Growth and Characterization of (Tb,Yb) Co-Doping Sprayed ZnO Thin Films
Abstract
1. Introduction
2. Experimental Details
3. Results and Discussion
3.1. Micro-Structural and Morphological Properties
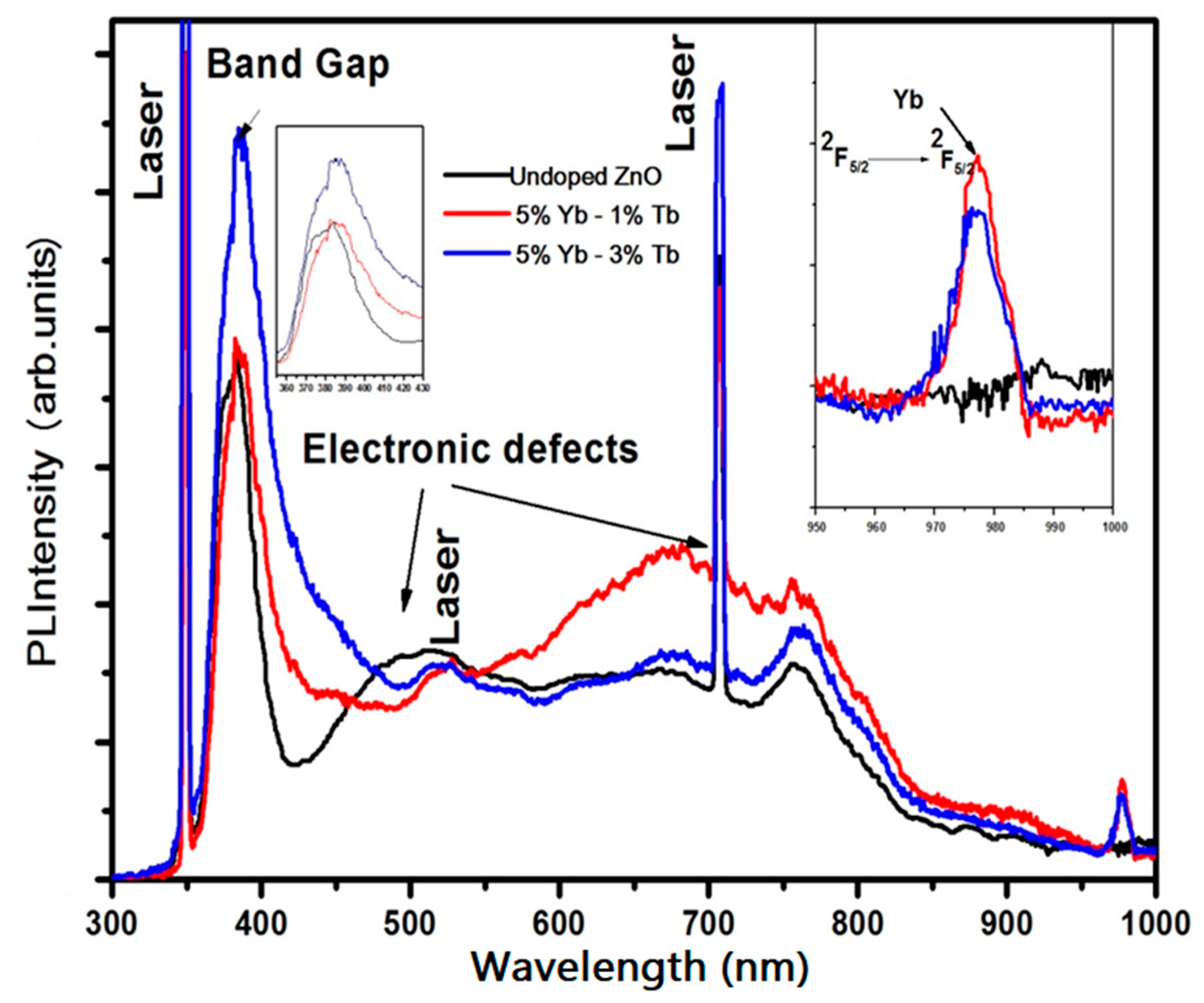
3.2. Optical Transmittance and Photoluminescence
3.3. Electrical Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lu, Y.M.; Li, X.P.; Cao, P.J.; Su, S.C.; Jia, F.; Han, S.; Liu, W.J.; Zhu, D.L.; Ma, X.C. Study of ultraviolet emission spectra in ZnO thin films. J. Spectrosc. 2013, 2013, 797232. [Google Scholar] [CrossRef]
- Caglara, M.; Ilican, S.; Caglar, Y.; Yakuphanoglu, F. Boron doped nanostructure ZnO films onto ITO substrate. J. Alloys Compd. 2011, 509, 3177–3182. [Google Scholar] [CrossRef]
- Robles-Águila, M.J.; Luna-López, J.A.; de la Luz, Á.D.H.; Martínez-Juárez, J.; Rabanal, M.E. Synthesis and characterization of nanocrystalline ZnO doped with Al3+ and Ni2+ by a sol–gel method coupled with ultrasound irradiation. Crystals 2018, 8, 406. [Google Scholar] [CrossRef]
- Ahn, K.J.; Lee, S.; Kim, W.J.; Yeom, G.Y.; Lee, W. Characteristics of Ga-doped ZnO films deposited by pulsed DC magnetron sputtering at low temperature. Mater. Sci. Semicond. Process. 2013, 16, 1957–1963. [Google Scholar] [CrossRef]
- Douayar, A.; Diaz, R.; Prieto, P.; Abd-Lefdil, M. Structural, optical and electrical properties of ZnO sprayed thin films doped with fluorine. Adv. Mater. Res. 2011, 324, 253–256. [Google Scholar] [CrossRef]
- Liu, J.P.; Qu, S.C.; Zeng, X.B.; Xu, Y.; Gou, X.F.; Wang, Z.J.; Zhou, H.Y.; Wang, Z.G. Fabrication of ZnO and its enhancement of charge injection and transport in hybrid organic/inorganic light emitting devices. Appl. Surf. Sci. 2007, 253, 7506–7509. [Google Scholar] [CrossRef]
- Chirakkara, S.; Krupanidhi, S.B. Pulsed laser deposited ZnO/ZnO: Li multilayer for blue light emitting diodes. J. Lumin. 2011, 131, 1649–1654. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, G.; Yu, L.; Bai, X.; Zhang, Z.; Zhao, X. Field emission properties of zinc oxide nanowires fabricated by thermal evaporation. Physica E 2007, 36, 86–91. [Google Scholar] [CrossRef]
- Lingling, W.; Maiphi, H.; Gennady, N.P.; Taewon, K.; Fu, D.J. Enhanced field emission from self-assembled ZnO nanorods on graphene/Ni/Si substrates. Mater. Lett. 2013, 112, 183–186. [Google Scholar]
- Breivik, T.H.; Diplas, S.; Ulyashin, A.G.; Gunnæs, A.E.; Olaisen, B.R.; Wright, D.N.; Holt, A.; Olsen, A. Nano-structural properties of ZnO films for Si based heterojunction solar cells. Thin Solid Films 2007, 515, 8479–8483. [Google Scholar] [CrossRef]
- Giannouli, M.; Spiliopoulou, F. Effects of the morphology of nanostructured ZnO films on the efficiency of dye-sensitized solar cells. Renew. Energy 2012, 41, 115–122. [Google Scholar] [CrossRef]
- Lupan, O.; Chai, G.; Chow, L. Fabrication of ZnO nanorod-based hydrogen gas nanosensor. Microelectron. J. 2007, 38, 1211–1216. [Google Scholar] [CrossRef]
- Rambu, A.P.; Ursu, L.; Iftimie, N.; Nica, V.; Dobromir, M.; Iacomi, F. Study on Ni-doped ZnO films as gas sensors. Appl. Surf. Sci. 2013, 280, 598–604. [Google Scholar] [CrossRef]
- Vettumperumal, R.; Kalyanaraman, S.; Thangavel, R. Optical constants and near infrared emission of Er doped ZnO sol–gel thin films. J. Lumin. 2015, 158, 493–500. [Google Scholar] [CrossRef]
- Sahu, D.R.; Lin, S.Y.; Huang, J.L. Improved properties of Al-doped ZnO film by electron beam evaporation technique. Microelectron. J. 2007, 38, 245–250. [Google Scholar] [CrossRef]
- Chaki, I.; Belayachi, A.; el Bahraoui, T.; Regragui, M.; Abd-Lefdil, M. Semiconducting properties of Tm doped Yb-ZnO films by spray pyrolysis. Eur. Phys. J. Appl. Phys. 2014, 68, 30301. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Yang, J.R.; Cheng, S.L.; Shiojiri, M. Structural investigation of ZnO: Al films deposited on the Si substrates by radio frequency magnetron sputtering. Thin Solid Films 2013, 545, 183–187. [Google Scholar] [CrossRef]
- Kaur, G.; Dwivedi, Y.; Rai, S.B. Gd3+ Sensitized Enhanced Green Luminescence in Gd:Tb(Sal)3 Phen Complex in PVA. J. Fluoresc. 2011, 21, 423–432. [Google Scholar] [CrossRef]
- Salley, G.M.; Valiente, R.; Gudel, H.U. Luminescence upconversion mechanisms in Yb3+–Tb3+ systems. J. Lumin. 2001, 94–95, 305–309. [Google Scholar] [CrossRef]
- John, R.; Rajakumari, R. Synthesis and characterization of rare earth ion doped nano ZnO. Nano-Micro Lett. 2012, 4, 65–72. [Google Scholar] [CrossRef]
- Douayar, A.; Prieto, P.; Schmerber, G.; Nouneh, K.; Diaz, R.; Chaki, I.; Colis, S.; el Fakir, A.; Hassanain, N.; Belayachi, A.; et al. Investigation of the structural, optical and electrical properties of Nd-doped ZnO thin films deposited by spray pyrolysis. Eur. Phys. J. Appl. Phys. 2013, 61, 10304–10306. [Google Scholar] [CrossRef][Green Version]
- Yang, Y.H.; Zhu, H.G.; Dong, H.M.; Yang, G.W. Growth and luminescence of Tb-doped ZnO nanocones. Mater. Lett. 2014, 124, 32–35. [Google Scholar] [CrossRef]
- Lv, Z.J.; Liu, Y.; Yu, H.; Dong, X.T.; Yang, H. Hollow tubular Tb3+ and Yb3+ co-doped ordered mesoporous ZnO composite materials and its luminescent properties. Opt. Mater. 2019, 89, 528–535. [Google Scholar] [CrossRef]
- Florêncio, L.D.A.; Gómez-Malagón, L.A.; Lima, B.C.; Gomes, A.S.; Garcia, J.A.M.; Kassab, L.R. Efficiency enhancement in solar cells using photon down-conversion in Tb/Yb-doped tellurite glass. Sol. Energy Mater. Sol. Cells 2016, 157, 468–475. [Google Scholar] [CrossRef]
- Shinde, S.S.; Shinde, P.S.; Pawar, S.M.; Moholkar, A.V.; Bhosale, C.H.; Rajpure, K.Y. Physical properties of transparent and conducting sprayed fluorine doped zinc oxide thin films. Solid State Sci. 2008, 10, 1209–1214. [Google Scholar] [CrossRef]
- Elfakir, A.; Douayar, A.; Diaz, R.; Chaki, I.; Prieto, P.; Loghmarti, M.; Belayachi, A.; Abd-lefdil, M. Elaboration and characterization of sprayed Tb-doped ZnO thin films. Thin Film. Sens. Transducers 2014, 27, 161–164. [Google Scholar]
- Soumahoro, I.; Schmerber, G.; Douayar, A.; Colis, S.; Abd-Lefdil, M.; Hassanain, N.; Berrada, A.; Muller, D.; Slaoui, A.; Rinnert, H.; et al. Structural, optical, and electrical properties of Yb-doped ZnO thin films prepared by spray pyrolysis method. J. Appl. Phys. 2011, 109, 033708. [Google Scholar] [CrossRef]
- Scherrer, P. Prinzipalle mechanismen in Physics. Göttinger Nachrichten Math. Phys. 1918, 2, 98–100. [Google Scholar]
- Yogamalar, N.R.; Sadhanandham, K.; Bose, A.C.; Jayavel, R. Band alignment and depletion zone at ZnO/CdS and ZnO/CdSe hetero-structures for temperature independent ammonia vapor sensing. Phys. Chem. Chem. Phys. 2016, 18, 32057–32071. [Google Scholar] [CrossRef]
- Petersen, J.; Brimont, C.; Gallart, M.; Crégut, O.; Schmerber, G.; Gilliot, P.; Hönerlage, B.; Ulhaq-Bouillet, C.; Rehspringer, J.L.; Leuvrey, C.; et al. Optical properties of ZnO thin films prepared by sol–gel process. Microelectron. J. 2009, 40, 239–241. [Google Scholar] [CrossRef]
- Balestrieri, M.; Ferblantier, G.; Colis, S.; Schmerber, G.; Ulhaq-Bouillet, C.; Muller, D.; Slaoui, A.; Dinia, A. Structural and optical properties of Yb-doped ZnO films deposited by magnetron reactive sputtering for photon conversion. Sol. Energy Mater. Sol. Cells 2013, 117, 363–371. [Google Scholar] [CrossRef]
- Zheng, J.H.; Song, J.L.; Zhao, Z.; Jiang, Q.; Lian, J.S. Optical and magnetic properties of Nd-doped ZnO nanoparticles. Cryst. Res. Technol. 2012, 47, 713–718. [Google Scholar] [CrossRef]
- Thangaraju, B. Structural and electrical studies on highly conducting spray deposited fluorine and antimony doped SnO2 thin films from SnCl2 precursor. Thin Solid Films 2002, 402, 71–78. [Google Scholar] [CrossRef]
- Swapna, R.; Ashok, M.; Muralidharan, G.; SanthoshKumar, M.C. Microstructural, electrical and optical properties of ZnO: Mo thin films with various thickness by spray pyrolysis. J. Anal. Appl. Pyrolysis 2013, 102, 68–75. [Google Scholar] [CrossRef]
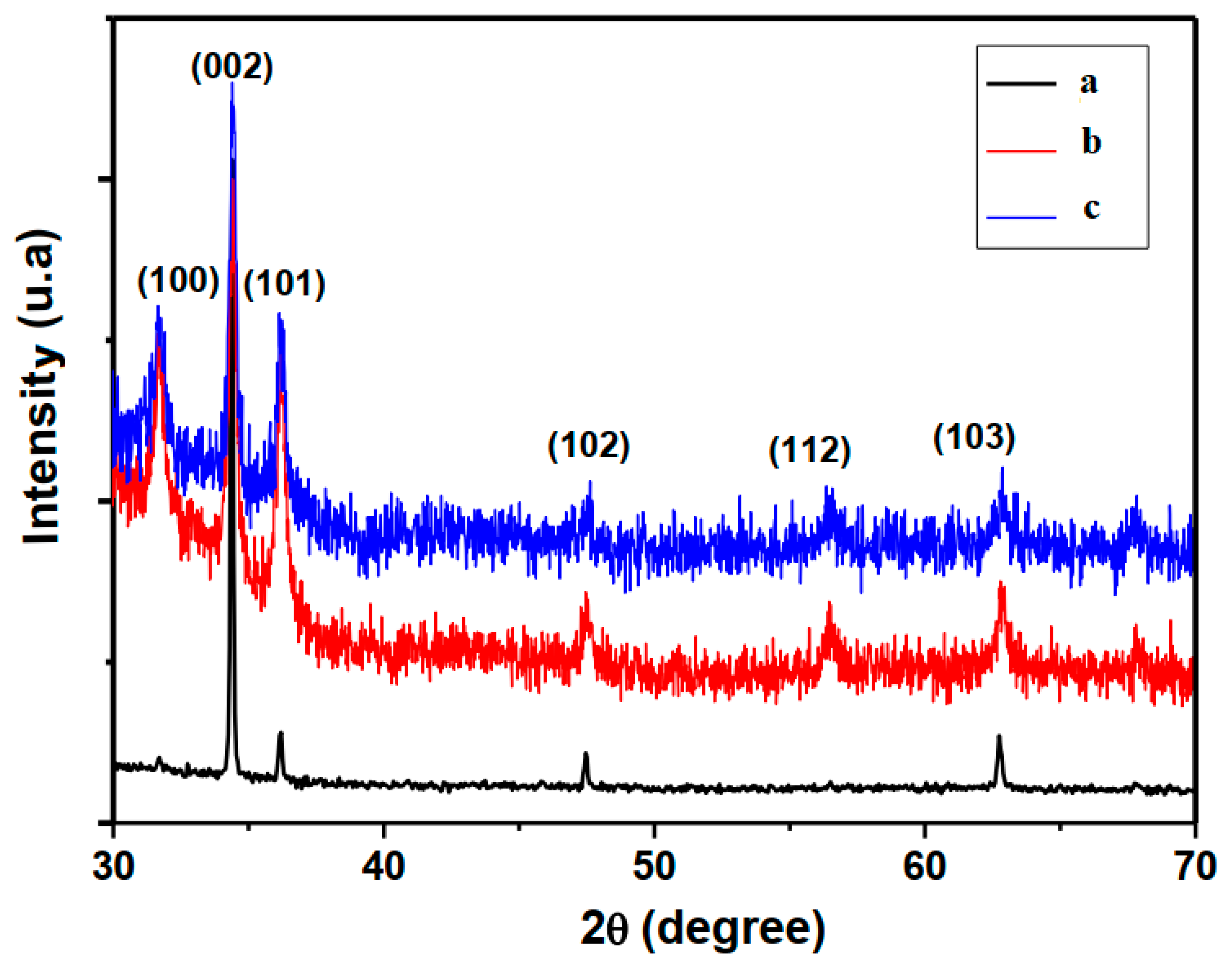
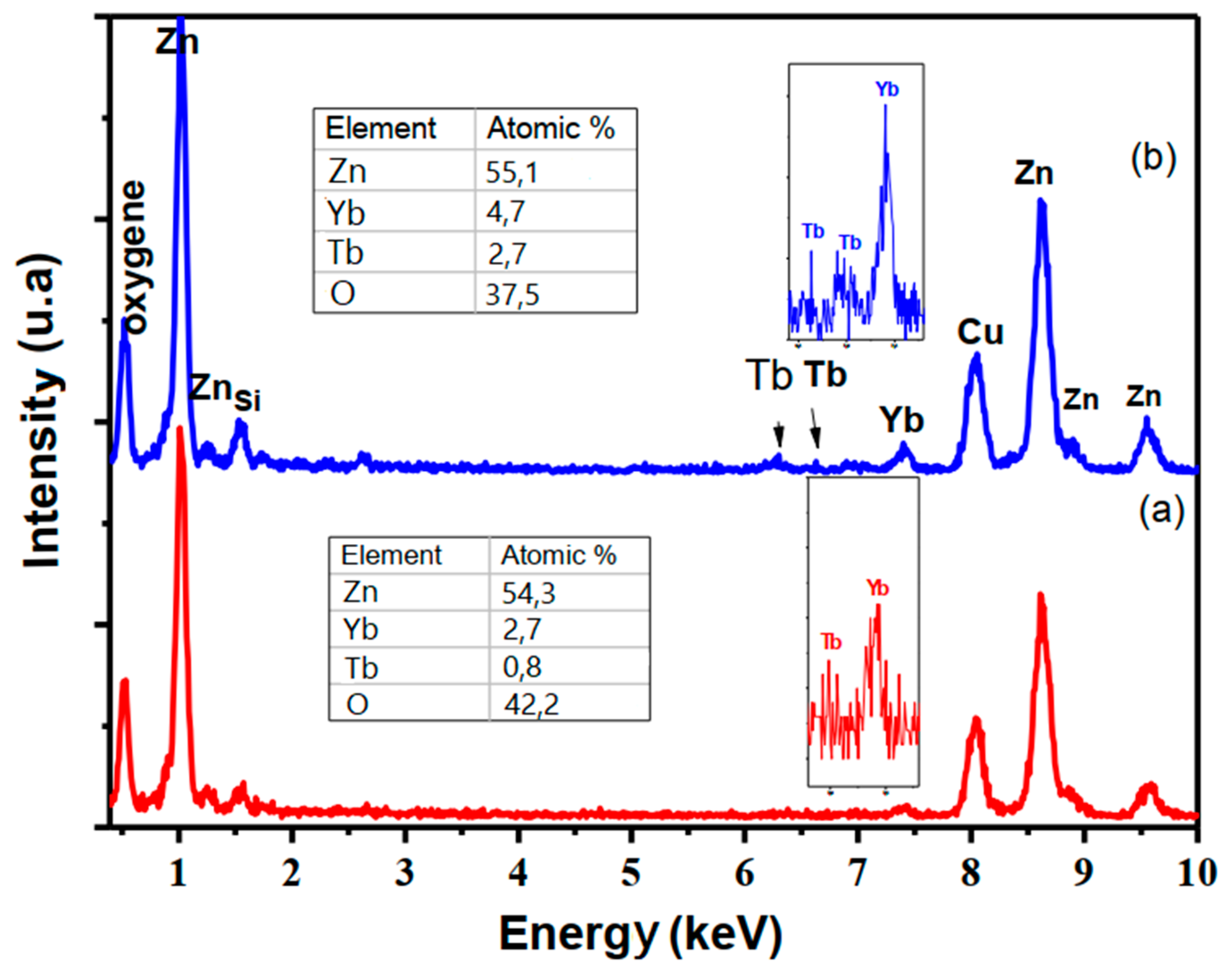
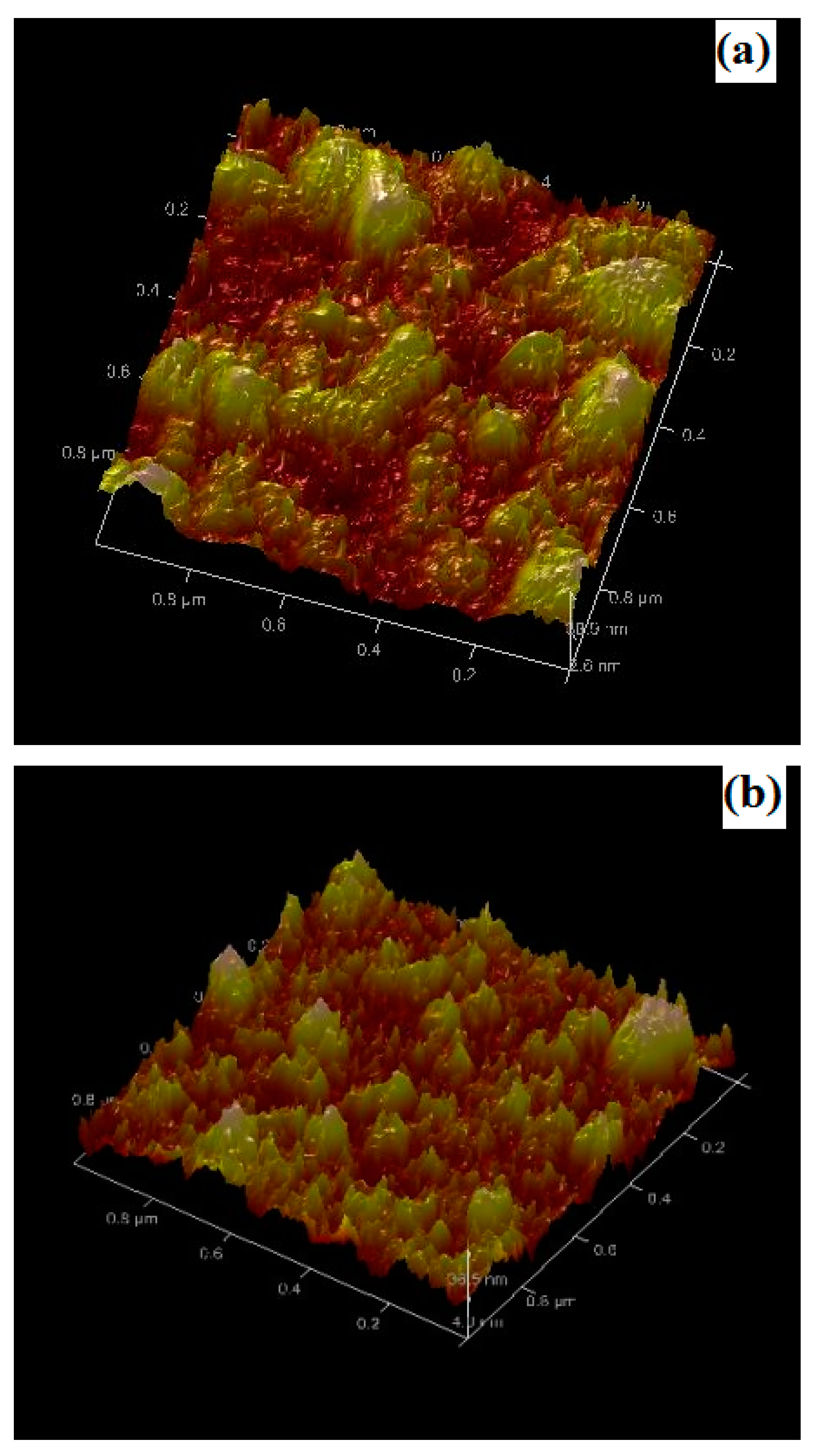
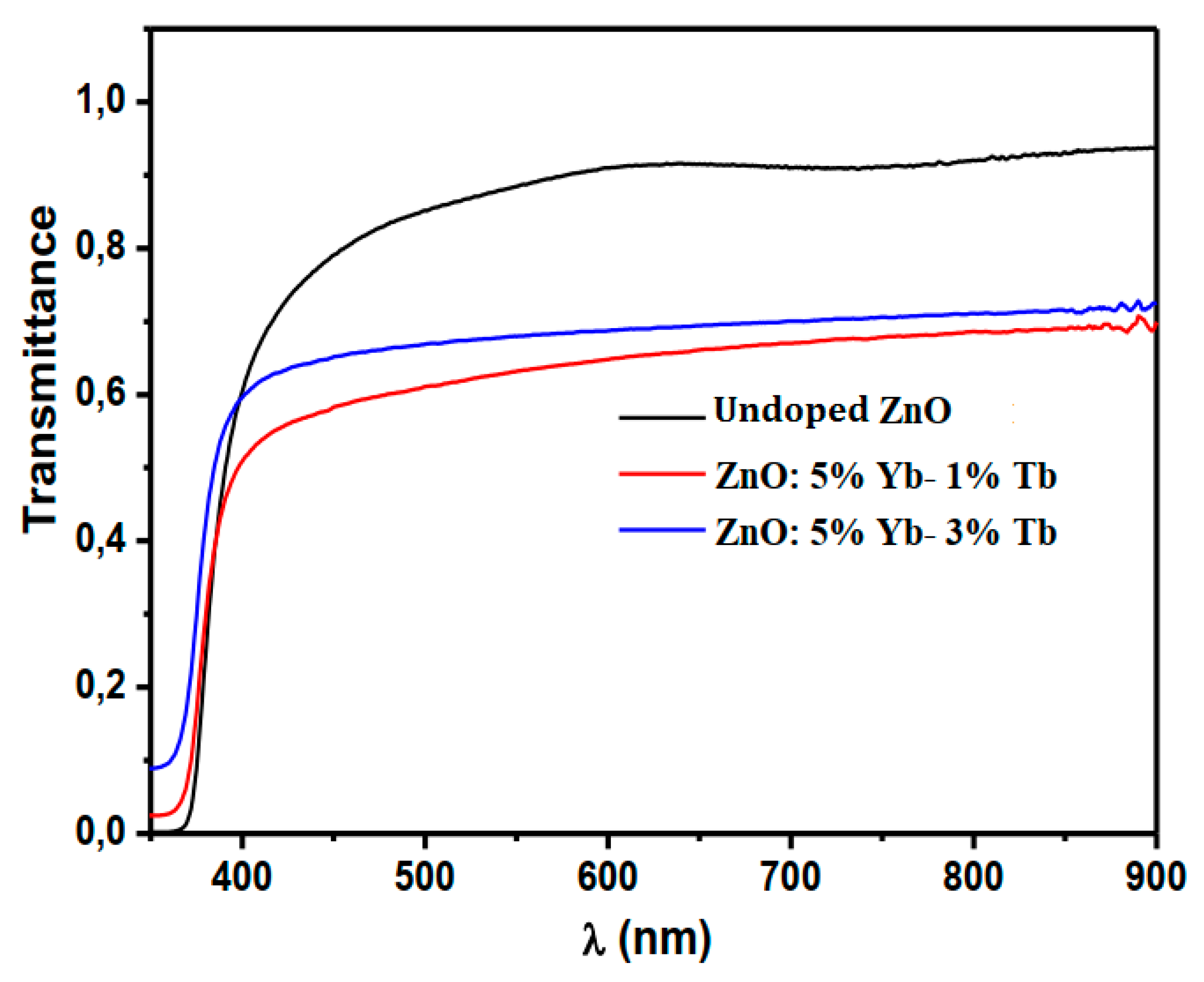
| x Nominal | Thickness (nm) | Grain Size D (nm) | Tc (002) | RMS Roughness (nm) | Reference |
|---|---|---|---|---|---|
| Undoped ZnO | 430 | 76 | 3.0 | 30.0 | This work |
| 1% Tb3+ | _ | _ | 2.9 | 36 | [26] |
| 5% Yb3+ | 455 | 83 | 3.5 | _ | [27] |
| 5% Yb3+-1% Tb3+ | 450 | 30 | 1.6 | 10.6 | This work |
| 3% Tb3+ | _ | _ | 2.7 | 77 | [26] |
| 5%Yb3+-3%Tb3+ | 462 | 23 | 1.6 | 8.8 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El hat, A.; Chaki, I.; Essajai, R.; Mzerd, A.; Schmerber, G.; Regragui, M.; Belayachi, A.; Sekkat, Z.; Dinia, A.; Slaoui, A.; et al. Growth and Characterization of (Tb,Yb) Co-Doping Sprayed ZnO Thin Films. Crystals 2020, 10, 169. https://doi.org/10.3390/cryst10030169
El hat A, Chaki I, Essajai R, Mzerd A, Schmerber G, Regragui M, Belayachi A, Sekkat Z, Dinia A, Slaoui A, et al. Growth and Characterization of (Tb,Yb) Co-Doping Sprayed ZnO Thin Films. Crystals. 2020; 10(3):169. https://doi.org/10.3390/cryst10030169
Chicago/Turabian StyleEl hat, A., I. Chaki, R. Essajai, A. Mzerd, G. Schmerber, M. Regragui, A. Belayachi, Z. Sekkat, A. Dinia, A. Slaoui, and et al. 2020. "Growth and Characterization of (Tb,Yb) Co-Doping Sprayed ZnO Thin Films" Crystals 10, no. 3: 169. https://doi.org/10.3390/cryst10030169
APA StyleEl hat, A., Chaki, I., Essajai, R., Mzerd, A., Schmerber, G., Regragui, M., Belayachi, A., Sekkat, Z., Dinia, A., Slaoui, A., & Abd-Lefdil, M. (2020). Growth and Characterization of (Tb,Yb) Co-Doping Sprayed ZnO Thin Films. Crystals, 10(3), 169. https://doi.org/10.3390/cryst10030169





