Structural, Optoelectrical, Linear, and Nonlinear Optical Characterizations of Dip-Synthesized Undoped ZnO and Group III Elements (B, Al, Ga, and In)-Doped ZnO Thin Films
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Preparation of Undoped ZnO Solution
2.2. Preparation of Group III Elements-Doped ZnO Solutions
2.3. Preparation of Undoped ZnO and Group III Elements-ZnO Thin Films
2.4. Characterization of Undoped ZnO and Group III Elements-ZnO Thin Films
3. Results and Discussion
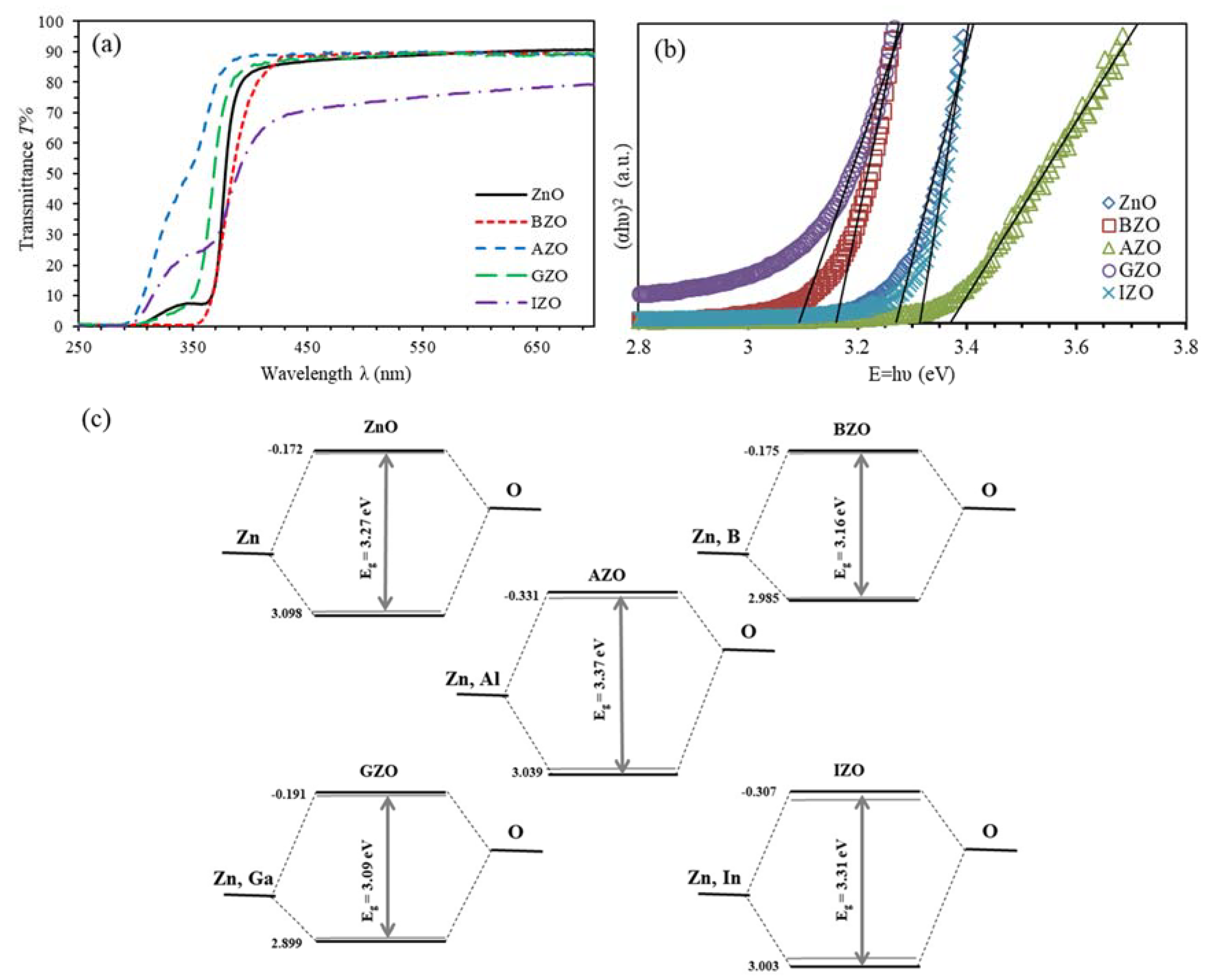
3.1. UV-Vis Spectroscopy
3.2. Dispersion of Refractive Index
3.3. Nonlinear Optical Properties
3.4. Optical and Electrical Conductivities
3.5. X-Ray Diffraction Analysis
3.6. Scanning Electron Microscopy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ko, S.H.; Lee, D.; Kang, H.W.; Nam, K.H.; Yeo, J.Y.; Hong, S.J.; Grigoropoulos, C.P.; Sung, H.J. Nanoforest of hydrothermally grown hierarchical ZnO nanowires for a high efficiency dye-sensitized solar cell. Nano Lett. 2011, 11, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y.; Zeng, H. ZnO-based transparent conductive thin films: Doping, performance, and processing. J. Nanomater. 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
- Gong, H.; Song, H.; Zhang, S.; Ni, K.; Dong, X. An optical liquid level sensor based on polarization-maintaining fiber modal interferometer. Sens. Actuators A Phys. 2014, 205, 204–207. [Google Scholar]
- Azam, A.; Ahmed, F.; Habib, S.S.; Khan, Z.H.; Salah, N.A. Fabrication of Co-doped ZnO nanorods for spintronic devices. Met. Mater. Int. 2013, 19, 845–850. [Google Scholar] [CrossRef]
- Kolhe, P.S.; Shinde, A.B.; Kulkarni, S.; Maiti, N.; Koinkar, P.M.; Sonawane, K.M. Gas sensing performance of Al doped ZnO thin film for H2S detection. J. Alloy. Compd. 2018, 748, 6–11. [Google Scholar]
- Sahay, P.; Nath, R. Al-doped zinc oxide thin films for liquid petroleum gas (LPG) sensors. Sens. Actuators B Chem. 2008, 133, 222–227. [Google Scholar] [CrossRef]
- Karpina, V.A.; Lazorenko, V.I.; Lashkarev, C.V.; Dobrowolski, V.D.; Kopylova, L.I.; Baturin, V.A.; Pustovoytov, S.A.; Karpenko, A.J.; Eremin, S.A.; Lytvyn, P.M.; et al. Zinc oxide–analogue of GaN with new perspective possibilities. Cryst. Res. Technol. 2004, 39, 980–992. [Google Scholar] [CrossRef]
- Asl, H.Z.; Rozati, S.M. High-Performance Spray-Deposited Indium Doped ZnO Thin Film: Structural, Morphological, Electrical, Optical, and Photoluminescence Study. J. Electron. Mater. 2018, 47, 3568–3576. [Google Scholar] [CrossRef]
- Gonçalves, R.; Barrozo, P.; Brito, G.; Viana, B.; Cunha, F. The effect of thickness on optical, structural and growth mechanism of ZnO thin film prepared by magnetron sputtering. Thin Solid Film 2018, 661, 40–45. [Google Scholar] [CrossRef]
- Liang, W.; Yoffe, A. Transmission spectra of ZnO single crystals. Phys. Rev. Lett. 1968, 20, 59. [Google Scholar] [CrossRef]
- Park, Y.; Litton, C.; Collins, T.; Reynolds, D. Exciton spectrum of ZnO. Phys. Rev. 1966, 143, 512. [Google Scholar] [CrossRef]
- Potter, D.B.; Powell, M.J.; Parkin, I.P.; Carmalt, C.J. Aluminium/gallium, indium/gallium, and aluminium/indium co-doped ZnO thin films deposited via aerosol assisted CVD. J. Mater. Chem. C 2018, 6, 588–597. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Singh, R.; Purohit, L.; Mehra, R. Structural, transport and optical properties of boron-doped zinc oxide nanocrystalline. J. Mater. Sci. Technol. 2011, 27, 481–488. [Google Scholar] [CrossRef]
- Wen, B.; Liu, C.Q.; Wang, N.; Wang, H.L.; Liu, S.M.; Jiang, W.W.; Ding, W.Y.; Fei, W.D.; Chai, W.P. Crystallization Behavior and Properties of B-Doped ZnO Thin Films Prepared by Sol-Gel Method with Different Pyrolysis Temperatures. Chin. J. Chem. Phys. 2016, 29, 229–233. [Google Scholar] [CrossRef]
- Wong, L.H.; Lai, Y.S. Characterization of boron-doped ZnO thin films prepared by magnetron sputtering with (100− x) ZnO–xB 2 O 3 ceramic targets. Thin Solid Film 2015, 583, 205–211. [Google Scholar] [CrossRef]
- Djelloul, A.; Larbah, Y.; Adnane, M.; Labdelli, B.; Ziane, M.; Manseri, A.; Messaoud, A. Properties of Undoped and (Al, In) Doped ZnO Thin Films Prepared by Ultrasonic Spray Pyrolysis for Solar Cell Applications. J. Nano Electron. Phys. 2018, 10, 02036-1–02036-5. [Google Scholar] [CrossRef]
- Varghese, J.; Aswathy, N.; Saji, S.D.; Vinodkumar, R. Structural and optical modification of ZnO: Al thin films by molybdenum co-doping and the origin of green emission. AIP Conf. Proc. 2018, 1953, 030079. [Google Scholar]
- Serrao, F.J.; Sandeep, K.; Bhat, S.; Dharmaprakash, S. High energy electron irradiation effects on Ga-doped ZnO thin films for optoelectronic space applications. Appl. Phys. A 2018, 124, 224. [Google Scholar] [CrossRef]
- Kayani, Z.N.; Yaseen, N.; Riaz, S.; Naseem, S. Investigation of Fe doping on the magnetic and optical properties of ZnO thin films. Mater. Res. Express 2018, 5, 036418. [Google Scholar] [CrossRef]
- Jo, G.H.; Kim, S.-H.; Koh, J.-H. Enhanced electrical and optical properties based on stress reduced graded structure of Al-doped ZnO thin films. Ceram. Int. 2018, 44, 735–741. [Google Scholar] [CrossRef]
- Lee, C.-S.; Yoon, K.-H.; Ahn, B. Improved optical transmittance of boron doped ZnO thin films by low pressure chemical vapor deposition with pulse boron doping. J. Electrochem. Soc. 2011, 158, H482–H486. [Google Scholar] [CrossRef]
- Wen, B.; Liu, C.Q.; Wang, N.; Wang, H.L.; Liu, S.M.; Jiang, W.W.; Ding, W.Y.; Fei, W.D.; Chai, W.P. Properties of boron-doped ZnO thin films deposited by pulsed DC magnetron sputtering at different substrate temperatures. Appl. Phys. A 2015, 121, 1147–1153. [Google Scholar] [CrossRef]
- Kaur, G.; Mitra, A.; Yadav, K. Pulsed laser deposited Al-doped ZnO thin films for optical applications. Prog. Nat. Sci. Mater. Int. 2015, 25, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Tsin, F.; Venerosy, A.; Vidal, J.; Collin, S.; Clatot, J.; Lombez, L.; Paire, M.; Borensztajn, S.; Broussillou, C.; Grand, P.-P. Electrodeposition of ZnO window layer for an all-atmospheric fabrication process of chalcogenide solar cell. Sci. Rep. 2015, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Yoon, H.; Kim, D.Y.; Kim, S.-O.; Leem, J.-Y. Optical properties and electrical resistivity of boron-doped ZnO thin films grown by sol–gel dip-coating method. Opt. Mater. 2013, 35, 2418–2424. [Google Scholar] [CrossRef]
- Al Sanableh, A. Structural and Optical Properties of ZnO Thin Films Deposited by Sol Gel Caoting Technique. Master’s Thesis, Jordan Univesity of Science and Technology Irbid, Physics Department, Irbid, Jordan, 2006. [Google Scholar]
- Ahmad, A.; Alsaad, A.; Al-Bataineh, Q.; Al-Naafa, M. Optical and structural investigations of dip-synthesized boron-doped ZnO-seeded platforms for ZnO nanostructures. Appl. Phys. A 2018, 124, 458. [Google Scholar] [CrossRef]
- Lee, J.-H.; Park, B.-O. Transparent conducting ZnO: Al, In and Sn thin films deposited by the sol–gel method. Thin Solid Film 2003, 426, 94–99. [Google Scholar] [CrossRef]
- Ahmad, A.; Alsaad, A.; Al-Bataineh, Q.; Bani-Salameh, A.; Al-Khateeb, H.; Al-Naafa, M. Optical and Structural Characterization of Dip Synthesized Al-B Co-doped ZnO Seeded Platforms for ZnO Nanostructures. Jordan J. Phys. 2017, 10, 33–48. [Google Scholar]
- Schropp, R.E.; Zeman, M. Amorphous and microcrystalline silicon solar cells: Modeling, materials and device technology. Springer 1998, 8. [Google Scholar] [CrossRef]
- Ellipsometry, S.; Boccara, A.C.; Pickering, C.; Rivory, J. (Eds.) Proceedings of the First International Conference on Spectroscopic Ellipsometry; Elsevier: Amsterdam, The Netherlands, 1993. [Google Scholar]
- Jellison, G., Jr. Data analysis for spectroscopic ellipsometry. Thin Solid Film 1993, 234, 416–422. [Google Scholar] [CrossRef]
- Jellison, G., Jr.; Chisholm, M.; Gorbatkin, S. Optical functions of chemical vapor deposited thin-film silicon determined by spectroscopic ellipsometry. Appl. Phys. Lett. 1993, 62, 3348–3350. [Google Scholar] [CrossRef]
- Jellison, G.; Boatner, L.; Lowndes, D.; McKee, R.; Godbole, M. Optical functions of transparent thin films of SrTiO3, BaTiO3, and SiOx determined by spectroscopic ellipsometry. Appl. Opt. 1994, 33, 6053–6058. [Google Scholar] [CrossRef] [PubMed]
- Hassanien, A.S.; Akl, A.A. Influence of composition on optical and dispersion parameters of thermally evaporated non-crystalline Cd50S50−xSex thin films. J. Alloy. Compd. 2015, 648, 280–290. [Google Scholar] [CrossRef]
- Hassanien, A.; Akl, A.A. Effect of Se addition on optical and electrical properties of chalcogenide CdSSe thin films. Superlattices Microstruct. 2016, 89, 153–169. [Google Scholar] [CrossRef]
- Appani, S.K.; Rayapati, S.V.; Sutar, D.; Major, S. Study of transparent conducting Ga-doped ZnO films grown by reactive co-sputtering of Zn and GaAs. AIP Conf. Proc. 2018, 1942, 120009. [Google Scholar]
- Salem, M.; Akir, S.; Ghrib, T.; Daoudi, K.; Gaidi, M. Fe-doping effect on the photoelectrochemical properties enhancement of ZnO films. J. Alloy. Compd. 2016, 685, 107–113. [Google Scholar] [CrossRef]
- Kafle, B.; Acharya, S.; Thapa, S.; Poudel, S. Structural and optical properties of Fe-doped ZnO transparent thin films. Ceram. Int. 2016, 42, 1133–1139. [Google Scholar] [CrossRef]
- Khan, S.A.; Al-Hazmi, F.; Al-Heniti, S.; Faidah, A.; Al-Ghamdi, A. Effect of cadmium addition on the optical constants of thermally evaporated amorphous Se–S–Cd thin films. Curr. Appl. Phys. 2010, 10, 145–152. [Google Scholar] [CrossRef]
- Urbach, F. The long-wavelength edge of photographic sensitivity and of the electronic absorption of solids. Phys. Rev. 1953, 92, 1324. [Google Scholar] [CrossRef]
- El-Hagary, M.; Emam-Ismail, M.; Shaaban, E.; El-Taher, A. Effect of γ-irradiation exposure on optical properties of chalcogenide glasses Se70S30−xSbx thin films. Radiat. Phys. Chem. 2012, 81, 1572–1577. [Google Scholar] [CrossRef]
- Parmar, R.; Kundu, R.; Punia, R.; Aghamkar, P.; Kishore, N. Iron modified structural and optical spectral properties of bismuth silicate glasses. Phys. B Condens. Matter 2014, 450, 39–44. [Google Scholar] [CrossRef]
- Melsheimer, J.; Ziegler, D. Band gap energy and Urbach tail studies of amorphous, partially crystalline and polycrystalline tin dioxide. Thin Solid Film 1985, 129, 35–47. [Google Scholar] [CrossRef]
- Ikhmayies, S.J.; Ahmad-Bitar, R.N. A study of the optical bandgap energy and Urbach tail of spray-deposited CdS: In thin films. J. Mater. Res. Technol. 2013, 2, 221–227. [Google Scholar] [CrossRef] [Green Version]
- Aly, K.; Elnaeim, A.A.; Uosif, M.; Abdel-Rahim, O. Optical properties of Ge–As–Te thin films. Phys. B Condens. Matter 2011, 406, 4227–4232. [Google Scholar] [CrossRef]
- Askari, M.; Soltani, N.; Saion, E.; Yunus, W.M.M.; Erfani, H.M.; Dorostkar, M. Structural and optical properties of PVP-capped nanocrystalline ZnxCd1−xS solid solutions. Superlattices Microstruct. 2015, 81, 193–201. [Google Scholar] [CrossRef]
- Xing, C.; Zhang, Y.; Yan, W.; Guo, L. Band structure-controlled solid solution of Cd1-xZnxS photocatalyst for hydrogen production by water splitting. Int. J. Hydrog. Energy 2006, 31, 2018–2024. [Google Scholar] [CrossRef]
- Eloy, J. Power Lasers, National School of Physics, Grenoble, France; John Wiley and Sons: Somerset, NJ, USA, 1984. [Google Scholar]
- Sutcliffe, B.T.; Wilson, S. Potential energy curves and surfaces. Handb. Mol. Phys. Quantum Chem. 2003, 574–587. [Google Scholar]
- Wemple, S.; DiDomenico, M., Jr. Behavior of the electronic dielectric constant in covalent and ionic materials. Phys. Rev. B 1971, 3, 1338. [Google Scholar] [CrossRef]
- Fu, D.W.; Zhang, W.; Cai, H.L.; Ge, J.Z.; Zhang, Y.; Xiong, R.G. Diisopropylammonium chloride: A ferroelectric organic salt with a high phase transition temperature and practical utilization level of spontaneous polarization. Adv. Mater. 2011, 23, 5658–5662. [Google Scholar] [CrossRef]
- Girisun, T.S.; Dhanuskodi, S. Linear and nonlinear optical properties of tris thiourea zinc sulphate single crystals. Cryst. Res. Technol. J. Exp. Ind. Crystallogr. 2009, 44, 1297–1302. [Google Scholar] [CrossRef]
- El Radaf, I. Structural, optoelectrical, linear, and nonlinear optical characterizations of the Cu2ZnGeSe4 thin films. J. Mater. Sci. Mater. Electron. 2020, 31, 3228–3237. [Google Scholar] [CrossRef]
- Kumarasinghe, P.; Dissanayake, A.; Pemasiri, B.; Dassanayake, B. Effect of post deposition heat treatment on microstructure parameters, optical constants and composition of thermally evaporated CdTe thin films. Mater. Sci. Semicond. Process. 2017, 58, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.; Huda, Q.; Hossain, M.; Aliyu, M.; Karim, M.; Sopian, K.; Amin, N. High quality 1 μm thick CdTe absorber layers grown by magnetron sputtering for solar cell application. Curr. Appl. Phys. 2013, 13, S115–S121. [Google Scholar] [CrossRef]
- Enriquez, J.P.; Mathews, N.; Hernández, G.P.; Mathew, X. Influence of the film thickness on structural and optical properties of CdTe thin films electrodeposited on stainless steel substrates. Mater. Chem. Phys. 2013, 142, 432–437. [Google Scholar] [CrossRef]
- Eid, A.; Seddek, M.; Salem, A.; Dahy, T. Structural characterization and optical properties of Cd(1−x)MnxSe thin films. Vacuum 2008, 83, 401–407. [Google Scholar] [CrossRef]
- Caglar, Y. Sol–gel derived nanostructure undoped and cobalt doped ZnO: Structural, optical and electrical studies. J. Alloy. Compd. 2013, 560, 181–188. [Google Scholar] [CrossRef]
- Barrett, C.; Massalski, T. Structure of Metals: Crystallographic Methods. Available online: https://www.amazon.com/Structure-Metals-Third-Crystallographic-International/dp/0080261728 (accessed on 26 March 2020).
- Ramırez-Ortiz, J.; Ogura, T.; Medina-Valtierra, J.; Acosta-Ortiz, S.E.; Bosch, P.; de Los Reyes, J.A.; Lara, V.H. A catalytic application of Cu2O and CuO films deposited over fiberglass. Appl. Surf. Sci. 2001, 174, 177–184. [Google Scholar] [CrossRef]
- Li, J.; Li, H.; Xue, Y.; Fang, H.; Wang, W. Facile electrodeposition of environment-friendly Cu2O/ZnO heterojunction for robust photoelectrochemical biosensing. Sens. Actuators B Chem. 2014, 191, 619–624. [Google Scholar] [CrossRef]
- Habubi, N.; Oboudi, S.; Chiad, S. Study of some optical properties of mixed SnO2-CuO thin films. J. Nano-Electron. Phys. 2012, 4, 04008. [Google Scholar]
- Mott, N.F.; Davis, E.A. Electrical Process in Non-Crystalline Materials; Clarendon: Oxford, UK, 1979. [Google Scholar]
- The International Union of Crystallography is a Non-Profit Scientific Union Serving the World-Wide Interests of Crystallographers and Other Scientists Employing Crystallographic Methods. Available online: https://www.iucr.org/resources/other-directories/software/powderx (accessed on 22 March 2020).
- Znaidi, L. Sol–gel-deposited ZnO thin films: A. review. Mater. Sci. Eng. B 2010, 174, 18–30. [Google Scholar] [CrossRef]
- Takahashi, Y.; Kanamori, M.; Kondoh, A.; Minoura, H.; Ohya, Y. Photoconductivity of ultrathin zinc oxide films. Jpn. J. Appl. Phys. 1994, 33, 6611. [Google Scholar] [CrossRef]
- Lee, H.; Lee, J.; Kim, T.; Kim, D.; Cho, W. Formation mechanism of preferential c-axis oriented ZnO thin films grown on p-Si substrates. J. Mater. Sci. 2004, 39, 3525–3528. [Google Scholar] [CrossRef]
- Xu, L.; Li, X.; Chen, Y.; Xu, F. Structural and optical properties of ZnO thin films prepared by sol–gel method with different thickness. Appl. Surf. Sci. 2011, 257, 4031–4037. [Google Scholar] [CrossRef]
- Tsai, D.-C.; Chang, Z.-C.; Kuo, B.-H.; Wang, Y.-H.; Chen, E.-C.; Shieu, F.-S. Thickness dependence of the structural, electrical, and optical properties of amorphous indium zinc oxide thin films. J. Alloy. Compd. 2018, 743, 603–609. [Google Scholar] [CrossRef]
- Sandeep, K.; Bhat, S.; Dharmaprakash, S. Nonlinear absorption properties of ZnO and Al doped ZnO thin films under continuous and pulsed modes of operations. Opt. Laser Technol. 2018, 102, 147–152. [Google Scholar] [CrossRef]
- Hadimani, P.; Ghosh, S.; Sil, A. Preparation of Fe doped ZnO thin films and their structural, magnetic, electrical characterization. Superlattices Microstruct. 2018, 120, 199–208. [Google Scholar] [CrossRef]
- Ivanova, T.; Harizanova, A.; Koutzarova, T.; Vertruyen, B.; Stefanov, B. Structural and morphological characterization of sol-gel ZnO: Ga films: Effect of annealing temperatures. Thin Solid Film 2018, 646, 132–142. [Google Scholar] [CrossRef]
- Sarma, H.; Sarma, K. X-ray Peak Broadening Analysis of ZnO Nanoparticles Derived by Precipitation method. Int. J. Sci. Res. Publ. 2014, 4, 1–7. [Google Scholar]
- Ramakanth, K. Basics of X-ray Diffraction and its Application; I K International Publishing House Pvt. Ltd.: New Delhi, India, 2007. [Google Scholar]
- Khan, Z.R.; Khan, M.S.; Zulfequar, M.; Khan, M.S. Optical and structural properties of ZnO thin films fabricated by sol-gel method. Mater. Sci. Appl. 2011, 2, 340–345. [Google Scholar] [CrossRef] [Green Version]
- Horiuchi, S.; Tokunaga, Y.; Giovannetti, G.; Picozzi, S.; Itoh, H.; Shimano, R.; Kumai, R.; Tokura, Y. Above-room-temperature ferroelectricity in a single-component molecular crystal. Nature 2010, 463, 789–792. [Google Scholar] [CrossRef] [PubMed]




| Sample | Egap (eV) | EU (eV) | X (ZnO) (eV) | X (Z) (eV) | X (tot) (eV) | ECB (eV) | EVB (eV) |
|---|---|---|---|---|---|---|---|
| ZnO | 3.270 | 0.169 | 5.963 | 0.000 | 5.963 | −0.172 | 3.098 |
| BZO | 3.161 | 0.150 | 5.963 | 4.286 | 5.905 | −0.175 | 2.985 |
| AZO | 3.369 | 0.301 | 5.963 | 3.224 | 5.854 | −0.331 | 3.039 |
| GZO | 3.093 | 0.131 | 5.963 | 3.218 | 5.854 | −0.191 | 2.899 |
| IZO | 3.314 | 0.384 | 5.963 | 3.104 | 5.848 | −0.307 | 3.003 |
| Parameter | ZnO | BZO | AZO | GZO | IZO |
|---|---|---|---|---|---|
| Effective single oscillator, (eV) | 4.616 | 5.015 | 4.609 | 4.732 | 4.011 |
| Dispersion energy, (eV) | 6.261 | 9.362 | 4.976 | 6.328 | 7.990 |
| Zero-frequency refractive index, | 1.535 | 1.693 | 1.442 | 1.529 | 1.729 |
| Zero-frequency dielectric constant, | 2.357 | 2.867 | 2.080 | 2.337 | 2.992 |
| Optical oscillator strengths f | 28.902 | 46.948 | 22.936 | 29.940 | 32.051 |
| Linear optical susceptibility, | 0.108 | 0.149 | 0.086 | 0.106 | 0.159 |
| Third-order nonlinear optical susceptibility, (esu) | 2.309 | 8.287 | 9.264 | 2.181 | 1.074 |
| The nonlinear refractive index, | 5.668 | 1.844 | 2.421 | 5.375 | 2.339 |
| Samples | Crystalline Size D (nm) | Dislocation Density δ (1015) | Strain (10−3) | Crystalline Density (1016) |
|---|---|---|---|---|
| ZnO | 19.298 | 2.693 | 6.353 | 6.998 |
| BZO | 24.932 | 1.609 | 5.286 | 3.226 |
| AZO | 14.132 | 5.118 | 8.938 | 18.490 |
| GZO | 11.877 | 7.197 | 10.636 | 30.746 |
| IZO | 66.995 | 0.223 | 1.744 | 1.663 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsaad, A.M.; Ahmad, A.A.; Qattan, I.A.; Al-Bataineh, Q.M.; Albataineh, Z. Structural, Optoelectrical, Linear, and Nonlinear Optical Characterizations of Dip-Synthesized Undoped ZnO and Group III Elements (B, Al, Ga, and In)-Doped ZnO Thin Films. Crystals 2020, 10, 252. https://doi.org/10.3390/cryst10040252
Alsaad AM, Ahmad AA, Qattan IA, Al-Bataineh QM, Albataineh Z. Structural, Optoelectrical, Linear, and Nonlinear Optical Characterizations of Dip-Synthesized Undoped ZnO and Group III Elements (B, Al, Ga, and In)-Doped ZnO Thin Films. Crystals. 2020; 10(4):252. https://doi.org/10.3390/cryst10040252
Chicago/Turabian StyleAlsaad, A. M., A. A. Ahmad, I. A. Qattan, Qais M. Al-Bataineh, and Zaid Albataineh. 2020. "Structural, Optoelectrical, Linear, and Nonlinear Optical Characterizations of Dip-Synthesized Undoped ZnO and Group III Elements (B, Al, Ga, and In)-Doped ZnO Thin Films" Crystals 10, no. 4: 252. https://doi.org/10.3390/cryst10040252
APA StyleAlsaad, A. M., Ahmad, A. A., Qattan, I. A., Al-Bataineh, Q. M., & Albataineh, Z. (2020). Structural, Optoelectrical, Linear, and Nonlinear Optical Characterizations of Dip-Synthesized Undoped ZnO and Group III Elements (B, Al, Ga, and In)-Doped ZnO Thin Films. Crystals, 10(4), 252. https://doi.org/10.3390/cryst10040252





