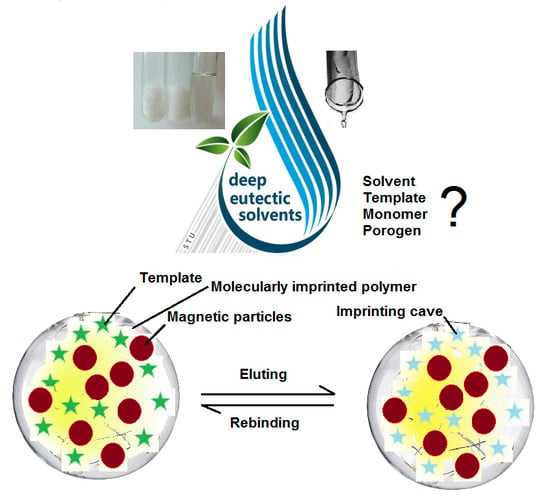
Involvement of Deep Eutectic Solvents in Extraction by Molecularly Imprinted Polymers—A Minireview
Abstract
:1. Introduction
2. Deep Eutectic Solvents
3. Extraction by Deep Eutectic Solvents
4. Selective Sorbents Based on Molecularly Imprinted Polymers
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | acrylamide |
| AC | acrylic acid |
| AcA | acetic acid |
| AIBN | 2,2-azobisisobutyronitrile |
| B | Betaine |
| Bud | 1,4-butanediol |
| CfA | caffeic acid |
| ChCl | choline chloride |
| CHL | chloromycetin |
| DES | deep eutectic solvent |
| EG | ethylene glycol |
| EGDMA | ethylene glycol dimethacrylate |
| Gl | glycerol |
| HBA | hydrogen bond acceptor |
| HBD | hydrogen bond donor |
| HMIPs | hybrid molecular imprinted polymers |
| MAA | methacrylic acid |
| MIP | molecularly imprinted polymers, polymer prepared without addition of template in polymerization mixture |
| MMIP | magnetic molecularly imprinted polymers |
| NADES | naturally deep eutectic solvent |
| NIP | not imprinted polymer |
| OA | oxalic acid |
| PA | propionic acid |
| PG | propylene glycol |
| TEOS | tetraethoxysilane |
| THI | thiamphenicol |
| U | urea |
| W | water |
References
- Mendonça, P.V.; Lima, M.S.; Guliashvili, T.; Serra, A.C.; Coelho, J.F.J. Deep eutectic solvents (DES): Excellent green solvents for rapid SARA ATRP of biorelevant hydrophilic monomers at ambient temperature. Polymer 2017, 132, 114–121. [Google Scholar] [CrossRef]
- Raks, V.; Al-Suod, H.; Buszewski, B. Isolation, separation, and preconcentration of biologically active compounds from plant matrices by extraction techniques. Chromatographia 2018, 81, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jablonsky, M.; Skulcova, A.; Malvis, A.; Sima, J. Extraction of value- added components from food industry based and agro-forest biowastes by deep eutectic solvents. J. Biotechnol. 2018, 282, 46–66. [Google Scholar] [CrossRef]
- Jablonský, M.; Šima, J. Deep Eutectic Solvents in Biomass Valorization; Spektrum STU: Bratislava, Slovakia, 2019; p. 176. [Google Scholar]
- Jablonský, M.; Škulcová, A.; Šima, J. Use of deep eutectic solvents in polymer chemistry—A review. Molecules 2019, 24, 3978. [Google Scholar] [CrossRef] [Green Version]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 1, 70–71. [Google Scholar] [CrossRef] [Green Version]
- Jablonsky, M.; Haz, A.; Majova, V. Assessing the opportunities for applying deep eutectic solvents for fractionation of beech wood and wheat straw. Cellulose 2019, 26, 7675–7684. [Google Scholar] [CrossRef]
- Jablonsky, M.; Majova, V.; Ondrigova, K.; Sima, J. Preparation and characterization of physicochemical properties and application of novel ternary deep eutectic solvents. Cellulose 2019, 26, 3031–3045. [Google Scholar] [CrossRef]
- Shishov, A.; Bulatov, A.; Locatelli, M.; Carradori, S.; Andruch, V. Application of deep eutectic solvents in analytical chemistry. A review. Microchem. J. 2017, 135, 33–38. [Google Scholar] [CrossRef]
- Ekezie, F.G.C.; Sun, D.W.; Cheng, J.H. Acceleration of microwave-assisted extraction processes of food components by integrating technologies and applying emerging solvents: A review of latest developments. Trends Food Sci. Technol. 2017, 67, 160–172. [Google Scholar] [CrossRef]
- Chen, Z.; Wan, C. Ultrafast fractionation of lignocellulosic biomass by microwave-assisted deep eutectic solvent pretreatment. Bioresour. Technol. 2018, 250, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Bajkacz, S.; Adamek, J. Evaluation of new natural deep eutectic solvents for the extraction of isoflavones from soy products. Talanta 2017, 168, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Bosiljkov, T.; Dujmić, F.; Bubalo, M.C.; Hribar, J.; Vidrih, R.; Brnčić, M.; Zlatic, E.; Redovniković, I.R.; Jokić, S. Natural deep eutectic solvents and ultrasound-assisted extraction: Green approaches for extraction of wine lees anthocyanins. Food Bioprod. Process. 2017, 102, 195–203. [Google Scholar] [CrossRef]
- Zhuang, B.; Dou, L.-L.; Li, P.; Liu, E.-H. Deep eutectic solvents as green media for extraction of flavonoid glycosides and aglycones from Platycladi Cacumen. J. Pharm. Biomed. 2017, 134, 214–219. [Google Scholar] [CrossRef]
- Paulaitis, M.; Krukonis, V.J.; Kurnik, R.T.; Reid, R.C. Supercritical fluid extraction. Rev. Chem. Eng. 1983, 1, 179–250. [Google Scholar]
- Yiin, C.L.; Quitain, A.T.; Yusup, S.; Sasaki, M.; Uemura, Y.; Kida, T. Characterization of natural low transition temperature mixtures (LTTMs): Green solvents for biomass delignification. Bioresour. Technol. 2016, 199, 258–264. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Taha, A.A.; Ying, Y.; Li, X.; Chen, X.; Ma, C. Subcritical water extraction of bioactive components from ginseng roots (Panax ginseng CA Mey). Ind. Crop. Prod. 2018, 117, 118–127. [Google Scholar] [CrossRef]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef] [Green Version]
- Bakirtzi, C.; Triantafyllidou, K.; Makris, D.P. Novel lactic acid-based natural deep eutectic solvents: Efficiency in the ultrasound-assisted extraction of antioxidant polyphenols from common native Greek medicinal plants. J. Appl. Res. Med. Arom. Plants 2016, 3, 120–127. [Google Scholar] [CrossRef]
- Zhao, B.-Y.; Xu, P.; Yang, F.-X.; Wu, H.; Zong, M.-H.; Lou, W.-Y. Biocompatible deep eutectic solvents based on choline chloride: Characterization and application to the extraction of rutin from Sophora japonica. ACS Sustain. Chem. Eng. 2015, 3, 2746–2755. [Google Scholar] [CrossRef]
- Duan, L.; Dou, L.-L.; Guo, L.; Li, P.; Liu, E.-H. Comprehensive evaluation of deep eutectic solvents in extraction of bioactive natural products. ACS Sustain. Chem. Eng. 2016, 4, 2405–2411. [Google Scholar] [CrossRef]
- Fu, N.; Lv, R.; Guo, Z.; Guo, Y.; You, X.; Tang, B.; Han, D.; Yan, H.; Row, K.H. Environmentally friendly and non-polluting solvent pretreatment of palm samples for polyphenol analysis using choline chloride deep eutectic solvents. J. Chromatogr. A 2017, 1492, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.M.; Ko, J.; Zhao, J.; Jin, Y.; Han, S.Y.; Lee, J. Multi-functioning deep eutectic solvents as extraction and storage media for bioactive natural products that are readily applicable to cosmetic products. J. Clean. Prod. 2017, 151, 87–95. [Google Scholar] [CrossRef]
- Škulcova, A.; Haščičová, Z.; Hrdlička, L.; Šima, J.; Jablonský, M. Green solvents based on choline chloride for the extraction of spruce bark (Picea abies). Cell. Chem. Technol. 2017, 52, 3–4. [Google Scholar]
- Jablonský, M.; Škulcová, A.; Kamenská, L.; Vrška, M.; Šima, J. Deep eutectic solvents: Fractionation of wheat straw. BioResources 2015, 10, 8039–8047. [Google Scholar] [CrossRef] [Green Version]
- Lomenova, A.; Hroboňová, K. Polyméry s odtlačkami molekúl ako chirálne stacionárne fázy v HPLC. Chem. Listy 2019, 113, 156–164. [Google Scholar]
- Machyňáková, A.; Hroboňová, K. Možnosti prípravy polymérov s odtlačkami molekúl. Chem. Listy 2016, 110, 609–615. [Google Scholar]
- BelBruno, J.J. Molecularly imprinted polymers. Chem. Rev. 2018, 119, 94–119. [Google Scholar] [CrossRef]
- Cormack, P.A.; Elorza, A.Z. Molecularly imprinted polymers: Synthesis and characterisation. J. Chromatogr. B 2004, 804, 173–182. [Google Scholar] [CrossRef]
- Huang, S.; Xu, J.; Zheng, J.; Zhu, F.; Xie, L.; Ouyang, G. Synthesis and application of magnetic molecularly imprinted polymers in sample preparation. Anal. Bioanal. Chem. 2018, 410, 3991–4014. [Google Scholar] [CrossRef]
- Li, G.; Wang, W.; Wang, Q.; Zhu, T. Deep eutectic solvents modified molecular imprinted polymers for optimized purification of chlorogenic acid from honeysuckle. J. Chromatogr. Sci. 2016, 54, 271–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, N.; Li, L.; Liu, X.; Fu, N.; Zhang, C.; Hu, L.; Li, D.; Tang, B.; Zhu, T. Specific recognition of polyphenols by molecularly imprinted polymers based on a ternary deep eutectic solvent. J. Chromatogr. A 2017, 1530, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Dai, Y.; Row, K.H. Molecular imprinted polymers based on magnetic chitosan with different deep eutectic solvent monomers for the selective separation of catechins in black tea. Electrophoresis 2018, 39, 2039–2046. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Ahn, W.S.; Row, K.H. Hybrid molecularly imprinted polymers modified by deep eutectic solvents and ionic liquids with three templates for the rapid simultaneous purification of rutin, scoparone, and quercetin from Herba Artemisiae Scopariae. J. Sep. Sci. 2016, 39, 4465–4473. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Row, K.H. Application of deep eutectic solvents in hybrid molecularly imprinted polymers and mesoporous siliceous material for solid-phase extraction of levofloxacin from green bean extract. Anal. Sci. 2017, 33, 611–617. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Row, K.H. Magnetic molecularly imprinted polymers for recognition and enrichment of polysaccharides from seaweed. J. Sep. Sci. 2017, 40, 4765–4772. [Google Scholar] [CrossRef]
- Li, G.; Wang, X.; Row, K.H. Magnetic molecularly imprinted polymers based on silica modified by deep eutectic solvents for the rapid simultaneous magnetic-based solid-phase extraction of Salvia miltiorrhiza bunge, Glycine max (Linn.) Merr and green tea. Electrophoresis 2018, 39, 1111–1118. [Google Scholar] [CrossRef]
- Fu, N.; Li, L.; Liu, K.; Kim, C.K.; Li, J.; Zhu, T.; Li, J.; Tang, B. A choline chloride-acrylic acid deep eutectic solvent polymer based on Fe3O4 particles and MoS2 sheets (poly (ChCl-AA DES)@ Fe3O4@ MoS2) with specific recognition and good antibacterial properties for β-lactoglobulin in milk. Talanta 2019, 197, 567–577. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Dai, Q.; Zhou, Y. Magnetic deep eutectic solvents molecularly imprinted polymers for the selective recognition and separation of protein. Anal. Chim. Acta 2016, 936, 168–178. [Google Scholar] [CrossRef]
- Li, G.; Dai, Y.; Wang, X.; Row, K. Molecularly imprinted polymers modified by deep eutectic solvents and ionic liquids with two templates for the simultaneous solid-phase extraction of fucoidan and laminarin from marine kelp. Anal. Lett. 2019, 52, 511–525. [Google Scholar] [CrossRef]
- Li, X.; Row, K.H. Application of novel ternary deep eutectic solvents as a functional monomer in molecularly imprinted polymers for purification of levofloxacin. J. Chromatogr. B 2017, 1068, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Row, K.H. Purification of antibiotics from the millet extract using hybrid molecularly imprinted polymers based on deep eutectic solvents. RSC Adv. 2017, 7, 16997–17004. [Google Scholar] [CrossRef] [Green Version]
- Meng, J.; Wang, X. Microextraction by Packed Molecularly Imprinted Polymer Combined Ultra-High-Performance Liquid Chromatography for the Determination of Levofloxacin in Human Plasma. J. Chem. 2019, 2019, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Tang, W.; Cao, W.; Wang, Q.; Zhu, T. Molecularly imprinted polymers combination with deep eutectic solvents for solid-phase extraction of caffeic acid from hawthorn. Chin. J. Chromatogr. 2015, 33, 792–798. [Google Scholar] [CrossRef]
- Ge, Y.-H.; Shu, H.; Xu, X.-Y.; Guo, P.-Q.; Liu, R.-L.; Luo, Z.-M.; Chang, C.; Fu, Q. Combined magnetic porous molecularly imprinted polymers and deep eutectic solvents for efficient and selective extraction of aristolochic acid I and II from rat urine. Mater. Sci. Eng. C 2019, 97, 650–657. [Google Scholar] [CrossRef]
- Li, X.; Row, K.H. Preparation of deep eutectic solvent-based hexagonal boron nitride-molecularly imprinted polymer nanoparticles for solid phase extraction of flavonoids. Microchim. Acta 2019, 186, 753. [Google Scholar] [CrossRef]
- Li, G.; Wang, X.; Row, K.H. Magnetic solid-phase extraction with Fe3O4/molecularly imprinted polymers modified by deep eutectic solvents and ionic liquids for the rapid purification of alkaloid isomers (theobromine and theophylline) from green tea. Molecules 2017, 22, 1061. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Row, K.H. Ternary deep eutectic solvent magnetic molecularly imprinted polymers for the dispersive magnetic solid-phase microextraction of green tea. J. Sep. Sci. 2018, 41, 3424–3431. [Google Scholar] [CrossRef]
- Tang, W.; Gao, F.; Duan, Y.; Zhu, T.; Ho Row, K. Exploration of deep eutectic solvent-based molecularly imprinted polymers as solid-phase extraction sorbents for screening chloramphenicol in milk. J. Chrom. Sci. 2017, 55, 654–661. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Zhu, T.; Row, K.H. Deep eutectic solvents for the purification of chloromycetin and thiamphenicol from milk. J. Sep. Sci. 2017, 40, 625–634. [Google Scholar] [CrossRef]

| DES | Molar Ratio | MIPs | Substrate | Target Compounds | Ref. |
|---|---|---|---|---|---|
| ChCl:Gl | 1:2 | Template: chlorogenic acid Monomers: AA Modifier: DES Crosslinker: EGDMA Initiator: AIBN | Honeysuckle | Chlorogenic acid | [32] |
| ChCl:EG ChCl:Gl ChCl:Bud | 1:3 n/n | Template: rutin, scoparone, quercetin Carrier: γ-aminopropyltriethoxysilane-methacrylic acid Monomer: MAA Modifier: DES Crosslinker: EGDMA Initiator: AIBN | Herba Artemisiae Scopariae | Rutin, scoparone, quercetin | [35] |
| B:EG:W | 1:2:1 | Template: levofloxacin Monomers: 3-aminopropyltriethoxysilane, MAA, TEOS Modifier: DES Crosslinker: EGDMA Initiator: AIBN Porogen: methanol | Green bean extract | Levofloxacin | [36] |
| ChCl:EG ChCl:Gl ChCl:Bud ChCl:U ChCl:FA ChCl:AcA ChCl:PA | 1:3 n/n | Template: fucodain, alginic acid Carrier: Fe3O4@3-aminopropyltriethoxysilane Monomer: MAA Modifier: DES Crosslinker: EGDMA Initiator: AIBN | Seaweed | Fucodain, alginic acid | [37] |
| ChCl:EG ChCl:Gl ChCl:U ChCl:Bud | 1:2 n/n | Template I: tanshinone I, tanshinone IIA, and cryptotanshinone Template II: glycitein, genistein, and daidzein Template III: epicatechin, epigallocatechin gallate, and epicatechin gallate Carrier: Fe3O4@SiO2 Monomers: MAA, DES Crosslinker: EGDMA Initiator: AIBN Porogen: acetonitrile | Salvia miltiorrhiza bunge, Glycine max (Linn.) Merr and green tea | Tanshinone I, tanshinone IIA, and cryptotanshinone from Salvia miltiorrhiza bunge; glycitein, genistein, and daidzein from Glycine max (Linn.) Merr; and epicatechin, epigallocatechin gallate, and epicatechin gallate from green tea | [38] |
| ChCl:AC | 1:2 | Template: β-lactoglobulin Carrier: Fe3O4@MoS2 Monomers: DES Crosslinker: EGDMA Initiator: benzyolperoxide, N,N-dimethylaniline Porogen: ethanol:water (9:1) | Milk | β-lactoglobulin, albumin, conalbumin | [39] |
| ChCl:MAA | 1:2 | Template: bovine hemoglobin Carrier: Fe3O4@AA Monomers: DES Crosslinker: N,N-methylenebidacrylamide, Initiator: ammonium persulfate, N, N, N´, N´-tetramethylenediamine | Protein solution | Protein | [40] |
| ChCl:FA ChCl:AcA ChCl:PA ChCl:U | 1:2 n/n | Template: laminarin, fucoidan Monomers: MAA, glycidil methacrylate Modifier: DES Crosslinker: EGDMA Initiator: AIBN | Marine kelp | Laminarin, fucoidan | [41] |
| CfA:ChCl:FA | 1:1:0.5 1:2:1 1:3:1.5 1:4:2 1:6:3 1:8:4 | Template: levoflocaxin Monomer: DES Crosslinker: EGDMA Initiator: AIBN | Millet extract | Levofloxacin | [42] |
| B:EG:W | 1:2:1 | Template: levofloxacin, tetracycline Monomers: 3-aminopropyltriethoxysilane, MAA, TEOS Modifier: DES Crosslinker: EGDMA Initiator: AIBN | Millet extract | Levofloxacin, tetracycline | [43] |
| ChCl:EG | 1:2 | Template: gatifloxacin Monomers: 3-aminopropyltriethoxysilane, MAA, TEOS Crosslinker: EGDMA Initiator: AIBN Porogen: DES | Human plasma | Levofloxacin | [44] |
| ChCl:Gl ChCl:U | (v/v) 0.5:1 1:1 1:2 1:3 1:4 1:5 | Template: caffeic acid Monomers: AA, Crosslinker: EGDMA Initiator: AIBN Elution solvent: DES | Hawthorn | Caffeic acid | [45] |
| ChCl:EG ChCl:Gl ChCl:U | 1:2 | Template: indomethacin Carrier: mesoporous carbon@MIPS Monomers: MAA, Crosslinker: EGDMA Initiator: AIBN Washing agent: DES | Rat urine | Aristolochic acid I, II | [46] |
| CfA:ChCl:EG | 1:1:1 1:2:2 1:3:3 1:5:5 1:8:8 1:10:10 1:15:15 | Template: quercetin Carrier: hexagonal boron nitride Monomers: DES Crosslinker: EGDMA Initiator: AIBN Porogen: methanol | Ginko biloba tea | Quercetin, isorhamnetin, kaempferol | [47] |
| ChCl:EG ChCl:Gl ChCl:Bud ChCl:U ChCl:FA ChCl:AcA ChCl:PA | 1:2 | Template: theobromine, theophylline Carrier: Fe3O4@MIPs Monomers: MAA Modifier: DES, isopropanol Crosslinker: EGDMA Initiator: AIBN | Green tea | Theobromine, theophylline | [48] |
| ChCl:OA:EG ChCl:OA:Gl ChCl:OA:PG ChCl:CfA:EG | 1:1:1 1:1:2 1:1:3 | Template: theophylline, theobromine, (+)-catechin hydrate, caffeic acid Carrier: Fe3O4@SiO2 Monomers: DES Crosslinker: EGDMA Initiator: AIBN Porogen: methanol | Green tea | Theophylline, theobromine, (+)-catechin hydrate, caffeic acid | [49] |
| ChCl:EG ChCl:Gl ChCl:PG | 1:1 | Template: chloramphenicol Monomers: AA Auxiliary monomer: DES Crosslinker: divinilbenzene Initiator: AIBN Porogen: acetonitrile | Milk | Chloramphenicol | [50] |
| ChCl:Gl | 1:2 n/n | Template: chloromycetin, thiamphenicol Monomer: AA Modifier: DES Crosslinker: EGDMA Initiator: 2-methylpropionitrile | Milk | Chloromycetin, thiamphenicol | [51] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jablonský, M.; Majová, V.; Šima, J.; Hroboňová, K.; Lomenová, A. Involvement of Deep Eutectic Solvents in Extraction by Molecularly Imprinted Polymers—A Minireview. Crystals 2020, 10, 217. https://doi.org/10.3390/cryst10030217
Jablonský M, Majová V, Šima J, Hroboňová K, Lomenová A. Involvement of Deep Eutectic Solvents in Extraction by Molecularly Imprinted Polymers—A Minireview. Crystals. 2020; 10(3):217. https://doi.org/10.3390/cryst10030217
Chicago/Turabian StyleJablonský, Michal, Veronika Majová, Jozef Šima, Katarína Hroboňová, and Anna Lomenová. 2020. "Involvement of Deep Eutectic Solvents in Extraction by Molecularly Imprinted Polymers—A Minireview" Crystals 10, no. 3: 217. https://doi.org/10.3390/cryst10030217