Shock Damage Analysis in Serial Femtosecond Crystallography Data Collected at MHz X-ray Free-Electron Lasers
Abstract
:1. Introduction
2. Materials and Methods
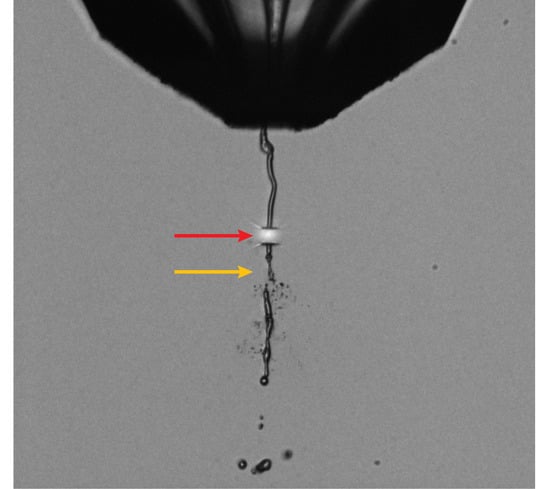
2.1. Sample Preparation, Injection, and Jet Imaging
2.2. Data Collection
2.3. Data Analysis for Number of Prior Pulses Launching Shocks
2.4. Data Processing
3. Results
3.1. Analysis of all Crystal Hits
3.2. Analysis of Hits in Continuous Jets
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Weierstall, U.; Spence, J.C.H.; Doak, R.B. Injector for scattering measurements on fully solvated biospecies. Rev. Sci. Instrum. 2012, 83, 035108. [Google Scholar] [CrossRef] [PubMed]
- Stan, C.A.; Milathianaki, D.; Laksmono, H.; Sierra, R.G.; McQueen, T.A.; Messerschmidt, M.; Williams, G.J.; Koglin, J.E.; Lane, T.J.; Hayes, M.J.; et al. Liquid explosions induced by X-ray laser pulses. Nat. Phys. 2016, 12, 966–971. [Google Scholar] [CrossRef]
- Blaj, G.; Liang, M.; Aquila, A.L.; Willmott, P.R.; Koglin, J.E.; Sierra, R.G.; Robinson, J.S.; Boutet, S.; Stan, C.A. Generation of high-intensity ultrasound through shock propagation in liquid jets. Phys. Fluids 2019, 4, 043401. [Google Scholar] [CrossRef]
- Grünbein, M.L.; Bielecki, J.; Gorel, A.; Stricker, M.; Bean, R.; Cammarata, M.; Dörner, K.; Fröhlich, L.; Hartmann, E.; Hauf, S.; et al. Megahertz data collection from protein microcrystals at an X-ray free-electron laser. Nat. Commun. 2018, 9, 3487. [Google Scholar] [CrossRef] [PubMed]
- Wiedorn, M.O.; Oberthür, D.; Bean, R.; Schubert, R.; Werner, N.; Abbey, B.; Aepfelbacher, M.; Adriano, L.; Allahgholi, A.; Al-Qudami, N.; et al. Megahertz serial crystallography. Nat. Commun. 2018, 9, 4025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yefanov, O.; Oberthür, D.; Bean, R.; Wiedorn, M.O.; Knoska, J.; Pena, G.; Awel, S.; Gumprecht, L.; Domaracky, M.; Sarrou, I.; et al. Evaluation of serial crystallographic structure determination within megahertz pulse trains. Struct. Dyn. 2019, 6, 064702. [Google Scholar] [CrossRef]
- Decking, W.; Abeghyan, S.; Abramian, P.; Abramsky, A.; Aguirre, A.; Albrecht, C.; Alou, P.; Altarelli, M.; Altmann, P.; Amyan, K.; et al. A MHz-repetition-rate hard X-ray free-electron laser driven by a superconducting linear accelerator. Nat. Photonics 2020, 14, 391–397. [Google Scholar] [CrossRef]
- Mancuso, A.P.; Aquila, A.; Batchelor, L.; Bean, R.J.; Bielecki, J.; Borchers, G.; Doerner, K.; Giewekemeyer, K.; Graceffa, R.; Kelsey, O.D.; et al. The Single Particles, Clusters and Biomolecules and Serial Femtosecond Crystallography instrument of the European XFEL: Initial installationThis article will form part of a virtual special issue on X-ray free-electron lasers. J. Synchrotron Radiat. 2019, 26, 660–676. [Google Scholar] [CrossRef]
- Obier, F.; Decking, W.; Hüning, M.; Wortmann, J. Fast Kicker System for European XFEL Beam Distribution. In Proceedings of the 39th International Free-Electron Laser Conference, Hamburg, Germany, 26–30 August 2019; JACOW Publishing: Geneva, Switzerland, 2019; pp. 353–356. [Google Scholar] [CrossRef]
- Boutet, S.; Lomb, L.; Williams, G.J.; Barends, T.R.; Aquila, A.; Doak, R.B.; Weierstall, U.; DePonte, D.P.; Steinbrener, J.; Shoeman, R.L. High-resolution protein structure determination by serial femtosecond crystallography. Science 2012, 337, 362–364. [Google Scholar] [CrossRef] [Green Version]
- Barends, T.R.; Foucar, L.; Ardevol, A.; Nass, K.; Aquila, A.; Botha, S.; Doak, R.B.; Falahati, K.; Hartmann, E.; Hilpert, M. Direct observation of ultrafast collective motions in CO myoglobin upon ligand dissociation. Science 2015, 350, 445–450. [Google Scholar] [CrossRef]
- Grünbein, M.L.; Bielecki, J.; Gorel, A.; Stricker, M.; Bean, R.; Cammarata, M.; Dörner, K.; Fröhlich, L.; Hartmann, E.; Hauf, S.; et al. MHz data collection of a microcrystalline mixture of different jack bean proteins. Sci. Data 2019, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Lomb, L.; Steinbrener, J.; Bari, S.; Beisel, D.; Berndt, D.; Kieser, C.; Lukat, M.; Neef, N.; Shoeman, R.L. An anti-settling sample delivery instrument for serial femtosecond crystallography. J. Appl. Crystallogr. 2012, 45, 674–678. [Google Scholar] [CrossRef]
- Palmer, G.; Kellert, M.; Wang, J.; Emons, M.; Wegner, U.; Kane, D.; Pallas, F.; Jezynski, T.; Venkatesan, S.; Rompotis, D.; et al. Pump-probe laser system at the FXE and SPB/SFX instruments of the European X-ray Free-Electron Laser FacilityThis article will form part of a virtual special issue on X-ray free-electron lasers. J. Synchrotron Radiat. 2019, 26, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Pergament, M.; Palmer, G.; Kellert, M.; Kruse, K.; Wang, J.; Wissmann, L.; Wegner, U.; Emons, M.; Kane, D.; Priebe, G.; et al. Versatile optical laser system for experiments at the European X-ray free-electron laser facility. Opt. Express 2016, 24, 29349–29359. [Google Scholar] [CrossRef] [PubMed]
- Maltezopoulos, T.; Dietrich, F.; Freund, W.; Jastrow, U.F.; Koch, A.; Laksman, J.; Liu, J.; Planas, M.; Sorokin, A.A.; Tiedtke, K.; et al. Operation of X-ray gas monitors at the European XFEL. J. Synchrotron Radiat. 2019, 26, 1045–1051. [Google Scholar] [CrossRef] [Green Version]
- Allahgholi, A.; Becker, J.; Delfs, A.; Dinapoli, R.; Goettlicher, P.; Greiffenberg, D.; Henrich, B.; Hirsemann, H.; Kuhn, M.; Klanner, R.; et al. The Adaptive Gain Integrating Pixel Detector at the European XFEL. J. Synchrotron Radiat. 2019, 26, 74–82. [Google Scholar] [CrossRef] [Green Version]
- Kuster, M.; Boukhelef, D.; Donato, M.; Dambietz, J.S.; Hauf, S.; Maia, L.; Raab, N.; Szuba, J.; Turcato, M.; Wrona, K.; et al. Detectors and Calibration Concept for the European XFEL. Synchrotron Radiation News 2014, 27, 35–38. [Google Scholar] [CrossRef]
- Foucar, L. CFEL-ASG Software Suite (CASS): Usage for free-electron laser experiments with biological focus. J. Appl. Crystallogr. 2016, 49, 1336–1346. [Google Scholar] [CrossRef] [Green Version]
- ZeroMQ Message Transport Protocol. Available online: https://rfc.zeromq.org/spec/23/ (accessed on 16 December 2020).
- Hintjens, P. Messaging for Many Applications. In ZeroMQ, 1st ed.; O‘Reilly Media, Inc.: Sebastopol, CA, USA, 2013. [Google Scholar]
- Fangohr, H.; Beg, M.; Bondar, V.; Boukhelef, D.; Brockhauser, S.; Danilevski, C.; Ehsan, W.; Esenov, S.; Flucke, G.; Giovanetti, G.; et al. Data Analysis Support in Karabo at European XFEL. In Proceedings of the ICALEPCS 2017: 16th International Conference on Accelerator and Large Experimental Control Systems, Barcelona, Spain, 8–13 October 2017; JACoW: Geneva, Switzerland. [Google Scholar] [CrossRef]
- White, T.A.; Kirian, R.A.; Martin, A.V.; Aquila, A.; Nass, K.; Barty, A.; Chapman, H.N. CrystFEL: A software suite for snapshot serial crystallography. J. Appl. Crystallogr. 2012, 45, 335–341. [Google Scholar] [CrossRef] [Green Version]
- Maia, F.R. The Coherent X-ray Imaging Data Bank. Nat. Methods 2012, 9, 854–855. [Google Scholar] [CrossRef]
- Karplus, P.A.; Diederichs, K. Assessing and maximizing data quality in macromolecular crystallography. Curr. Opin. Struct. Biol. 2015, 34, 60–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barends, T.R.; Foucar, L.; Shoeman, R.L.; Bari, S.; Epp, S.W.; Hartmann, R.; Hauser, G.; Huth, M.; Kieser, C.; Lomb, L.; et al. Anomalous signal from S atoms in protein crystallographic data from an X-ray free-electron laser. Acta Crystallogr. D 2013, 69, 838–842. [Google Scholar] [CrossRef] [PubMed]




| Lysozyme Run 20–52 | First Pulse of First Wagon | Second Pulse of First Wagon | Third Pulse of First Wagon | Fourth Pulse of First Wagon | Fifth Pulse of First Wagon |
| Space group | P43212 | P43212 | P43212 | P43212 | P43212 |
| Cell dimensions (Å, °) | 79. 79. 39. 90. 90. 90. | 79. 79. 39. 90. 90. 90. | 79. 79. 39. 90. 90. 90. | 79. 79. 39. 90. 90. 90. | 79. 79. 39. 90. 90. 90. |
| # indexed images | 843 | 896 | 831 | 808 | 881 |
| Resolution (Å) | 22.83–1.80 (1.85–1.80) | 26.36–1.80 (1.85–1.80) | 26.37–1.80 (1.85–1.80) | 21.95–1.80 (1.85–1.80) | 22.83–1.80 (1.85–1.80) |
| I/σ(I) | 1.6 (1.0) | 2.0 (1.0) | 1.5 (0.6) | 1.5 (1.2) | 1.5 (0.8) |
| Rsplit (%) | 60.0 (126.7) | 60.2 (112.8) | 60.9 (115.8) | 61.2 (116.5) | 58.7 (129.6) |
| CC1/2 | 0.604 (0.215) | 0.61 (0.296) | 0.585 (0.333) | 0.575 (0.265) | 0.608 (0.177) |
| CC* | 0.868 (0.595) | 0.87 (0.676) | 0.859 (0.707) | 0.854 (0.648) | 0.87 (0.548) |
| Completeness (%) | 99.4 (98.6) | 99.4 (99.1) | 99.3 (98.8) | 99.4 (98.8) | 99.5 (99.7) |
| Multiplicity | 11.9 (7.7) | 12.1 (7.9) | 11.9 (8.1) | 11.6 (7.6) | 12.8 (8.4) |
| Wilson B (Å2) | 14.9 | 14.9 | 15.3 | 15.0 | 15.1 |
| Correlation on Is with entire wagon (%) | 91.8 | 92.1 | 91.9 | 91.6 | 92.6 |
| Myoglobin Run 66–107 | First Pulse of Sixth Wagon | Second Pulse of Sixth Wagon | Third Pulse of Sixth Wagon | Fourth Pulse of Sixth Wagon | Fifth Pulse of Sixth Wagon |
| Space group | P21 | P21 | P21 | P21 | P21 |
| Cell dimensions (Å,°) | 63.6 28.8 35.6 90. 106.5 90. | 63.6 28.8 35.6 90. 106.5 90. | 63.6 28.8 35.6 90. 106.5 90. | 63.6 28.8 35.6 90. 106.5 90. | 63.6 28.8 35.6 90. 106.5 90. |
| # indexed images | 971 | 823 | 858 | 881 | 917 |
| Resolution (Å) | 21.26–1.80 (1.85–1.80) | 22.34–1.80 (1.85–1.80) | 21.26–1.80 (1.85–1.80) | 22.34–1.80 (1.85–1.80) | 19.85–1.80 (1.85–1.80) |
| I/σ(I) | 2.1 (1.3) | 2.0 (1.6) | 2.0 (1.8) | 2.0 (1.4) | 2.1 (1.7) |
| Rsplit (%) | 64.9 (95.0) | 66.4 (99.6) | 63.3 (92.4) | 64.0 (94.2) | 66.8 (90.3) |
| CC1/2 | 0.42 (0.102) | 0.417 (0.245) | 0.456 (0.534) | 0.427 (0.154) | 0.42 (0.251) |
| CC* | 0.769 (0.429) | 0.767 (0.628) | 0.792 (0.834) | 0.774 (0.517) | 0.769 (0.634) |
| Completeness (%) | 97.9 (93.3) | 96.6 (94.8) | 97.1 (94.4) | 96.9 (94.4) | 97.7 (95.5) |
| Multiplicity | 10.6 (7.0) | 8.8 (5.8) | 9.5 (6.3) | 9.2 (6.1) | 10.2 (6.4) |
| Wilson B (Å2) | 12.9 | 13.7 | 14.5 | 14.7 | 13.1 |
| Correlation on Is with entire wagon (%) | 91.2 | 89.6 | 90.2 | 90.6 | 91.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorel, A.; Grünbein, M.L.; Bean, R.; Bielecki, J.; Hilpert, M.; Cascella, M.; Colletier, J.-P.; Fangohr, H.; Foucar, L.; Hartmann, E.; et al. Shock Damage Analysis in Serial Femtosecond Crystallography Data Collected at MHz X-ray Free-Electron Lasers. Crystals 2020, 10, 1145. https://doi.org/10.3390/cryst10121145
Gorel A, Grünbein ML, Bean R, Bielecki J, Hilpert M, Cascella M, Colletier J-P, Fangohr H, Foucar L, Hartmann E, et al. Shock Damage Analysis in Serial Femtosecond Crystallography Data Collected at MHz X-ray Free-Electron Lasers. Crystals. 2020; 10(12):1145. https://doi.org/10.3390/cryst10121145
Chicago/Turabian StyleGorel, Alexander, Marie Luise Grünbein, Richard Bean, Johan Bielecki, Mario Hilpert, Michele Cascella, Jacques-Philippe Colletier, Hans Fangohr, Lutz Foucar, Elisabeth Hartmann, and et al. 2020. "Shock Damage Analysis in Serial Femtosecond Crystallography Data Collected at MHz X-ray Free-Electron Lasers" Crystals 10, no. 12: 1145. https://doi.org/10.3390/cryst10121145
APA StyleGorel, A., Grünbein, M. L., Bean, R., Bielecki, J., Hilpert, M., Cascella, M., Colletier, J.-P., Fangohr, H., Foucar, L., Hartmann, E., Hunter, M. S., Kirkwood, H., Kloos, M., Letrun, R., Michelat, T., Shoeman, R. L., Sztuk-Dambietz, J., Tetreau, G., Zimmermann, H., ... Schlichting, I. (2020). Shock Damage Analysis in Serial Femtosecond Crystallography Data Collected at MHz X-ray Free-Electron Lasers. Crystals, 10(12), 1145. https://doi.org/10.3390/cryst10121145