Structural Transformations in Crystals Induced by Radiation and Pressure. Part 10. The Crystallographic Picture of Photochemical Behaviour of bi(anthracene-9,10-dimethylene) under High Pressure
Abstract
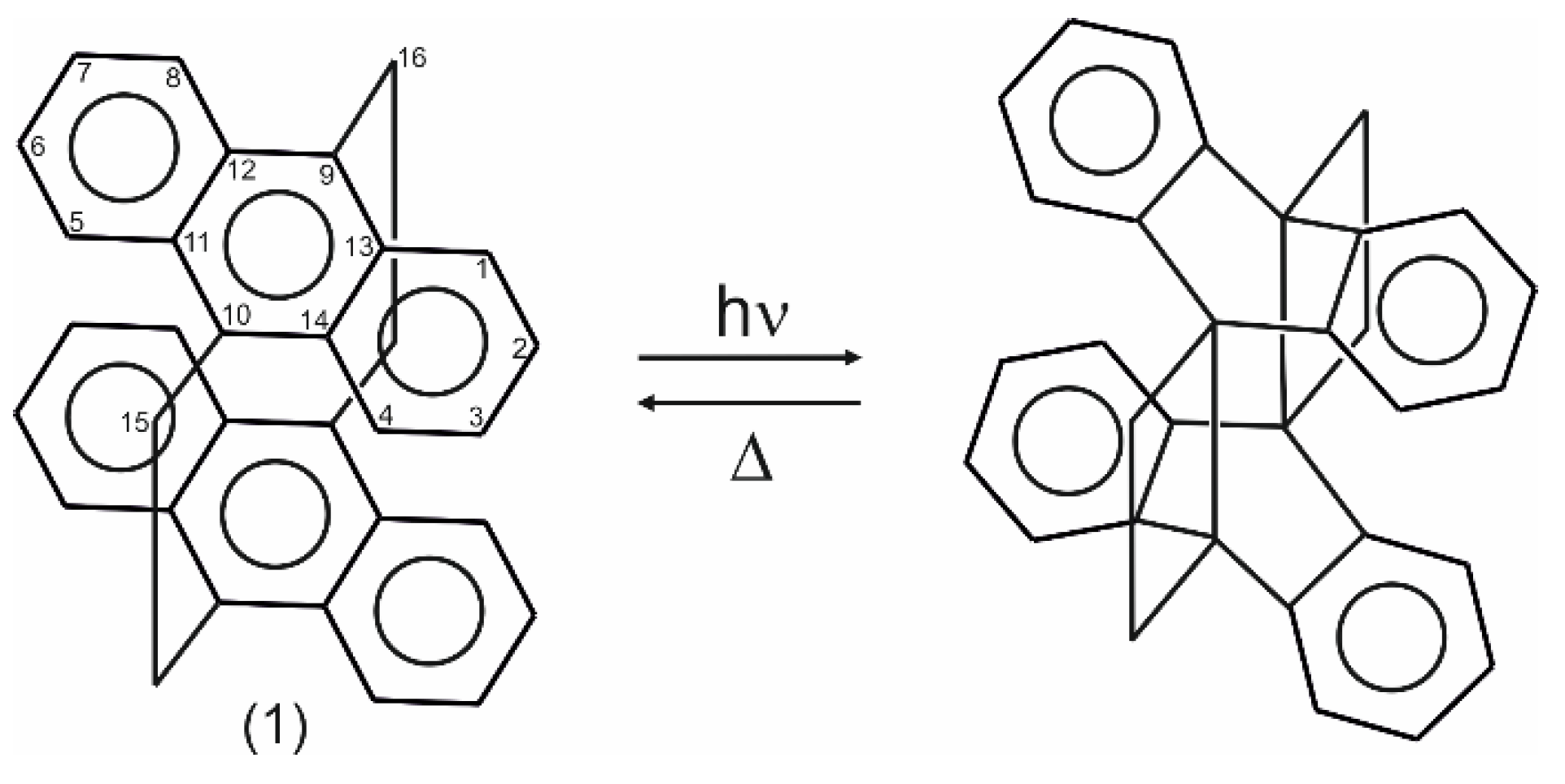
1. Introduction
2. Materials and Methods
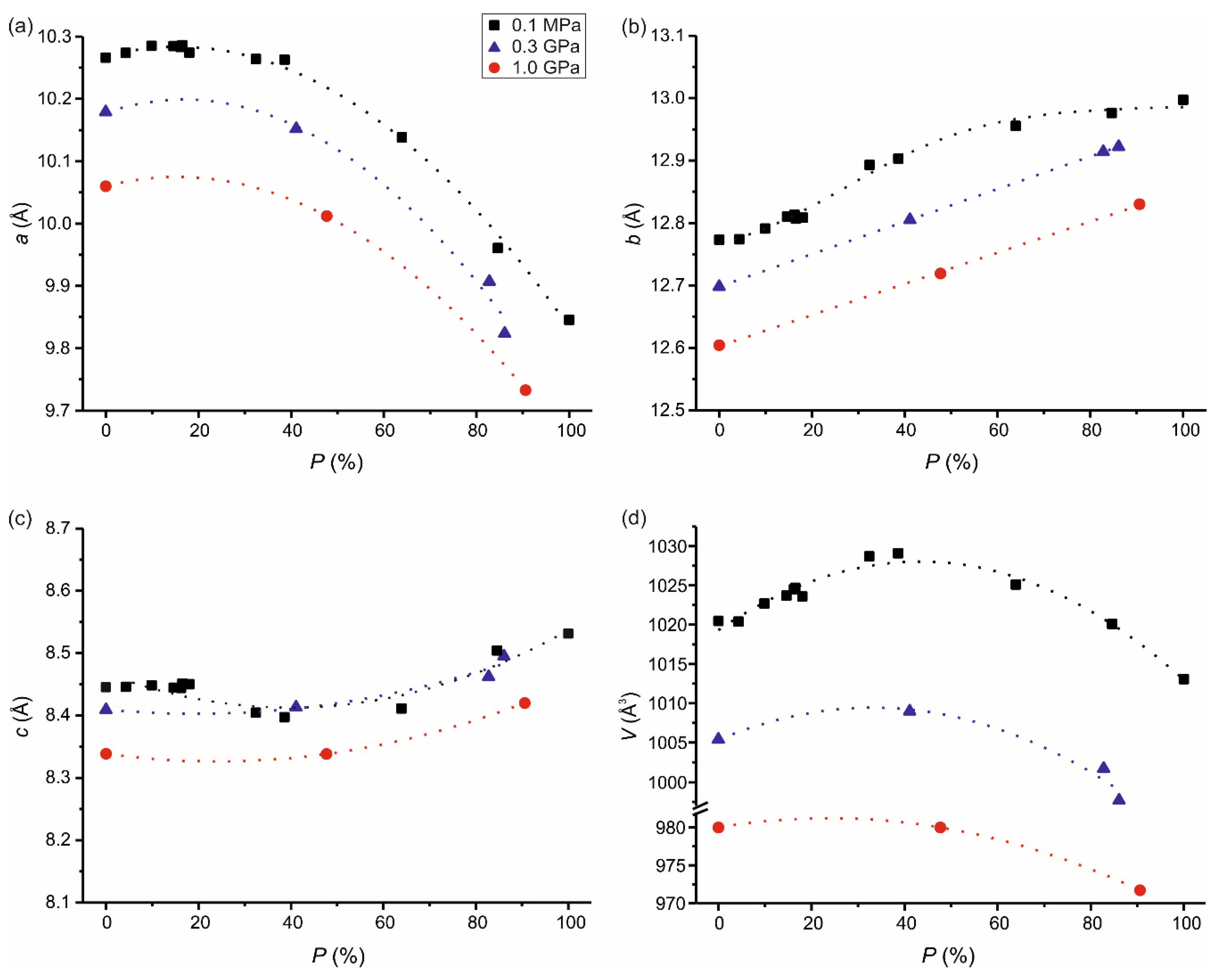
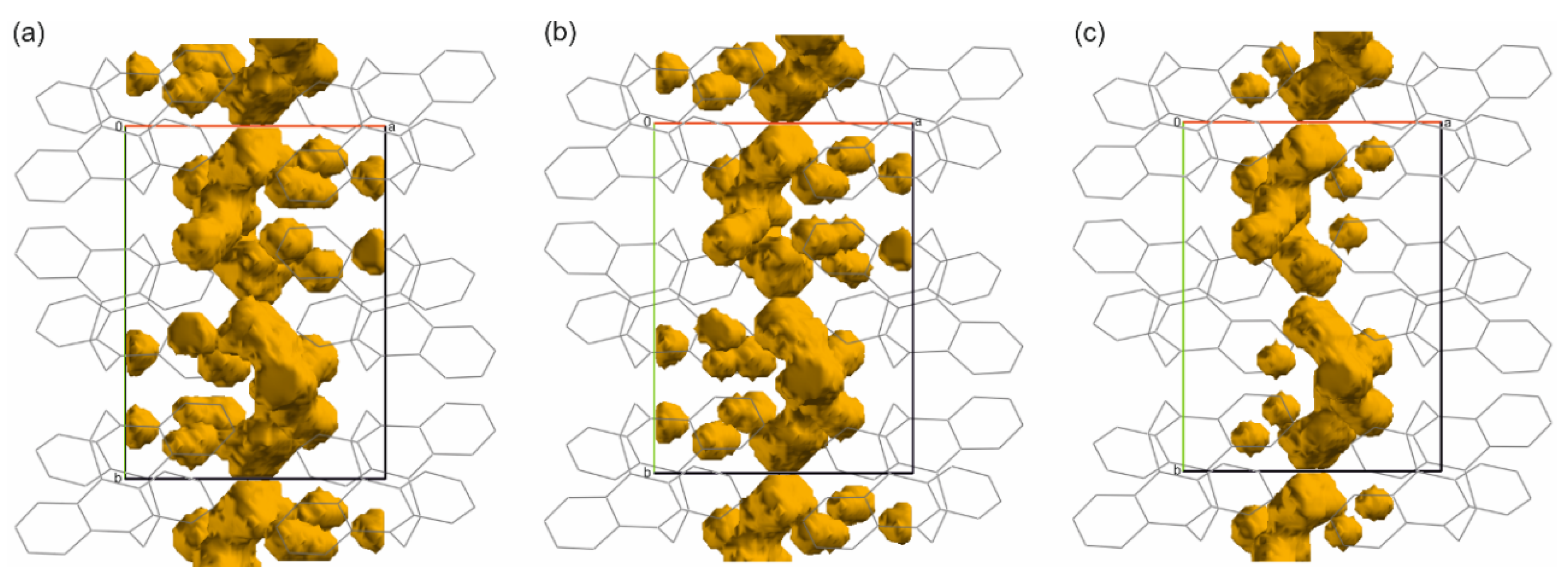
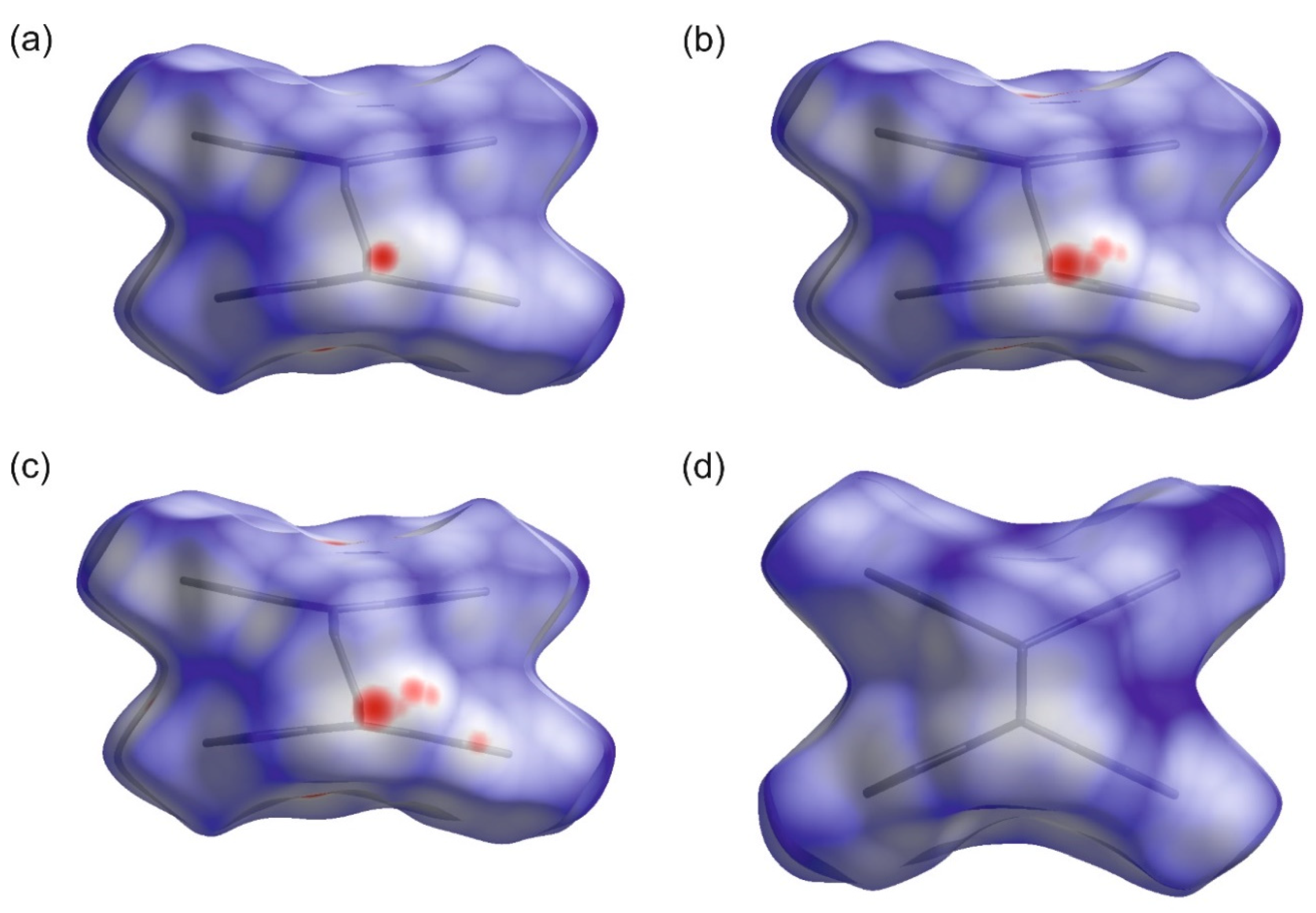
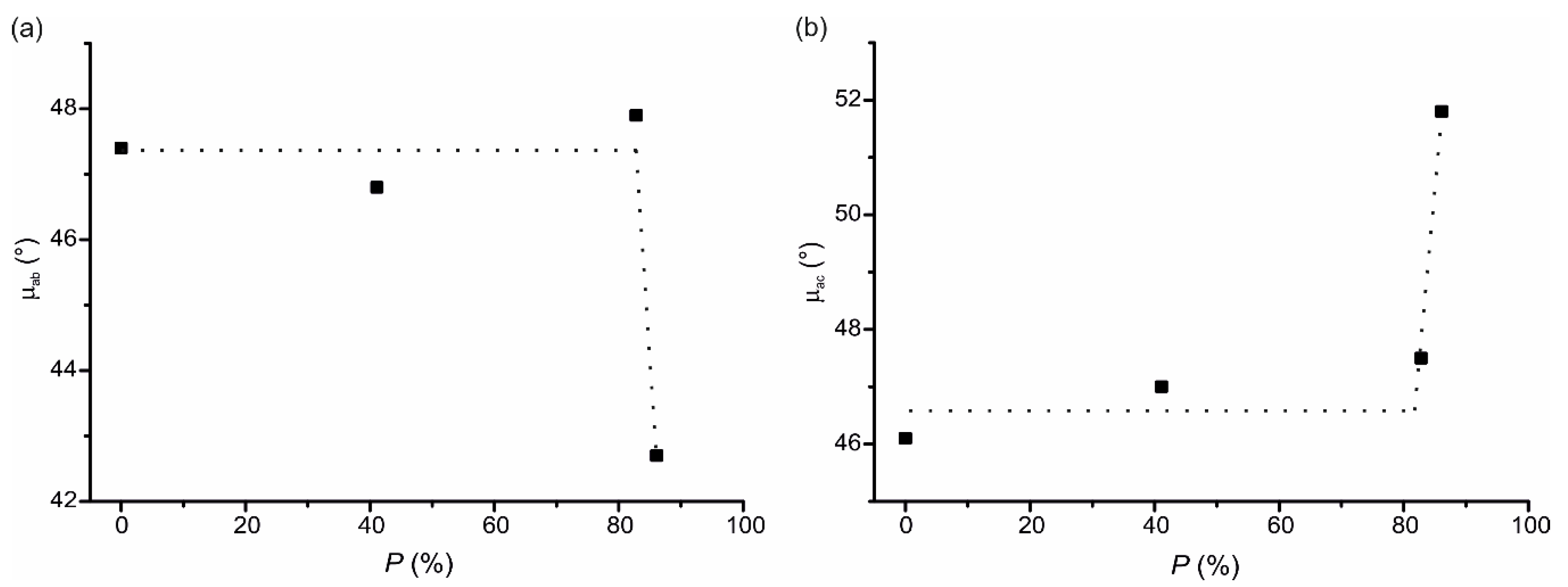
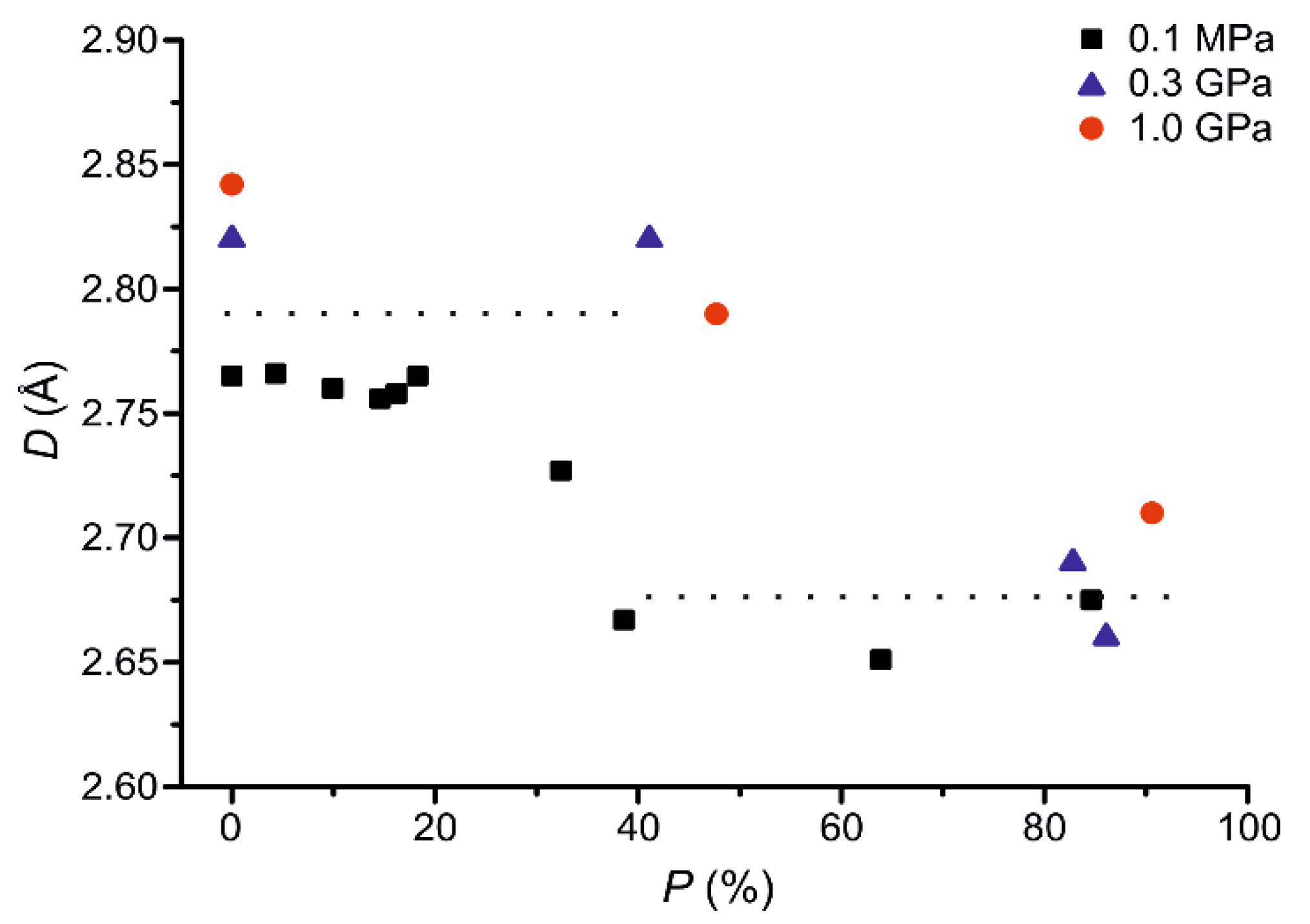
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kaupp, G. Mechanochemistry: The varied applications of mechanical bond-breaking. CrystEngComm 2009, 11, 388–403. [Google Scholar] [CrossRef]
- Jezowski, S.R.; Zhu, L.; Wang, Y.; Rice, A.P.; Scott, G.W.; Bardeen, C.J.; Chronister, E.L. Pressure catalyzed bond dissociation in an anthracene cyclophane photodimer. J. Am. Chem. Soc. 2012, 134, 7459–7466. [Google Scholar] [CrossRef] [PubMed]
- Tong, F.; Cruz, C.D.; Jezowski, S.R.; Zhou, X.; Zhu, L.; Al-Kaysi, R.O.; Chronister, E.L.; Bardeen, C.J. Pressure dependence of the forward and backward rates of 9-tert-butylanthracene dewar isomerization. J. Phys. Chem. A 2014, 118, 5349–5354. [Google Scholar] [CrossRef] [PubMed]
- James, S.L.; Friscic, T. Mechanochemistry. Chem. Soc. Rev. 2013, 42, 7494–7496. [Google Scholar] [CrossRef]
- Trzop, E.; Turowska-Tyrk, I. Monitoring structural transformations in crystals. 12. Course of an intramolecular [4 + 4] photocycloaddition in a crystal. Acta Crystallogr. Sect. B Struct. Sci. 2008, 64, 375–382. [Google Scholar] [CrossRef]
- Golden, J.H. Bi(anthracene-9,10-dimethylene) (Tetrabenzo-[2,2]paracyclophane]. J. Chem. Soc. 1961, 3741–3748. [Google Scholar] [CrossRef]
- Ferguson, J.; Mau, A.W.H. A spectroscopic study of the photodimerization of anthracene sandwich dimers in dianthracene. Mol. Phys. 1974, 27, 377–387. [Google Scholar] [CrossRef]
- Ferguson, J.; Mau, A.W.H.; Morris, J.M. Spectra of dimers of anthracene and its derivatives. I. Sandwich dimers. Aust. J. Chem. 1973, 26, 91–102. [Google Scholar] [CrossRef]
- Wada, A.; Tanaka, J. The molecular structure of bi(anthracene-9,10-dimethylene), C32H24. Acta Crystallogr. Sect. B 1977, 33, 355–360. [Google Scholar] [CrossRef]
- Dougherty, D.A.; Choi, C.S.; Kaupp, G.; Buda, A.B.; Rudziński, J.M.; Osawa, E. Effects of substituents on the length of central C(sp3)–C(sp3) bond in anthracene photodimers and related molecules. J. Chem. Soc. Perkin Trans. 2 1986, 1063–1070. [Google Scholar] [CrossRef]
- Kaupp, G. Cyclovinylogous [2π→2σ] Photoadditions. Angew. Chem. Int. Ed. 1972, 11, 313–314. [Google Scholar] [CrossRef]
- Kaupp, G. AFM and STM in photochemistry including photon tunneling. Adv. Photochem. 1995, 19, 119–177. [Google Scholar] [CrossRef]
- Milledge, H.J. The automatic selection of molecular crystal structures by combining stereochemical criteria and high-speed computing. Proc. Roy. Soc. A 1962, 267, 566–589. [Google Scholar] [CrossRef]
- Ehrenberg, M. The crystal structure of di-para-anthracene. Acta Crystallogr. 1966, 20, 182–186. [Google Scholar] [CrossRef]
- Harada, J.; Ogawa, K.; Tomoda, S. The central C–C bond length in the bi(anthracene-9,10-dimethylene) photoisomer: Unusual elongation and crystalline state reaction. Chem. Lett. 1995, 24, 751–752. [Google Scholar] [CrossRef]
- Merrill, L.; Bassett, W.A. Miniature diamond anvil pressure cell for single crystal X-ray diffraction studies. Rev. Sci. Instrum. 1974, 45, 290–294. [Google Scholar] [CrossRef]
- Angel, R.J.; Allan, D.R.; Miletich, R.; Finger, L.W. The use of quartz as an internal pressure standard in high-pressure crystallography. J. Appl. Crystallogr. 1997, 30, 461–466. [Google Scholar] [CrossRef]
- Rigaku Oxford Difraction, CrysAlisPro Rigaku Oxford Diffraction, Wrocław, Poland.
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Konieczny, K.; Bąkowicz, J.; Turowska-Tyrk, I. Structural transformations in crystals induced by radiation and pressure. Part 2. The path of the photochemical intramolecular reaction in crystals at different pressures. CrystEngComm 2015, 17, 7693–7701. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B Struct. Sci. 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Abrahams, S.C.; Robertson, J.M.; White, J.G. The crystal and molecular structure of naphthalene. I. X-ray measurements. Acta Crystallogr. 1949, 2, 233–238. [Google Scholar] [CrossRef]
- Fabbiani, F.P.A.; Allan, D.R.; Parsons, S.; Pulham, C.R. Exploration of the high-pressure behaviour of polycyclic aromatic hydrocarbons: Naphthalene, phenanthrene and pyrene. Acta Crystallogr. Sect. B Struct. Sci. 2006, 62, 826–842. [Google Scholar] [CrossRef] [PubMed]
- Budzianowski, A.; Katrusiak, A. Pressure-frozen benzene I revisited. Acta Crystallogr. Sect. B Struct. Sci. 2006, 62, 94–101. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer17. University of Western Australia. 2017. Available online: https://crystalexplorer.scb.uwa.edu.au/ (accessed on 8 June 2017).
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Fernandes, M.A.; Levendis, D.C. Photodimerisation of the α′-polymorph of ortho-ethoxy-trans-cinnamic acid occurs via a two-stage mechanism at 343 K yielding 100% α-truxillic acid. Acta Crystallogr. Sect. B Struct. Sci. 2004, 60, 315–324. [Google Scholar] [CrossRef]
- Turowska-Tyrk, I. Structural transformations in a crystal during the photochemical reaction of 2-benzyl-5-benzylidenecyclopentanone. Chem. Eur. J. 2001, 7, 3401–3405. [Google Scholar] [CrossRef]
- Turowska-Tyrk, I. Monitoring structural transformations in crystals. 5. A topotactic [2 + 2]-photodimerization reaction. Acta Crystallogr. Sect. B Struct. Sci. 2003, 59, 670–675. [Google Scholar] [CrossRef]
- Turowska-Tyrk, I.; Trzop, E. Monitoring structural transformations in crystals. 6. The [4 + 4] photodimerization of 9-methylanthracene. Acta Crystallogr. Sect. B Struct. Sci. 2003, 59, 779–786. [Google Scholar] [CrossRef]
- Turowska-Tyrk, I.; Trzop, E.; Scheffer, J.R.; Chen, S. Monitoring structural transformations in crystals. 8. Monitoring molecules and a reaction center during a solid-state Yang photocyclization. Acta Crystallogr. Sect. B Struct. Sci. 2006, 62, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Turowska-Tyrk, I.; Bąkowicz, J.; Scheffer, J.R. Monitoring structural transformations in crystals. 11. Yang photocyclizations—one type of reaction, but diversity of structural changes. Acta Crystallogr. Sect. B Struct. Sci. 2007, 63, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Konieczny, K.; Bąkowicz, J.; Siedlecka, R.; Galica, T.; Turowska-Tyrk, I. Photoinduced structural changes as the factor influencing the direction of the photochemical reaction in the crystal. Cryst. Growth Des. 2017, 17, 1347–1352. [Google Scholar] [CrossRef]


| Pressure | 0.3 GPa | 1.0 GPa | |||||
|---|---|---|---|---|---|---|---|
| Reaction progress/% | 0 | 41.1 | 82.8 | 86.1 | 0 | 47.7 | 90.6 |
| Chemical formula | C32H24 | C32H24 | C32H24 | C32H24 | C32H24 | C32H24 | C32H24 |
| Formula Weight | 408.51 | 408.51 | 408.51 | 408.51 | 408.51 | 408.51 | 408.51 |
| Crystal dimensions/mm | 0.40 × 0.14 × 0.05 | 0.40 × 0.14 × 0.05 | 0.40 × 0.14 × 0.05 | 0.40 × 0.14 × 0.05 | 0.34 × 0.13 × 0.06 | 0.34 × 0.13 × 0.06 | 0.34 × 0.13 × 0.06 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic | Monoclinic | Monoclinic | Monoclinic | Monoclinic |
| Space group | P21/c | P21/c | P21/c | P21/c | P21/c | P21/c | P21/c |
| a/Å | 10.181(2) | 10.152(4) | 9.907(3) | 9.824(3) | 10.0600(16) | 10.012(4) | 9.733(3) |
| b/Å | 12.713(6) | 12.805(9) | 12.914(8) | 12.922(8) | 12.604(7) | 12.719(11) | 12.830(9) |
| c/Å | 8.416(3) | 8.413(5) | 8.462(4) | 8.495(5) | 8.3382(18) | 8.338(3) | 8.420(3) |
| β/° | 112.44(3) | 112.70(5) | 112.30(4) | 112.31(4) | 112.03(2) | 112.69(4) | 112.46(3) |
| V/Å3 | 1006.8(7) | 1008.9(11) | 1001.6(9) | 997.7(9) | 980.1(6) | 979.6(10) | 971.7(8) |
| Z | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Dx/Mg m−3 | 1.348 | 1.345 | 1.354 | 1.360 | 1.384 | 1.385 | 1.396 |
| μ/mm−1 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 |
| T/K | 299 | 299 | 299 | 299 | 299 | 299 | 299 |
| Reflections collected | 4007 | 4192 | 4067 | 4059 | 3804 | 4036 | 3964 |
| Reflections independent | 795 | 820 | 792 | 783 | 715 | 759 | 758 |
| Reflections observed | 391 | 302 | 320 | 344 | 407 | 286 | 335 |
| Completeness/% | 43.2 | 43.7 | 43.3 | 42.8 | 39.4 | 43.2 | 42.8 |
| Rint | 0.111 | 0.226 | 0.186 | 0.154 | 0.134 | 0.232 | 0.186 |
| R (F2 > 2 σ(F2)), wR, S | 0.141, 0.433, 1.48 | 0.184, 0.511, 1.32 | 0.152, 0.455, 1.28 | 0.151, 0.458, 1.47 | 0.130, 0.390, 1.30 | 0.155, 0.456, 1.21 | 0.139, 0.444, 1.34 |
| Δρmax, Δρmin/eÅ−3 | 0.31, −0.30 | 0.23, −0.30 | 0.32, −0.31 | 0.43, −0.32 | 0.43, −0.48 | 0.22, −0.28 | 0.29, −0.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bąkowicz, J.; Turowska-Tyrk, I. Structural Transformations in Crystals Induced by Radiation and Pressure. Part 10. The Crystallographic Picture of Photochemical Behaviour of bi(anthracene-9,10-dimethylene) under High Pressure. Crystals 2020, 10, 1031. https://doi.org/10.3390/cryst10111031
Bąkowicz J, Turowska-Tyrk I. Structural Transformations in Crystals Induced by Radiation and Pressure. Part 10. The Crystallographic Picture of Photochemical Behaviour of bi(anthracene-9,10-dimethylene) under High Pressure. Crystals. 2020; 10(11):1031. https://doi.org/10.3390/cryst10111031
Chicago/Turabian StyleBąkowicz, Julia, and Ilona Turowska-Tyrk. 2020. "Structural Transformations in Crystals Induced by Radiation and Pressure. Part 10. The Crystallographic Picture of Photochemical Behaviour of bi(anthracene-9,10-dimethylene) under High Pressure" Crystals 10, no. 11: 1031. https://doi.org/10.3390/cryst10111031
APA StyleBąkowicz, J., & Turowska-Tyrk, I. (2020). Structural Transformations in Crystals Induced by Radiation and Pressure. Part 10. The Crystallographic Picture of Photochemical Behaviour of bi(anthracene-9,10-dimethylene) under High Pressure. Crystals, 10(11), 1031. https://doi.org/10.3390/cryst10111031






