Spectroscopic, Raman, EMPA, Micro-XRF and Micro-XANES Analyses of Sulphur Concentration and Oxidation State of Natural Apatite Crystals
Abstract
:1. Introduction
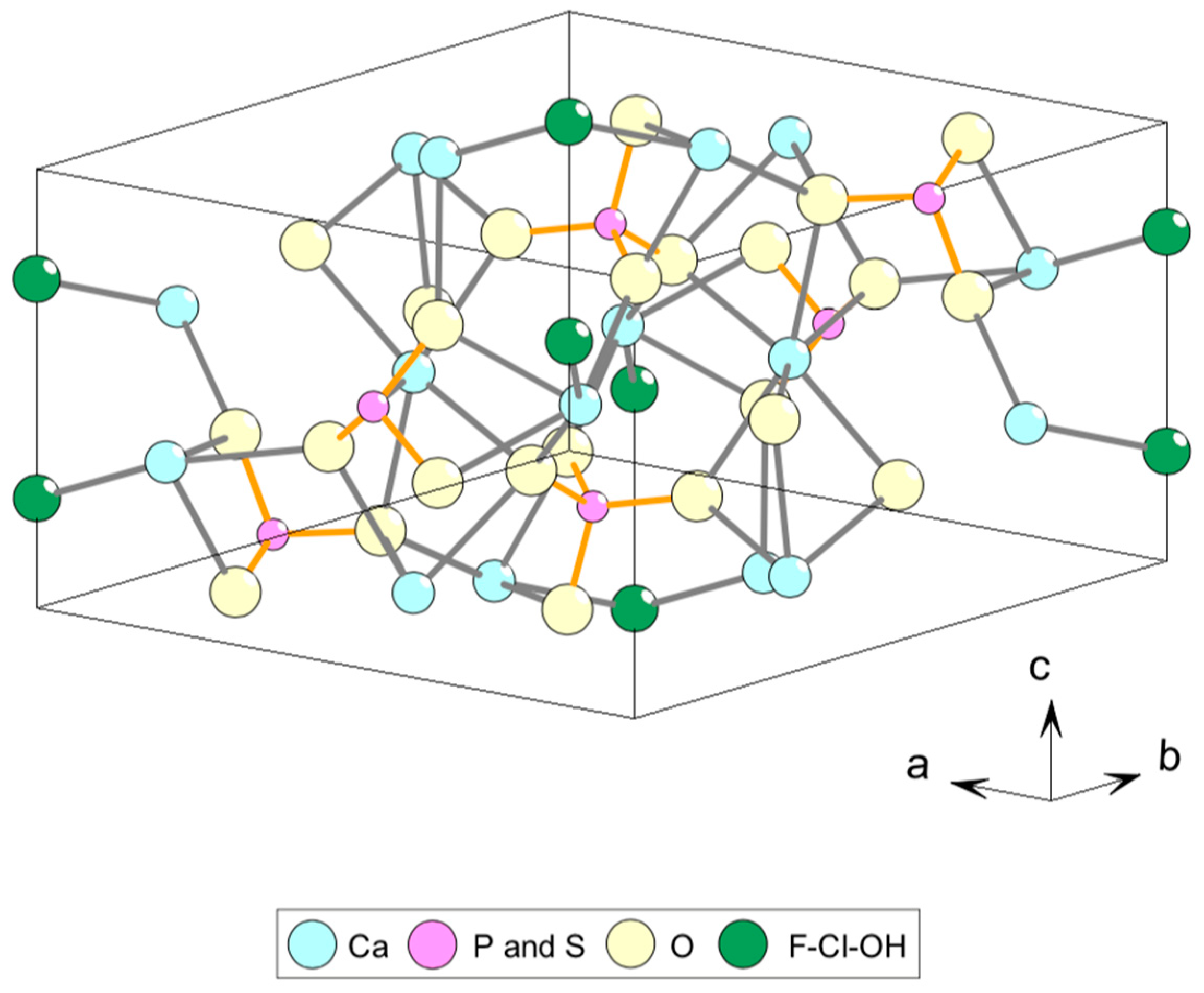
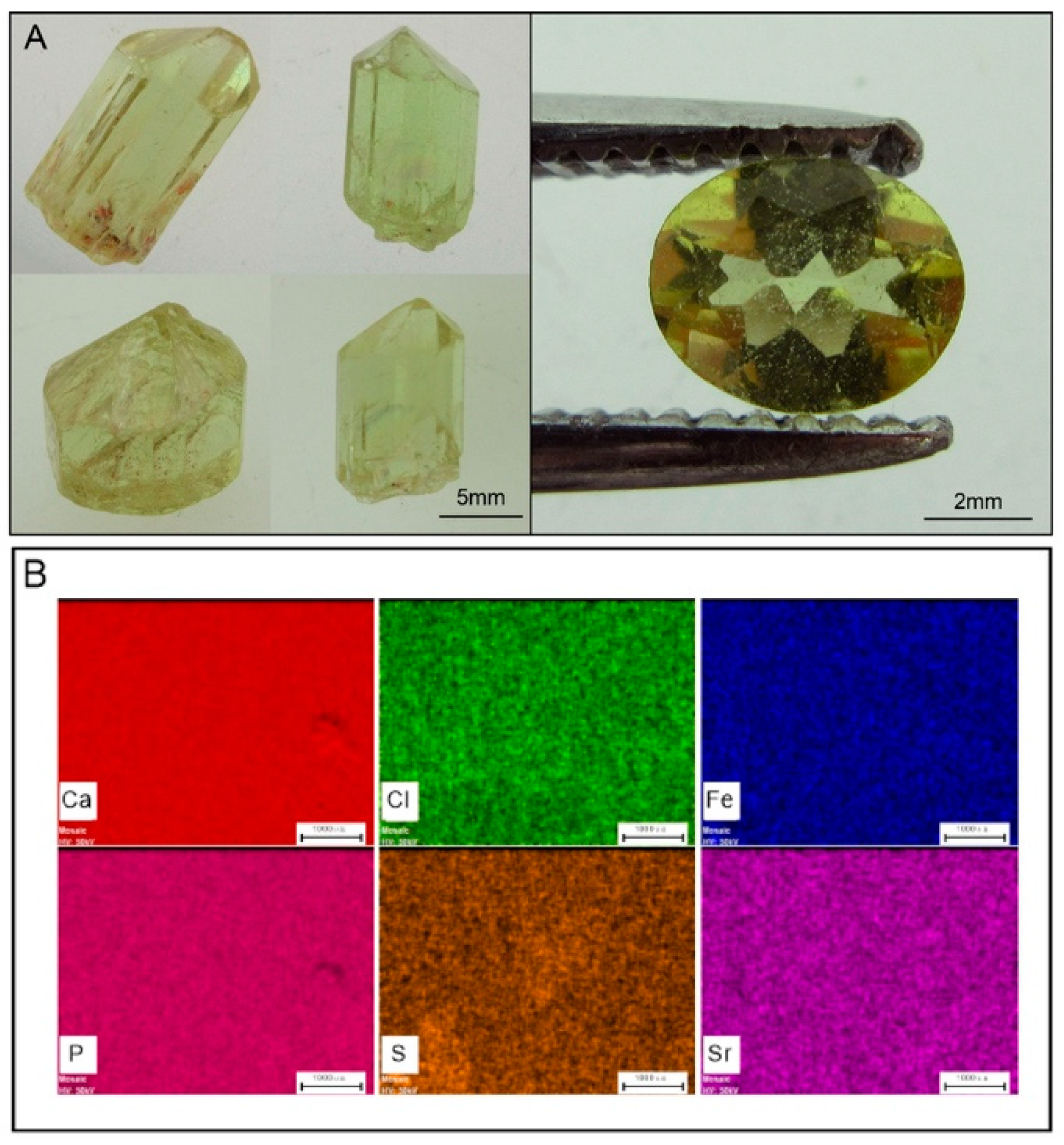
2. Standards and Natural Apatite
3. Experiment
3.1. X-ray Diffraction (XRD)
3.2. Infrared Spectra
3.3. Raman Spectra
3.4. EMPA
3.5. LA-ICP-MS
3.6. Micro-XRF Elemental Mapping
3.7. Sulphur X-ray Absorption Near-Edge Structure Spectroscopy (S XANES)
4. Results and Discussion
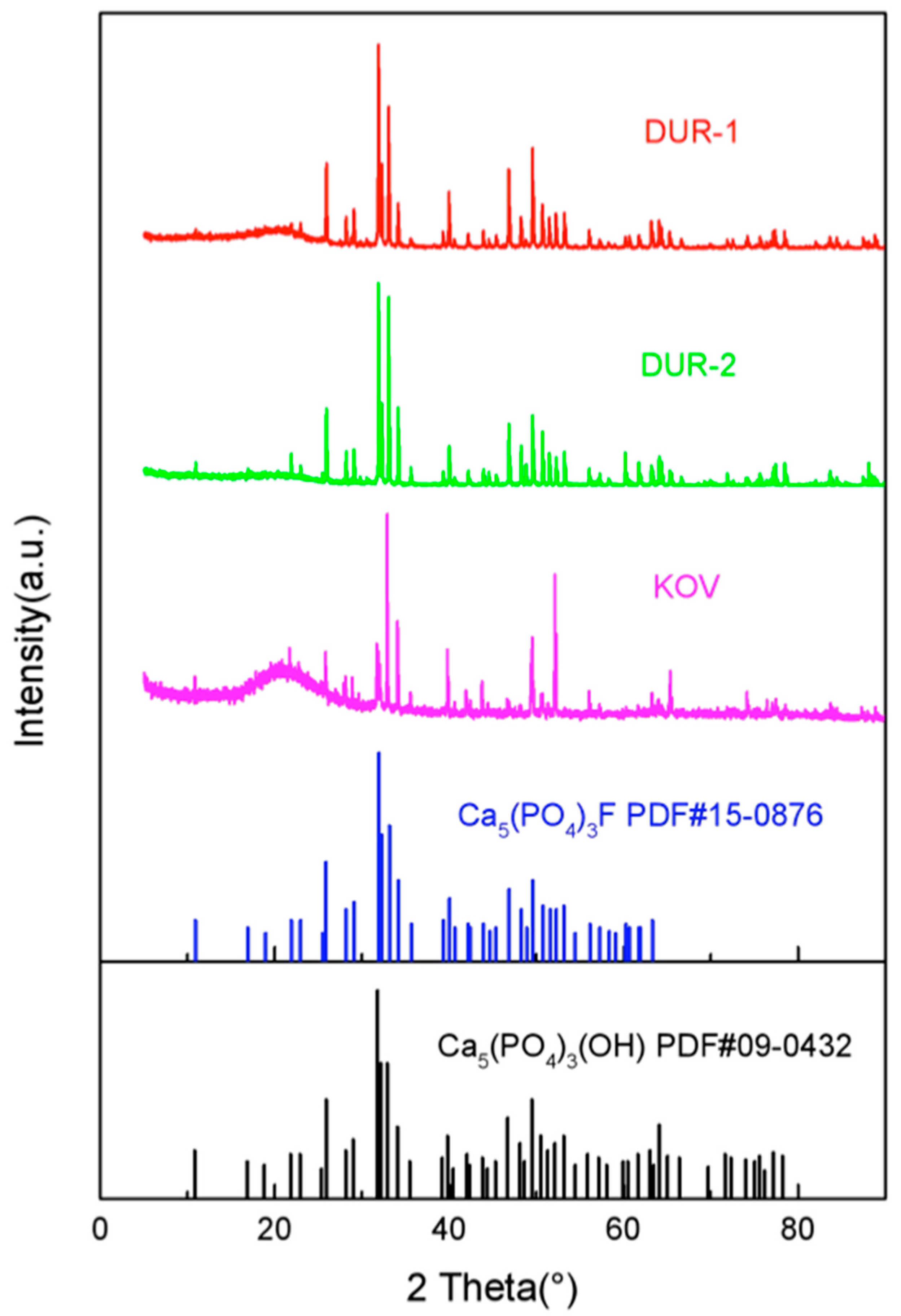
4.1. X-ray Diffraction (XRD)
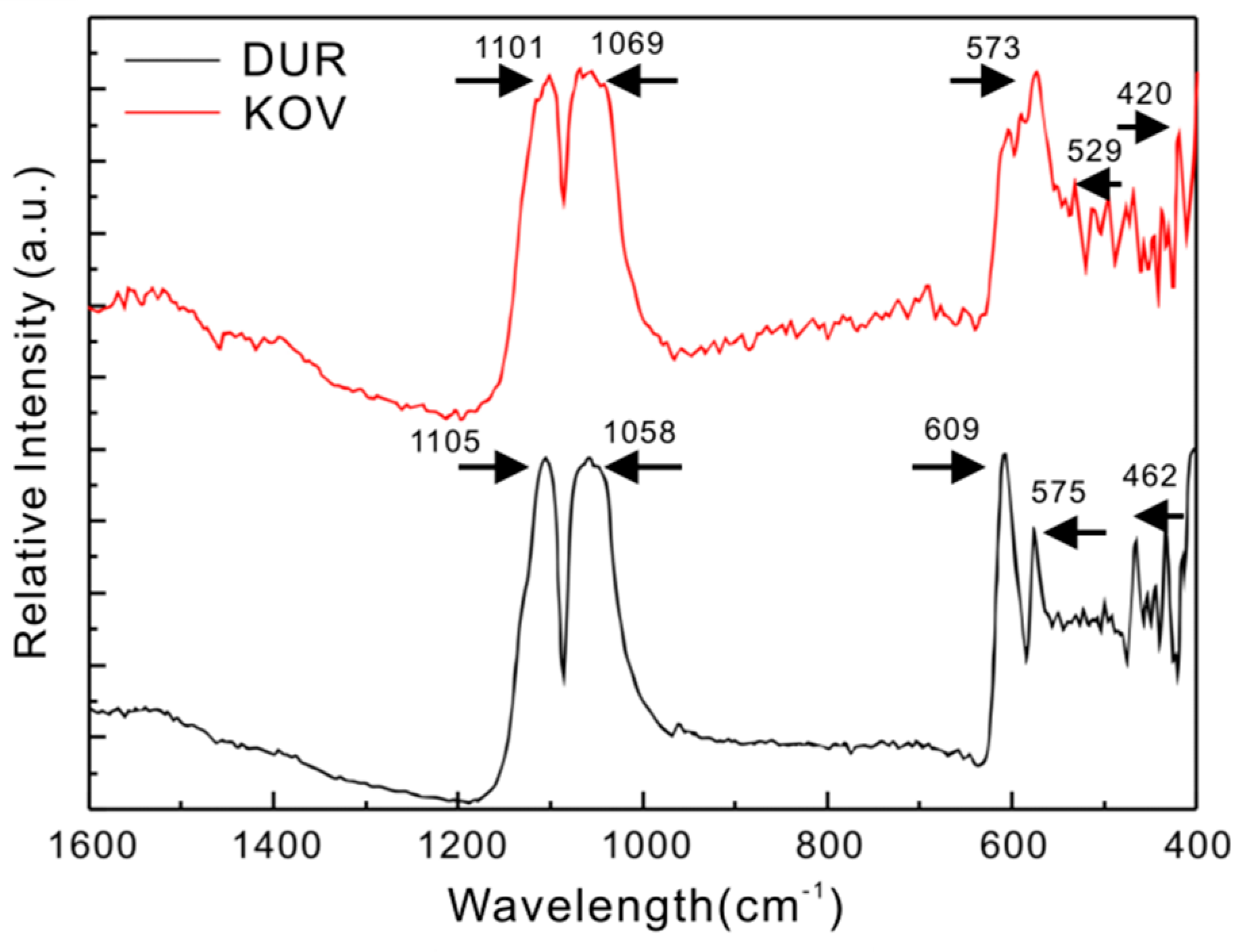
4.2. Infrared Spectra
4.3. Raman Spectra
4.4. Elemental Maps by Micro-XRF Mapping and Major-, Trace-Element Compositions of Apatite
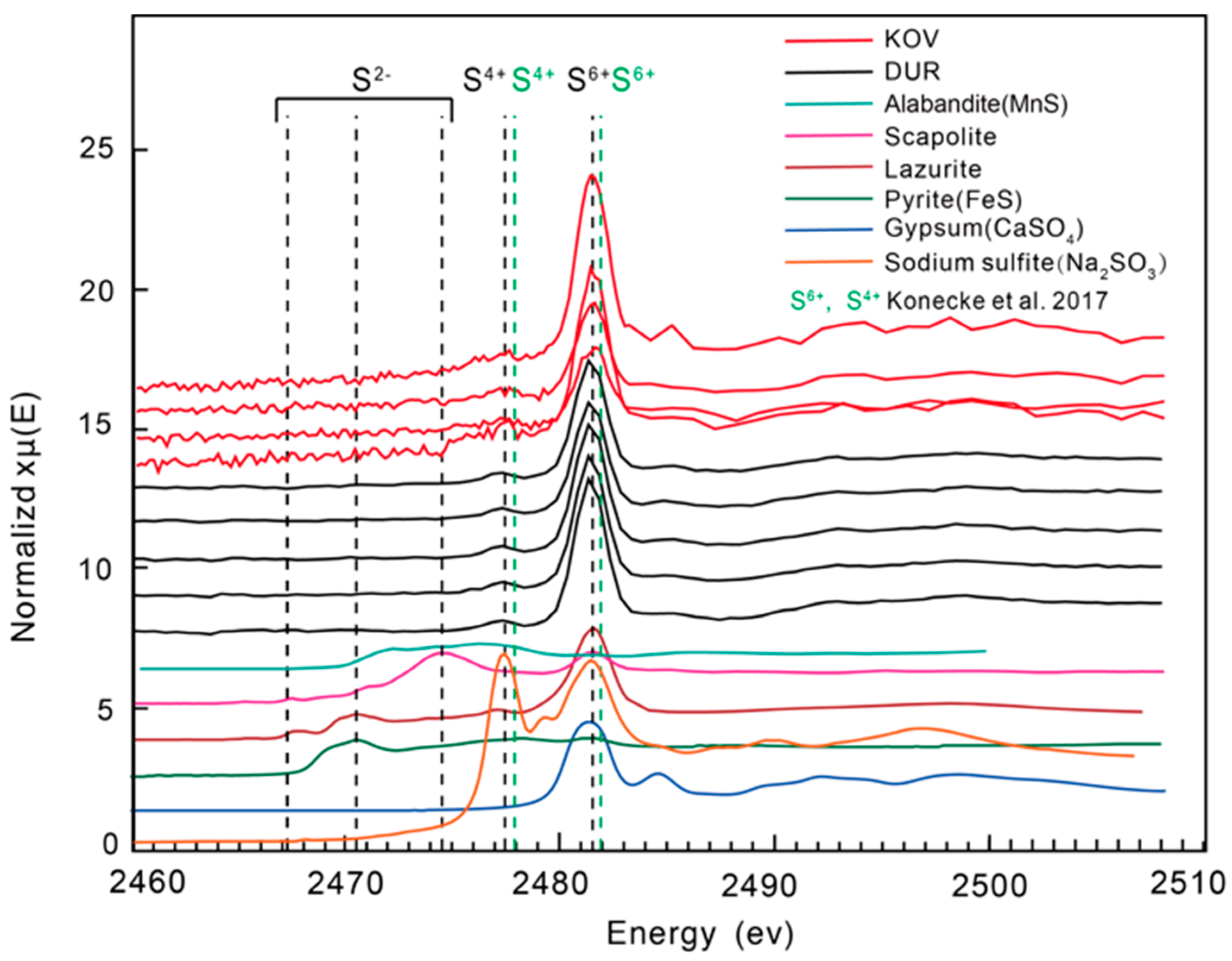
4.5. Sulphur X-ray Absorption Near-Edge Structure Spectroscopy (S XANES)
4.6. Homogeneous Chemistry and S Oxidation States, Implications for Potential Natural Apatite Reference Materials
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Simon, A.C.; Ripley, E.M. The Role of Magmatic Sulfur in the Formation of Ore Deposits. Rev. Mineral. Geochem. 2011, 73, 513–578. [Google Scholar] [CrossRef]
- Gustafson, L.B.; Hunt, J.P. The porphyry copper deposit at El Salvador, Chile. Econ. Geol. 1975, 70, 857–912. [Google Scholar] [CrossRef]
- Richards, J.P.; López, G.P.; Zhu, J.J.; Creaser, R.A.; Locock, A.J.; Mumin, A.H. Contrasting tectonic settings and sulfur contents of magmas associated with cretaceous porphyry Cu ± Mo ± Au and intrusion-related iron oxide Cu-Au deposits in northern Chile. Econ. Geol. 2017, 112, 295–318. [Google Scholar] [CrossRef] [Green Version]
- Candela, P.; Piccoli, P. Magmatic processes in the development of porphyry-type ore systems. One Hundredth Anniv. Vol. 2005, 25–37. Available online: https://pubs.geoscienceworld.org/books/book/1940/chapter/107706468/Magmatic-Processes-in-the-Development-of-Porphyry (accessed on 12 November 2020). [CrossRef]
- Parat, F.; Holtz, F. Sulfur partitioning between apatite and melt and effect of sulfur on apatite solubility at oxidizing conditions. Contrib. Mineral. Petr. 2004, 147, 201–212. [Google Scholar] [CrossRef]
- Marks, M.A.; Wenzel, T.; Whitehouse, M.J.; Loose, M.; Zack, T.; Barth, M.; Markl, G. The volatile inventory (F, Cl, Br, S, C) of magmatic apatite: An integrated analytical approach. Chem. Geol. 2012, 291, 241–255. [Google Scholar] [CrossRef]
- Rossi, M.; Ghiara, M.R.; Chita, G.; Capitelli, F. Crystal-chemical and structural characterization of fluorapatites in ejecta from Somma-Vesuvius volcanic complex. Am. Mineral. 2011, 96, 1828–1837. [Google Scholar] [CrossRef]
- Young, J.O.; Hanes, D.M.; Tappel, A.L. Chromatography of lysosomal enzymes on hydroxylapatite. J. Chromatogr. 1969, 44, 133–140. [Google Scholar] [CrossRef]
- Jarosewich, E.; Nelen, J.A.; Norberg, J.A. Reference samples for electron microprobe analysis. Geostand. Newsl. 1980, 4, 43–47. [Google Scholar] [CrossRef]
- Lapin, A.V.; Lyagushkin, A.P. The Kovdor Apatite–Francolite Deposit as a Prospective Source of Phosphate. Geol. Ore Deposit. 2014, 56, 61–80. [Google Scholar] [CrossRef]
- Fleet, M.E.; Liu, X.; Harmer, S.L.; Nesbitt, H.W. Chemical state of sulfur in natural and synthetic lazurite by S K-edge XANES and X-ray photoelectron spectroscopy. Can. Mineral. 2005, 43, 1589–1603. [Google Scholar] [CrossRef] [Green Version]
- Fleet, M.E. Xanes Spectroscopy of Sulfur in Earth Materials. Can. Mineral. 2005, 43, 1811–1838. [Google Scholar] [CrossRef] [Green Version]
- Farrell, S.P.; Fleet, M.E.; Stekhin, I.E.; Kravtsova, A.; Soldatov, A.V.; Liu, X.Y. Evolution of local electronic structure in alabandite and niningerite solid solutions [(Mn,Fe)S, (Mg,Mn)S, (Mg,Fe)S] using sulfur K- and L-edge XANES spectroscopy. Am. Mineral. 2002, 87, 1321–1332. [Google Scholar] [CrossRef]
- Callegaro, S.; Baker, D.R.; Min, A.D.; Marzoli, A.; Geraki, K.; Bertrand, H. Microanalyses link sulfur from large igneous provinces and mesozoic mass extinctions. Geology 2014, 42, 895–898. [Google Scholar] [CrossRef]
- Han, J.Y.; Guo, L.H.; Chen, W.S. Raman Spectra Atlas of Minerals; Geological Publishing House: Beijing, China, 2016. [Google Scholar]
- Chu, M.F.; Wang, K.L.; Griffin, W.L.; Chung, S.L.; O’Reilly, S.Y.; Pearson, N.J.; Iizuka, Y. Apatite composition: Tracing petrogenetic processes in Transhimalayan granitoids. J. Petrol. 2009, 50, 1829–1855. [Google Scholar] [CrossRef]
- Boyce, J.W.; Hervig, R.L. Apatite as a monitor of late-stage magmatic processes at Volcán Irazú, Costa Rica. Contrib. Mineral. Petrol. 2009, 157, 135. [Google Scholar] [CrossRef]
- Economos, R.; Boehnke, P.; Burgisser, A. Sulfur isotopic zoning in apatite crystals: A new record of dynamic sulfur behavior in magmas. Geochim. Cosmochim. Acta 2017, 215, 387–403. [Google Scholar] [CrossRef]
- Rukhlov, A.S.; Plouffe, A.; Ferbey, T.; Mao, M.; Spence, J. Application of Trace-Element Compositions of Detrital Apatite to Explore for Porphyry Deposits in Central British Columbia. 2016. Available online: https://www.semanticscholar.org/paper/Application-of-trace-element-compositions-of-to-for-Rukhlov-Plouffe/e3ea8c079ca63d0f74e1ee6286bfb51bbff18efe (accessed on 12 November 2020).
- Mao, M.; Rukhlov, A.S.; Rowins, S.M.; Spence, J.; Coogan, L.A. Apatite trace element compositions: A robust new tool for mineral exploration. Econ. Geol. 2016, 111, 1187–1222. [Google Scholar] [CrossRef]
- Rojas, P.A.; Barra, F.; Reich, M.; Deditius, A.; Simon, A.; Uribe, F.; Rojo, M. A genetic link between magnetite mineralization and diorite intrusion at the El Romeral iron oxide-apatite deposit, northern Chile. Miner. Deposita 2018, 53, 947–966. [Google Scholar] [CrossRef]
- Konecke, B.A.; Fiege, A.; Simon, A.C.; Parat, F.; Stechern, A. Co-variability of S6+, S4+, and S2− in apatite as a function of oxidation state: Implications for a new oxybarometer. Am. Mineral. 2017, 102, 548–557. [Google Scholar] [CrossRef]
- Boyce, J.W.; Liu, Y.; Rossman, G.R.; Guan, Y.; Eiler, J.M.; Stolper, E.M.; Taylor, L.A. Lunar apatite with terrestrial volatile abundances. Nature 2010, 466, 466–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanner, D.; Henley, R.W.; Mavrogenes, J.A.; Holden, P. Sulfur isotope and trace element systematics of zoned pyrite crystals from the el indio au-cu-ag deposit, chile. Contrib. Mineral. Petrol. 2016, 171, 33. [Google Scholar] [CrossRef] [Green Version]
- Zajacz, Z.; Candela, P.A.; Piccoli, P.M.; Sanchez-Valle, C. The partitioning of sulfur and chlorine between andesite melts and magmatic volatiles and the exchange coefficients of major cations. Geochim. Cosmochim. Acta 2012, 89, 81–101. [Google Scholar] [CrossRef]
- Burgisser, A.; Alletti, M.; Scaillet, B. Simulating the behavior of volatiles belonging to the C-O-H-S system in silicate melts under magmatic conditions with the software D-Compress. Compu. Geosci. 2015, 79, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Fiegea, A.; Holtza, F.; Behrensa, H.; Mandevilleb, C.W.; Shimizuc, N.; Credea, L.S.; Göttlicherd, J. Experimental investigation of the S and S-isotope distribution between H2O-S±Cl fluids and basaltic melts during decompression. Chem. Geol. 2015, 393–394, 36–54. [Google Scholar] [CrossRef]
- Harlov, D.E. Apatite: A Fingerprint for Metasomatic Processes. Elements 2015, 11, 171–176. [Google Scholar] [CrossRef]
- Tran, L.K.; Stepien, K.R.; Bollmeyer, M.M.; Yoder, C.H. Substitution of sulfate in apatite. Am. Mineral. 2017, 102, 1971–1976. [Google Scholar] [CrossRef]

| Samples | Unit-Cell Parameters | |
|---|---|---|
| a(Å) | c(Å) | |
| DUR-1 | 9.3657 (77) | 6.8685 (16) |
| DUR-2 | 9.3659 (74) | 6.8984 (1) |
| KOV | 9.3892 (25) | 6.8891(1) |
| Ca5(PO4)3(OH) PDF#09-0432 | 9.4180 | 6.8840 |
| Ca5(PO4)3F PDF#15-0876 | 9.3690 | 6.8840 |
| Sample | Na2O | SiO2 | CaO | P2O5 | SO3 | FeO | F | Cl | Total |
|---|---|---|---|---|---|---|---|---|---|
| DUR-1 | 0.23 | 0.5 | 53.6 | 39.97 | 0.32 | 0.07 | 2.97 | 0.43 | 99.04 |
| DUR-2 | 0.27 | 0.53 | 54.2 | 41.03 | 0.31 | 0.02 | 3.15 | 0.42 | 100.81 |
| DUR-3 | 0.28 | 0.51 | 53.82 | 40.26 | 0.32 | 0.01 | 3.05 | 0.44 | 99.73 |
| DUR-4 | 0.26 | 0.57 | 53.95 | 41 | 0.32 | 0.05 | 3.01 | 0.39 | 100.48 |
| DUR-5 | 0.28 | 0.41 | 54.15 | 40.99 | 0.32 | 0.07 | 3.17 | 0.43 | 100.6 |
| DUR-6 | 0.27 | 0.34 | 54.25 | 40.86 | 0.34 | 0.04 | 3.01 | 0.42 | 100.4 |
| DUR-7 | 0.25 | 0.49 | 54.12 | 40.66 | 0.32 | 0.02 | 3.03 | 0.4 | 100 |
| DUR-8 | 0.21 | 0.44 | 54.25 | 40.86 | 0.34 | 0.07 | 3.06 | 0.41 | 100.34 |
| DUR-9 | 0.25 | 0.49 | 54.07 | 40.62 | 0.34 | 0.03 | 3.08 | 0.42 | 100.01 |
| DUR-11 | 0.29 | 0.37 | 54.34 | 40.98 | 0.34 | 0.03 | 3.05 | 0.43 | 100.52 |
| DUR-12 | 0.28 | 0.34 | 54.29 | 41 | 0.33 | 0.04 | 3.03 | 0.42 | 100.43 |
| DUR-13 | 0.28 | 0.38 | 54.55 | 41.18 | 0.34 | 0.04 | 2.96 | 0.41 | 100.92 |
| DUR-14 | 0.26 | 0.43 | 54.44 | 40.67 | 0.35 | 0.03 | 3.01 | 0.41 | 100.34 |
| DUR-15 | 0.28 | 0.42 | 54.41 | 40.68 | 0.34 | 0.04 | 3 | 0.41 | 100.37 |
| DUR-16 | 0.3 | 0.36 | 54.45 | 40.94 | 0.35 | 0.03 | 3.11 | 0.41 | 100.69 |
| KOV1-1 | 0.269 | 0.294 | 52.208 | 40.151 | 0.015 | 0.007 | 1.496 | 0.014 | 94.621 |
| KOV1-2 | 0.301 | 0.259 | 52.375 | 39.979 | 0.025 | - | 1.595 | 0.013 | 94.694 |
| KOV1-3 | 0.227 | 0.476 | 52.266 | 39.83 | 0.011 | 0.009 | 1.557 | 0.013 | 94.676 |
| KOV2-1 | 0.272 | 0.61 | 52.007 | 39.265 | 0.019 | 0.002 | 1.63 | 0.018 | 94.065 |
| KOV2-2 | 0.251 | 0.554 | 52.111 | 39.738 | 0.016 | 0.03 | 1.638 | 0.027 | 94.738 |
| KOV2-3 | 0.231 | 0.579 | 52.211 | 39.733 | 0.025 | 0.009 | 1.572 | 0.017 | 94.731 |
| KOV3-1 | 0.218 | 0.447 | 52.448 | 39.941 | 0.017 | - | 1.625 | 0.02 | 94.92 |
| KOV3-2 | 0.234 | 0.527 | 52.259 | 40.227 | 0.013 | 0.021 | 1.877 | 0.017 | 95.252 |
| KOV3-3 | 0.258 | 0.495 | 52.112 | 39.968 | 0.013 | 0.028 | 1.894 | 0.017 | 94.923 |
| Samples | Sr (ppm) | Y (ppm) |
|---|---|---|
| DUR-01 | 477.86 | 555.13 |
| DUR-02 | 478.34 | 556.79 |
| DUR-03 | 477.73 | 574.91 |
| DUR-04 | 480.6 | 577.63 |
| DUR-05 | 480.55 | 577.54 |
| DUR-06 | 474.93 | 558.05 |
| DUR-07 | 477.54 | 560.46 |
| DUR-08 | 477.57 | 560.44 |
| DUR-09 | 482.11 | 550.49 |
| DUR-10 | 480.93 | 549.23 |
| DUR-11 | 480.76 | 549.08 |
| KOV-01 | 2767.97 | 222.11 |
| KOV-02 | 2771.66 | 222.77 |
| KOV-03 | 2726.27 | 261.3 |
| KOV-04 | 2739.79 | 262.33 |
| KOV-05 | 2740.4 | 262.35 |
| KOV-06 | 2771.72 | 229 |
| KOV-07 | 2784.01 | 229.8 |
| KOV-08 | 2785.06 | 229.85 |
| KOV-09 | 2612.97 | 194.45 |
| KOV-10 | 2604.21 | 193.44 |
| KOV-11 | 2601.36 | 193.28 |
| KOV-12 | 2801.04 | 230.65 |
| KOV-13 | 2785.84 | 228.78 |
| KOV-14 | 2781.77 | 228.53 |
| KOV-15 | 2688.25 | 207.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, B.; Kou, G.; Etschmann, B.; Liu, D.; Brugger, J. Spectroscopic, Raman, EMPA, Micro-XRF and Micro-XANES Analyses of Sulphur Concentration and Oxidation State of Natural Apatite Crystals. Crystals 2020, 10, 1032. https://doi.org/10.3390/cryst10111032
Xu B, Kou G, Etschmann B, Liu D, Brugger J. Spectroscopic, Raman, EMPA, Micro-XRF and Micro-XANES Analyses of Sulphur Concentration and Oxidation State of Natural Apatite Crystals. Crystals. 2020; 10(11):1032. https://doi.org/10.3390/cryst10111032
Chicago/Turabian StyleXu, Bo, Guanyu Kou, Barbara Etschmann, Daiyue Liu, and Joël Brugger. 2020. "Spectroscopic, Raman, EMPA, Micro-XRF and Micro-XANES Analyses of Sulphur Concentration and Oxidation State of Natural Apatite Crystals" Crystals 10, no. 11: 1032. https://doi.org/10.3390/cryst10111032




