Simple Preparation of Ceramic-Like Materials Based on 1D-Agx(x=0, 5, 10, 20, 40 mM)/TiO2 Nanostructures and Their Photocatalysis Performance
Abstract
:1. Introduction
2. Experimental Details
3. Results and Discussions
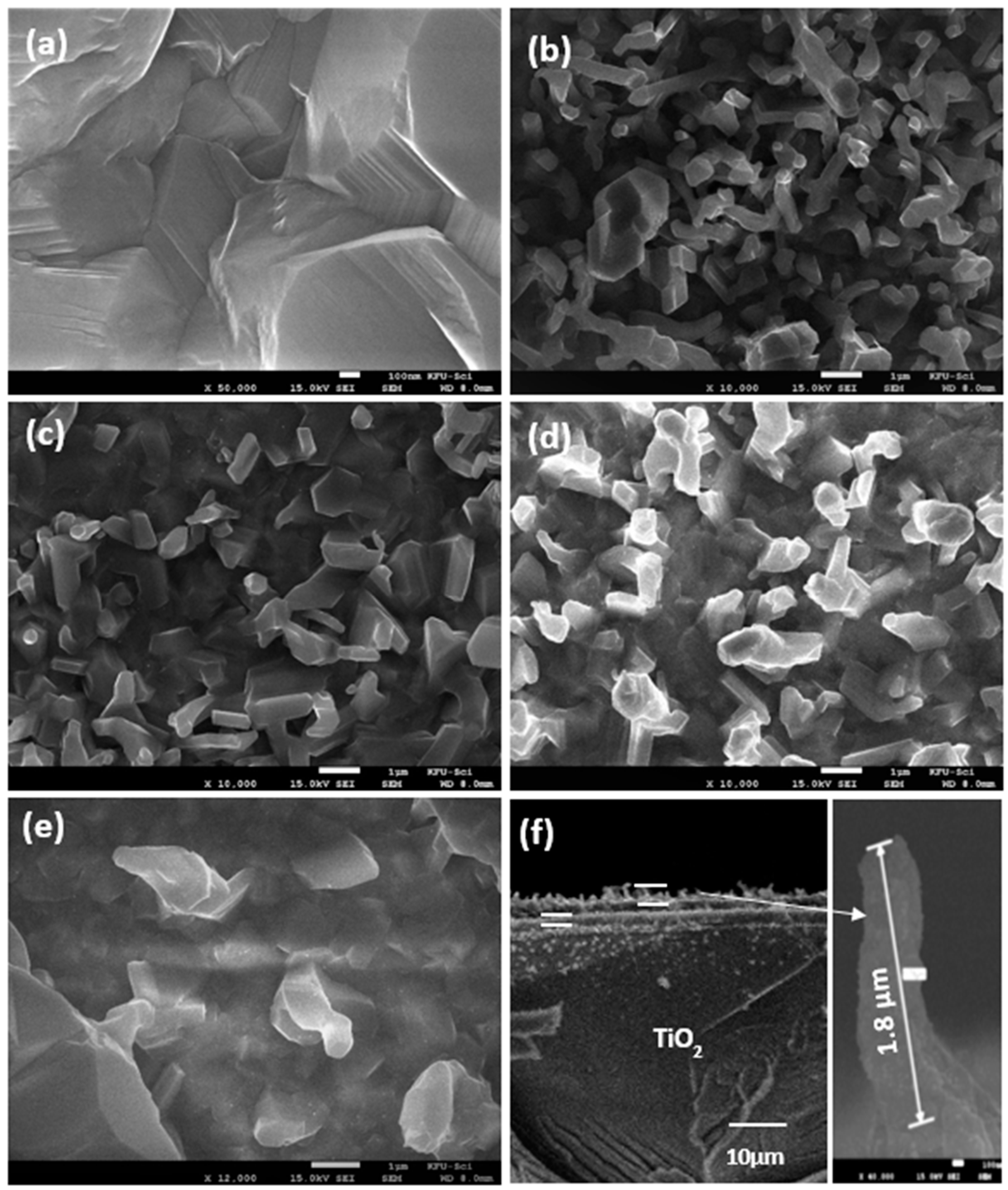
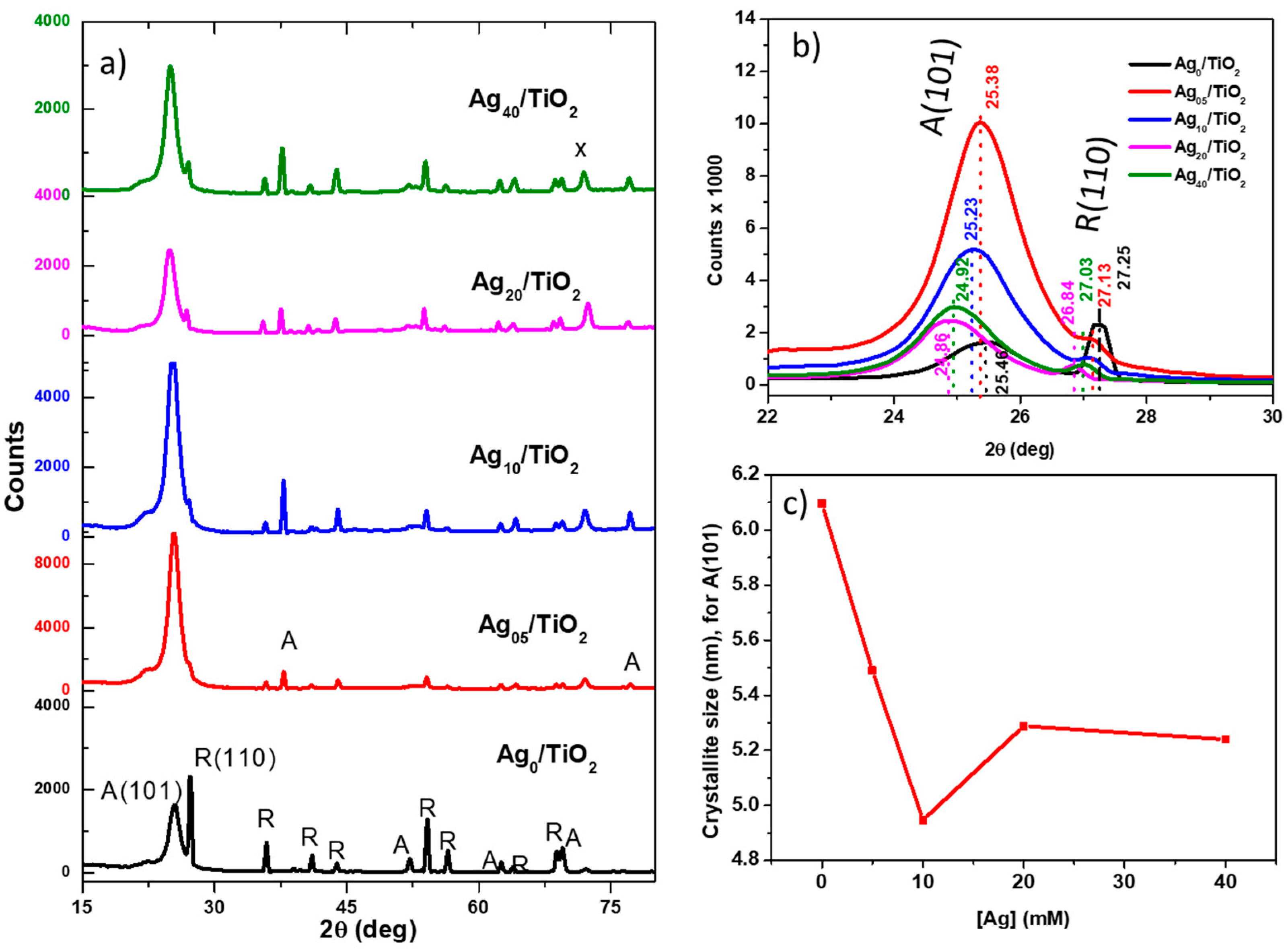
3.1. Structural Characteristics
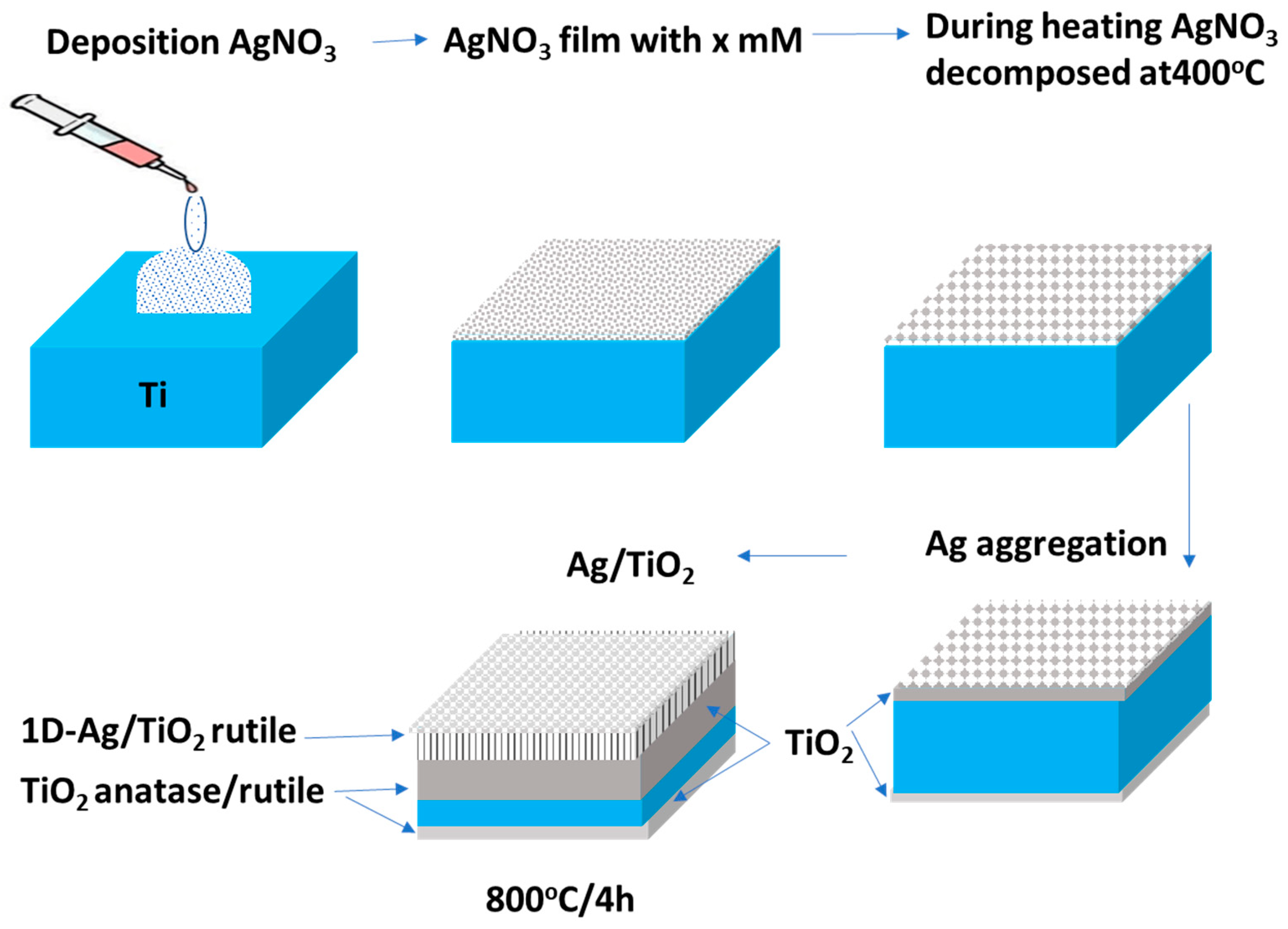
3.2. Growth Mechanism of Ag/TiO2 Nanorods
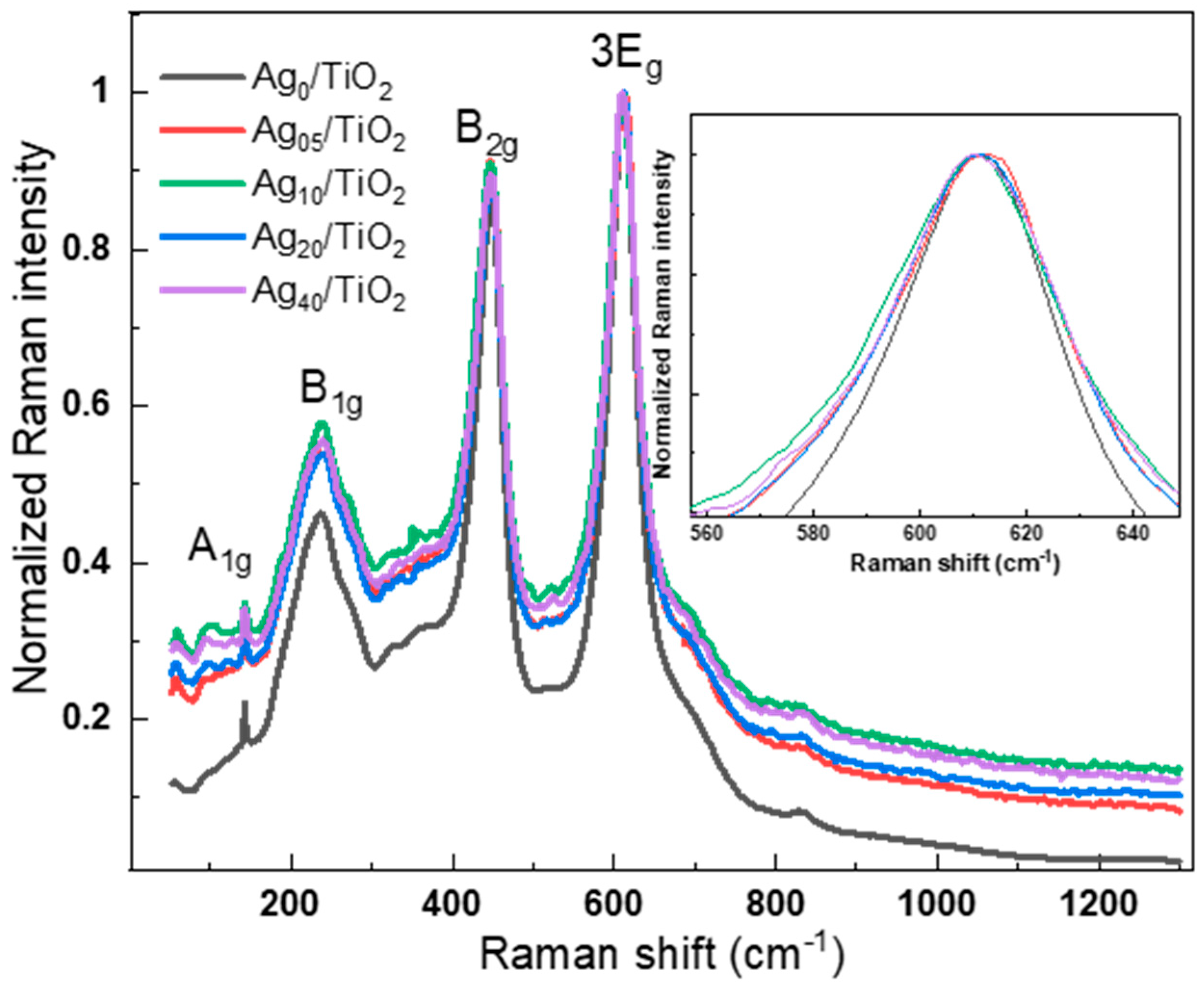
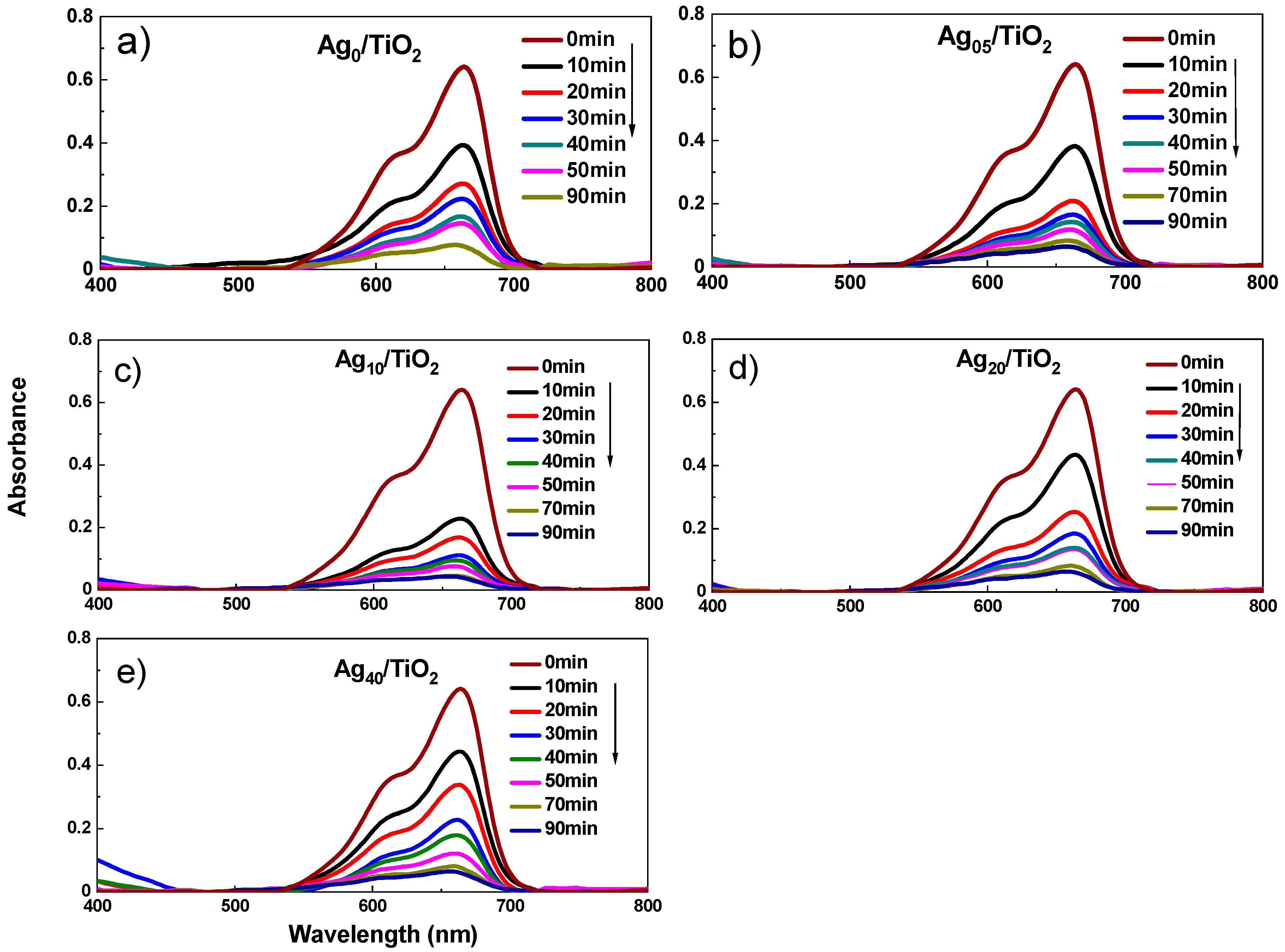
3.3. Optical Properties
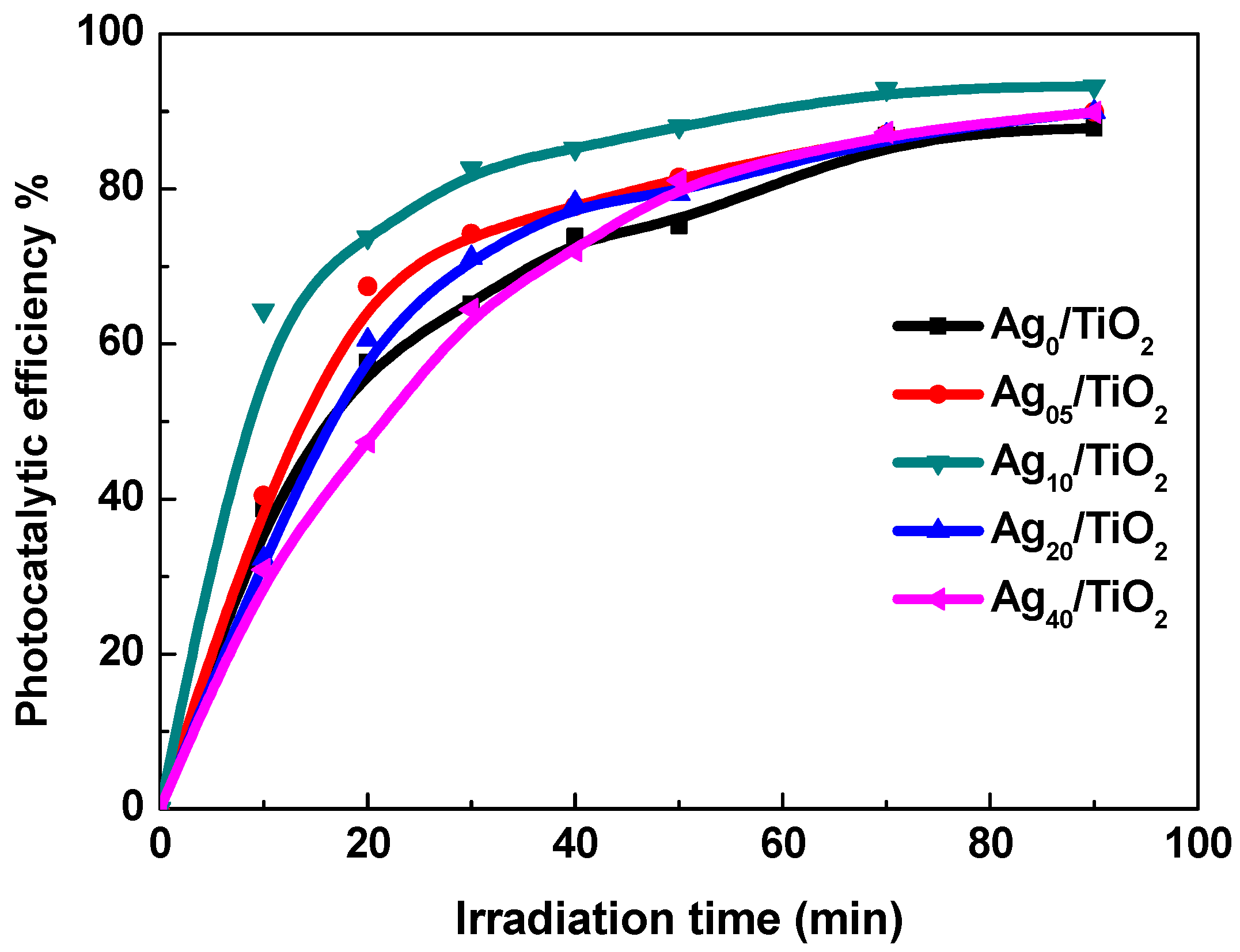
3.4. Photocatalysis Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Byrne, C.; Subramanian, G.; Pillai, S.C. Recent advances in photocatalysis for environmental applications. J. Environ. Chem. Eng. 2018, 6, 3531–3555. [Google Scholar] [CrossRef]
- Mills, A.; Le Hunte, S. An overview of semiconductor photocatalysis. J. Photochem. Photobiol. A Chem. 1997, 108, 1–35. [Google Scholar] [CrossRef]
- Khan, M.M.; Adil, S.F.; Al-Mayouf, A. Metal oxides as photocatalysts. J. Saudi Chem. Soc. 2015, 19, 462–464. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef] [Green Version]
- Daghrir, R.; Drogui, P.; Robert, D. Modified TiO2 For Environmental Photocatalytic Applications: A Review. Ind. Eng. Chem. Res. 2013, 52, 3581–3599. [Google Scholar] [CrossRef]
- Mahlambi, M.M.; Ngila, C.J.; Mamba, B.B. Recent Developments in Environmental Photocatalytic Degradation of Organic Pollutants: The Case of Titanium Dioxide Nanoparticles—A Review. J. Nanomater. 2015, 2015, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Hu, Y.; Han, J.; Guo, R.; Xiong, H.; Yin, Y. TiO2/NiO hybrid shells: P–n junction photocatalysts with enhanced activity under visible light. J. Mater. Chem. A 2015, 3, 20727–20735. [Google Scholar] [CrossRef]
- An, H.R.; Park, S.Y.; Huh, J.Y.; Kim, H.; Lee, Y.C.; Lee, Y.B.; Hong, Y.C.; Lee, H.U. Nanoporous hydrogenated TiO2 photocatalysts generated by underwater discharge plasma treatment for solar photocatalytic applications. Appl. Catal. B Environ. 2017, 211, 126–136. [Google Scholar] [CrossRef]
- Shao, J.; Sheng, W.; Wang, M.; Li, S.; Chen, J.; Zhang, Y.; Cao, S. In situ synthesis of carbon-doped TiO2 single-crystal nanorods with a remarkably photocatalytic efficiency. Appl. Catal. B Environ. 2017. [Google Scholar] [CrossRef]
- Hochbaum, A.I.; Chen, R.; Delgado, R.D.; Liang, W.; Garnett, E.C.; Najarian, M.; Majumdar, A.; Yang, P. Enhanced thermoelectric performance of rough silicon nanowires. Nature 2008, 451, 163–167. [Google Scholar] [CrossRef]
- Park, J.-Y.; Choi, K.-I.; Lee, J.-H.; Hwang, C.-H.; Choi, D.-Y.; Lee, J.-W. Fabrication and characterization of metal-doped TiO2 nanofibers for photocatalytic reactions. Mater. Lett. 2013, 97, 64–66. [Google Scholar] [CrossRef]
- Albu, S.P.; Ghicov, A.; Macak, J.M.; Hahn, R.; Schmuki, P. Self-Organized, Free-Standing TiO2 Nanotube Membrane for Flow-through Photocatalytic Applications. Nano Lett. 2007, 7, 1286–1289. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Wang, J.; Tafen, D.N.; Wang, H.; Zheng, J.-G.; Lewis, J.P.; Liu, X.; Leonard, S.S.; Manivannan, A. Shape-Enhanced Photocatalytic Activity of Single-Crystalline Anatase TiO2 (101) Nanobelts. J. Am. Chem. Soc. 2010, 132, 6679–6685. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Lu, W.; Tian, J.; Luo, Y.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Synthesis and Study of Plasmon-Induced Carrier Behavior at Ag/TiO2 Nanowires. Chem.-A Eur. J. 2012, 18, 8508–8514. [Google Scholar] [CrossRef]
- Wu, H.B.; Hng, H.H.; Lou, X.W.D. Direct Synthesis of Anatase TiO2 Nanowires with Enhanced Photocatalytic Activity. Adv. Mater. 2012, 24, 2567–2571. [Google Scholar] [CrossRef]
- Liu, G.; Yang, H.G.; Wang, X.; Cheng, L.; Pan, J.; Lu, G.Q.; Cheng, H.-M. Visible Light Responsive Nitrogen Doped Anatase TiO2 Sheets with Dominant {001} Facets Derived from TiN. J. Am. Chem. Soc. 2009, 131, 12868–12869. [Google Scholar] [CrossRef]
- Ge, M.; Cao, C.; Huang, J.; Li, S.; Chen, Z.; Zhang, K.-Q.; Al-Deyab, S.S.; Lai, Y. A review of one-dimensional TiO2 nanostructured materials for environmental and energy applications. J. Mater. Chem. A 2016, 4, 6772–6801. [Google Scholar] [CrossRef]
- Plodinec, M.; Grčić, I.; Willinger, M.G.; Hammud, A.; Huang, X.; Panžić, I.; Gajović, A. Black TiO2 nanotube arrays decorated with Ag nanoparticles for enhanced visible-light photocatalytic oxidation of salicylic acid. J. Alloys Compd. 2019, 776, 883–896. [Google Scholar] [CrossRef]
- Qi, H.-P.; Wang, H.-L.; Zhao, D.-Y.; Jiang, W.-F. Preparation and photocatalytic activity of Ag-modified GO-TiO2 mesocrystals under visible light irradiation. Appl. Surf. Sci. 2019, 480, 105–114. [Google Scholar] [CrossRef]
- Khan, S.B.; Hou, M.; Shuang, S.; Zhang, Z. Morphological influence of TiO2 nanostructures (nanozigzag, nanohelics and nanorod) on photocatalytic degradation of organic dyes. Appl. Surf. Sci. 2017, 400, 184–193. [Google Scholar] [CrossRef]
- Fan, X.; Wan, J.; Liu, E.; Sun, L.; Hu, Y.; Li, H.; Hu, X.; Fan, J. High-efficiency photoelectrocatalytic hydrogen generation enabled by Ag deposited and Ce doped TiO2 nanotube arrays. Ceram. Int. 2015, 41, 5107–5116. [Google Scholar] [CrossRef]
- Meng, L.; Huang, T.; Wang, X.; Chen, S.; Yang, Z.; Ren, B. Gold-coated AFM tips for tip-enhanced Raman spectroscopy: Theoretical calculation and experimental demonstration. Opt. Express 2015, 23, 13804–13813. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, C.; Liu, J.; Tian, Z.; Wang, G.; Cai, W. Defect-Mediated Formation of Ag Cluster-Doped TiO2 Nanoparticles for Efficient Photodegradation of Pentachlorophenol. Langmuir 2012, 28, 3938–3944. [Google Scholar] [CrossRef]
- Subrahmanyam, A.; KP, B.; Rajesh, P.; Kumar, K.J.; MR, K. Surface modification of sol gel TiO2 surface with sputtered metallic silver for Sun light photocatalytic activity: Initial studies. Sol. Energy Mater. Sol. Cells 2012, 101, 241–248. [Google Scholar] [CrossRef]
- Li, Y.; Ma, M.; Chen, W.; Li, L.; Zen, M. Preparation of Ag-doped TiO2 nanoparticles by a miniemulsion method and their photoactivity in visible light illuminations. Mater. Chem. Phys. 2011, 129, 501–505. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Chang, S.-Y.; Jehng, J.-M. Photocatalytic H2 Generation Efficiencies of TiO2 Nanotube-based Heterostructures Grafted with ZnO Nanorods, Ag Nanoparticles, or Pd Nanodendrites. J. Phys. Chem. C 2017, 121, 19063–19068. [Google Scholar] [CrossRef]
- Guan, B.; Yu, J.; Guo, S.; Yu, S.; Han, S. Porous nickel doped titanium dioxide nanoparticles with improved visible light photocatalytic activity. Nanoscale Adv. 2020, 2, 1352–1357. [Google Scholar] [CrossRef] [Green Version]
- Corredor, J.; Rivero, M.J.; Ortiz, I. New insights in the performance and reuse of rGO/TiO2 composites for the photocatalytic hydrogen production. Int. J. Hydrogen Energy 2020. [Google Scholar] [CrossRef]
- Ismael, A.M.; El-Shazly, A.N.; Gaber, S.E.; Rashad, M.M.; Kamel, A.H.; Hassan, S.S.M. Novel TiO2/GO/CuFe2O4 nanocomposite: A magnetic, reusable and visible-light-driven photocatalyst for efficient photocatalytic removal of chlorinated pesticides from wastewater. RSC Adv. 2020, 10, 34806–34814. [Google Scholar] [CrossRef]
- Lottici, P.P.; Bersani, D.; Braghini, M.; Montenero, A. Raman scattering characterization of gel-derived titania glass. J. Mater. Sci. 1993, 28, 177–183. [Google Scholar] [CrossRef]
- Mathew, S.; Ganguly, P.; Rhatigan, S.; Kumaravel, V.; Byrne, C.; Hinder, S.; Bartlett, J.; Nolan, M.; Pillai, S. Cu-Doped TiO2: Visible Light Assisted Photocatalytic Antimicrobial Activity. Appl. Sci. 2018, 8, 2067. [Google Scholar] [CrossRef] [Green Version]
- Parker, J.C.; Siegel, R.W. Calibration of the Raman spectrum to the oxygen stoichiometry of nanophase TiO2. Appl. Phys. Lett. 1990, 57, 943–945. [Google Scholar] [CrossRef]
- Parker, J.C.; Siegel, R.W. Raman microprobe study of nanophase TiO2 and oxidation-induced spectral changes. J. Mater. Res. 1990, 5, 1246–1252. [Google Scholar] [CrossRef]
- Melendres, C.A.; Narayanasamy, A.; Maroni, V.A.; Siegel, R.W. Raman spectroscopy of nanophase TiO2. J. Mater. Res. 1989, 4, 1246–1250. [Google Scholar] [CrossRef]
- Satoh, N.; Nakashima, T.; Kamikura, K.; Yamamoto, K. Quantum size effect in TiO2 nanoparticles prepared by finely controlled metal assembly on dendrimer templates. Nat. Nanotechnol. 2008, 3, 106–111. [Google Scholar] [CrossRef]
- Peng, H.; Li, J. Quantum Confinement and Electronic Properties of Rutile TiO2 Nanowires. J. Phys. Chem. C 2008, 112, 20241–20245. [Google Scholar] [CrossRef]
- Türkyılmaz, Ş.Ş.; Güy, N.; Özacar, M. Photocatalytic efficiencies of Ni, Mn, Fe and Ag doped ZnO nanostructures synthesized by hydrothermal method: The synergistic/antagonistic effect between ZnO and metals. J. Photochem. Photobiol. A Chem. 2017, 341, 39–50. [Google Scholar] [CrossRef]
- Skinner, A.W.; DiBernardo, A.M.; Masud, A.M.; Aich, N.; Pinto, A.H. Factorial design of experiments for optimization of photocatalytic degradation of tartrazine by zinc oxide (ZnO) nanorods with different aspect ratios. J. Environ. Chem. Eng. 2020, 8, 104235. [Google Scholar] [CrossRef]
- de Mendonça, V.R.; Ribeiro, C. Influence of TiO2 morphological parameters in dye photodegradation: A comparative study in peroxo-based synthesis. Appl. Catal. B Environ. 2011, 105, 298–305. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Suliman, N.; Awada, C.; Alshoaibi, A.; Shaalan, N.M. Simple Preparation of Ceramic-Like Materials Based on 1D-Agx(x=0, 5, 10, 20, 40 mM)/TiO2 Nanostructures and Their Photocatalysis Performance. Crystals 2020, 10, 1024. https://doi.org/10.3390/cryst10111024
Al Suliman N, Awada C, Alshoaibi A, Shaalan NM. Simple Preparation of Ceramic-Like Materials Based on 1D-Agx(x=0, 5, 10, 20, 40 mM)/TiO2 Nanostructures and Their Photocatalysis Performance. Crystals. 2020; 10(11):1024. https://doi.org/10.3390/cryst10111024
Chicago/Turabian StyleAl Suliman, Noura, Chawki Awada, Adil Alshoaibi, and Nagih M. Shaalan. 2020. "Simple Preparation of Ceramic-Like Materials Based on 1D-Agx(x=0, 5, 10, 20, 40 mM)/TiO2 Nanostructures and Their Photocatalysis Performance" Crystals 10, no. 11: 1024. https://doi.org/10.3390/cryst10111024
APA StyleAl Suliman, N., Awada, C., Alshoaibi, A., & Shaalan, N. M. (2020). Simple Preparation of Ceramic-Like Materials Based on 1D-Agx(x=0, 5, 10, 20, 40 mM)/TiO2 Nanostructures and Their Photocatalysis Performance. Crystals, 10(11), 1024. https://doi.org/10.3390/cryst10111024







