Characterization of High-Temperature Hierarchical Porous Mullite Washcoat Synthesized Using Aluminum Dross and Coal Fly Ash
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Characterization of Starting Materials
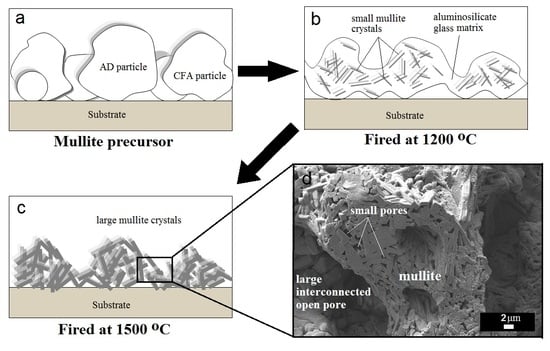
3.2. Sintering and Phase Evolution Characterization
3.3. Specific Surface Area and Pore Size Distribution
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Trueba, M.; Trasatti, S.P. γ-Alumina as a support for catalysts: A review of fundamental aspects. Eur. J. Inorg. Chem. 2005, 17, 3393–3403. [Google Scholar] [CrossRef]
- Huirache-Acuna, R.; Nava, R.; Peza-Ledesma, C.L.; Lara-Romero, J.; Alonso-Nunez, G.; Pawelec, B.; Rivera-Munoz, E.M. SBA-15 mesoporous silica as catalytic support for hydrodesulfurization catalysts—Review. Materials 2013, 6, 4139–4167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagheri, S.; Muhd Julkapli, N.; Bee Abd Hamid, S. Titanium dioxide as a catalyst support in heterogeneous catalysis. Sci. World J. 2014, 2014, 727496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, G.; Ong, P.P.; Chu, C. Thermal stability of mesoporous silica molecular sieve. J. Phys. Chem. Solids 1999, 60, 943–947. [Google Scholar] [CrossRef]
- Mahlambi, M.M.; Mishra, A.K.; Mishra, S.B.; Krause, R.W.; Mamba, B.B.; Raichur, A.M. Comparison of rhodamine B degradation under UV irradiation by two phases of titania nano-photocatalyst. J. Therm. Anal. Calorim. 2012, 110, 847–855. [Google Scholar] [CrossRef]
- Souto, A.; Guitian, F. Novel method for obtaining corundum layers of high surface area on ceramic supports for high-temperature catalysis. J. Am. Ceram. Soc. 2002, 85, 1823–1826. [Google Scholar] [CrossRef]
- Schneider, H.; Komarneni, S. Basic Properties of Mullite; Mullite Wiley VCH: Weinheim, Germany, 2005; p. 241. [Google Scholar]
- Pyzik, A.; Ziebarth, R.; Han, C.; Yang, K. High-porosity acicular mullite ceramics for multifunctional diesel particulate filters. Int. J. Appl. Ceram. Technol. 2011, 8, 1059–1066. [Google Scholar] [CrossRef]
- Choo, T.F.; Mohd Salleh, M.A.; Kok, K.Y.; Matori, K.A. Mineralogy and thermal expansion study of mullite-based ceramics synthesized from coal fly ash and aluminum dross industrial wastes. Ceram. Int. 2019, 45, 7488–7494. [Google Scholar]
- Choo, T.F.; Mohd Salleh, M.A.; Kok, K.Y.; Matori, K.A. Modified cenospheres as non-sacrificial pore-forming agent for porous mullite ceramics. Ceram. Int. 2019, 45, 21827–21834. [Google Scholar] [CrossRef]
- Agrafiotis, C.; Tsetsekou, A. The effect of powder characteristics on washcoat quality. Part I: Alumina washcoats. J. Eur. Ceram. Soc. 2000, 20, 815–824. [Google Scholar] [CrossRef]
- Liu, S.; Ma, W.; Zhang, Y.; Zhang, Y.; Qi, K. Sequential Transformation Behavior of Iron-Bearing Minerals during Underground Coal Gasification. Minerals 2018, 8, 90. [Google Scholar]
- Bressiani, J.C.; VIzhevskyi, V.; Bressiani, H.A. Development of the Microstructure of the Silicon Nitride Based Ceramics. Mater. Res. 1999, 2, 165–172. [Google Scholar] [CrossRef] [Green Version]
- Argyle, M.D.; Bartholomew, C.H. Heterogeneous Catalyst Deactivation and Regeneration: A Review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef] [Green Version]
- Johansson, E.M.; Papadias, D.; Thevenin, P.O.; Ersson, A.G.; Gabrielsson, R.; Menon, P.G.; Björnbom, P.H.; Järäs, S.G. Catalytic combustion for gas turbine applications. Catalysis 1999, 14, 183–235. [Google Scholar]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pieroti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Kušar, H.M.J.; Ersson, A.G.; Järäs, S.G. Catalytic combustion of gasified refuse-derived fuel. Appl. Catal. B Environ. 2003, 45, 1–11. [Google Scholar] [CrossRef]
- Johansson, E.M.; Danielsson, K.M.J.; Ersson, A.G.; Järäs, S.G. Development of hexaaluminate catalysts for combustion of gasified biomass in gas turbines. J. Eng. Gas Turbines Power 2002, 124, 235–238. [Google Scholar] [CrossRef]
- Inoue, H.; Sekizawa, K.; Eguchi, K.; Arai, H. Thermal stability of hexaaluminate film coated on SiC substrate for high-temperature catalytic application. J. Am. Ceram. Soc. 1997, 80, 584–588. [Google Scholar] [CrossRef]
- Kušar, H.M.J.; Ersson, A.G.; Thevenin, P.O.; Järäs, S.G. Sulfur poisoning in catalytic combustion of industrial waste. Studies in Surface Science and Catalysis 2001, 139, 463–470. [Google Scholar]








| Compound | Content (wt%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Al2O3 | SiO2 | K2O | Fe2O3 | TiO2 | P2O5 | CaO | CuO | |
| aluminum dross (AD) | 89.69 | 5.38 | 2.37 | 1.04 | 0.16 | 0.28 | 0.48 | 0.20 |
| coal fly ash (CFA) | 34.48 | 57.13 | 3.67 | 3.46 | 0.48 | 0.33 | 0.32 | <0.01 |
| Mullite precursor (MP) | 68.54 | 27.60 | 1.56 | 0.84 | 0.27 | 0.21 | 0.15 | 0.36 |
| No. | Firing Temperature | Composition of Mineralogical Phases (wt.%) | ||||
|---|---|---|---|---|---|---|
| Mullite | Quartz | Cristobalite | Corundum | Hercynite | ||
| 1 | 1200 °C | 36.7 | 17.4 | 14.6 | 24.7 | 6.7 |
| 2 | 1500 °C | 100 | 0 | 0 | 0 | 0 |
| Reference | Raw Materials | Ceramics Product | Synthesis Process | BET Specific Surface Area (m2g−1)/Temperature |
|---|---|---|---|---|
| This work | aluminum dross + coal fly ash | mullite | heating at 1500 °C | 4.9/1500 °C |
| [6] | kaolin | corundum | heating at 1300–1500 °C in a controlled reducing atmosphere | 10.5/1300 ° C8.5/1450 ° C3/1500 °C |
| [14] | boehmite | γ-alumina δ-alumina θ-alumina α-alumina | heating at 450 ° Cheating at 850 ° Cheating at 1000 ° Cheating at 1125 °C | 200/450 ° C120/850 ° C50/1000 ° C1/1125 °C |
| [17] | Aluminum nitrate nonahydrate + Magnesium nitrate hexahydrate | Magnesium aluminate | Precipitation and heating at 1400 °C | 7.5/1400 °C |
| [18] | Aluminum isopropoxide + lanthanum nitrate | lanthanum hexaaluminate | Precipitation and heating at 1400 °C | 17/1400 °C |
| [19] | BaCl2 + boehmite | Barium hexaaluminate | Precipitation and heating at 1300 °C | 6.6/1300 °C |
| [20] | Yttrium nitrate + Aluminum nitrate | Yttrium aluminum oxide | Precipitation and heating at 1400 °C | 2/1400 °C |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choo, T.F.; Mohd Salleh, M.A.; Kok, K.Y.; Matori, K.A.; Abdul Rashid, S. Characterization of High-Temperature Hierarchical Porous Mullite Washcoat Synthesized Using Aluminum Dross and Coal Fly Ash. Crystals 2020, 10, 178. https://doi.org/10.3390/cryst10030178
Choo TF, Mohd Salleh MA, Kok KY, Matori KA, Abdul Rashid S. Characterization of High-Temperature Hierarchical Porous Mullite Washcoat Synthesized Using Aluminum Dross and Coal Fly Ash. Crystals. 2020; 10(3):178. https://doi.org/10.3390/cryst10030178
Chicago/Turabian StyleChoo, Thye Foo, Mohamad Amran Mohd Salleh, Kuan Ying Kok, Khamirul Amin Matori, and Suraya Abdul Rashid. 2020. "Characterization of High-Temperature Hierarchical Porous Mullite Washcoat Synthesized Using Aluminum Dross and Coal Fly Ash" Crystals 10, no. 3: 178. https://doi.org/10.3390/cryst10030178
APA StyleChoo, T. F., Mohd Salleh, M. A., Kok, K. Y., Matori, K. A., & Abdul Rashid, S. (2020). Characterization of High-Temperature Hierarchical Porous Mullite Washcoat Synthesized Using Aluminum Dross and Coal Fly Ash. Crystals, 10(3), 178. https://doi.org/10.3390/cryst10030178