reduced to rGO using ascorbic acid, i.e., environment-tally benign reducing agent. | 2019 | Vartak et al. [129] |
| Electrochemical exfoliation | Controlled synthesis of graphene via electrochemical route and its use as efficient metal-free catalyst for oxygen reduction | (NH4)2SO4 used as electrolyte whereas Pt and graphite substrate served as the two electrodes in electrochemical setup. High standard graphene with governable layer number was fabricated using cost-effective precursors. | 2019 | Komba et al. [130] |
| Electrochemical exfoliation | A single-step strategy to fabricate graphene fibers via electrochemical exfoliation for micro-super capacitor applications | The simplistic, economical top-down synthesis method is used to fabricate porous, fiber-like graphene. Graphene fibers showed high specific capacitance, high electrochemical performance because of porous structure and fine electronic conductivity. | 2019 | He et al. [131] |
| Synthesis Strategy | Title of Paper | Specifications | Year of Publication | Author + Reference |
|---|---|---|---|---|
| Electrochemical exfoliation | Single-layer semiconducting nanosheets: high-yield preparation and device fabrication | The multi-layered bulk material (MoS2, WS2, Graphene, etc.) was used as a cathode in the electrochemical cell while lithium foil being used as an anode. LiPF6 used as an electrolyte. Monolayer yield is 92% in this method for MoS2. | 2011 | Zeng et al. [199] |
| Electrochemical exfoliation | Large-area atomically thin mos2 nanosheets prepared using electrochemical exfoliation | Na2SO4 being used as an electrolyte along with a pure single crystal of MoS2 as a working electrode and Pt wire as a counter electrode. This synthesis method ended up giving the yield of MoS2 monolayers up to 5–9% with 50 µm lateral size of sheets, little oxidation and acceptable quality. | 2014 | Liu et al. [201] |
| Electrochemical exfoliation | Electrochemical exfoliation of mos2 electrogeneration crystal for hydrogen | Pt foil and MoS2 crystal being used as electrodes in electrochemical setup including K2SO4 as the electrolyte. The obtained lateral size of MoS2 nanosheets was 100–200 nm with 15–20 layers. | 2018 | Ambrosi & Pumera [71] |
| Electrochemical exfoliation | Solution-processable 2d semiconductors for high-performance large-area electronics | In electrochemical setup, MoS2 crystal being intercalated by THAB (tetraheptylammonium bromide). After intercalation, the enlarged MoS2 crystal was sonicated in PVP/DMF (poly-vinylpyrrolidone solution in dimethylformamide). PVP provided stability to the monolayers of MoS2 from stacking back. Obtained nanosheets have had a thickness of 3.8 nm with a lateral size of 0.5–2 µm. | 2018 | Lin et al. [203] |
| Electrochemical exfoliation | Advanced composite 2d energy materials by simultaneous anodic and cathodic exfoliation | Single MoS2 crystal sank in TBA+ in electrochemical setup. Firstly, the bulk crystal was immersed in acetonitrile but later acetonitrile replaced by TBA+ for better expansion. Cathodic exfoliation gave MoS2 monolayers with great crystallinity and no defects were observed as well. | 2018 | Li et al. [204] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nawz, T.; Safdar, A.; Hussain, M.; Sung Lee, D.; Siyar, M. Graphene to Advanced MoS2: A Review of Structure, Synthesis, and Optoelectronic Device Application. Crystals 2020, 10, 902. https://doi.org/10.3390/cryst10100902
Nawz T, Safdar A, Hussain M, Sung Lee D, Siyar M. Graphene to Advanced MoS2: A Review of Structure, Synthesis, and Optoelectronic Device Application. Crystals. 2020; 10(10):902. https://doi.org/10.3390/cryst10100902
Chicago/Turabian StyleNawz, Tahreem, Amna Safdar, Muzammil Hussain, Dae Sung Lee, and Muhammad Siyar. 2020. "Graphene to Advanced MoS2: A Review of Structure, Synthesis, and Optoelectronic Device Application" Crystals 10, no. 10: 902. https://doi.org/10.3390/cryst10100902
APA StyleNawz, T., Safdar, A., Hussain, M., Sung Lee, D., & Siyar, M. (2020). Graphene to Advanced MoS2: A Review of Structure, Synthesis, and Optoelectronic Device Application. Crystals, 10(10), 902. https://doi.org/10.3390/cryst10100902





