Investigation of the Structural Changes and Catalytic Properties of FeNi Nanostructures as a Result of Exposure to Gamma Radiation
Abstract
:1. Introduction
2. Experimental Part
3. Results and Discussion
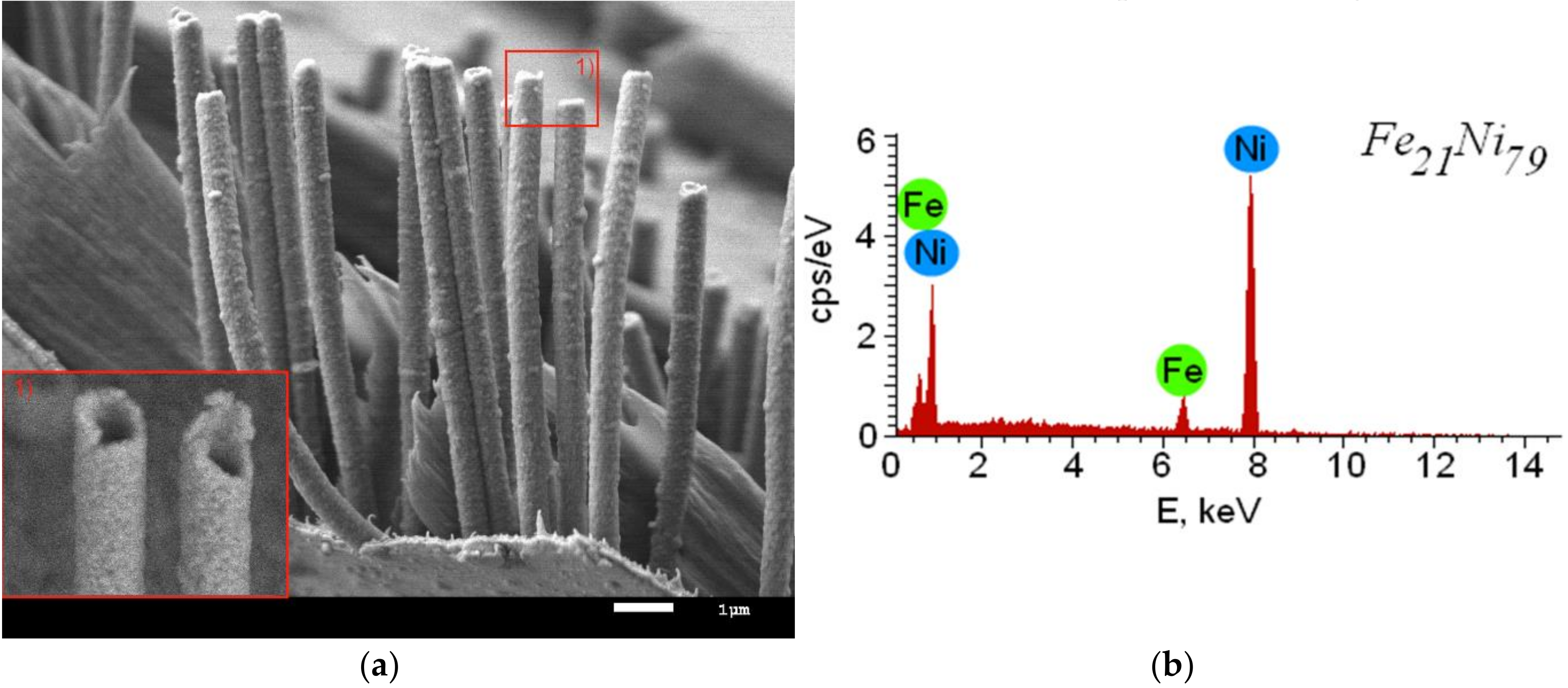
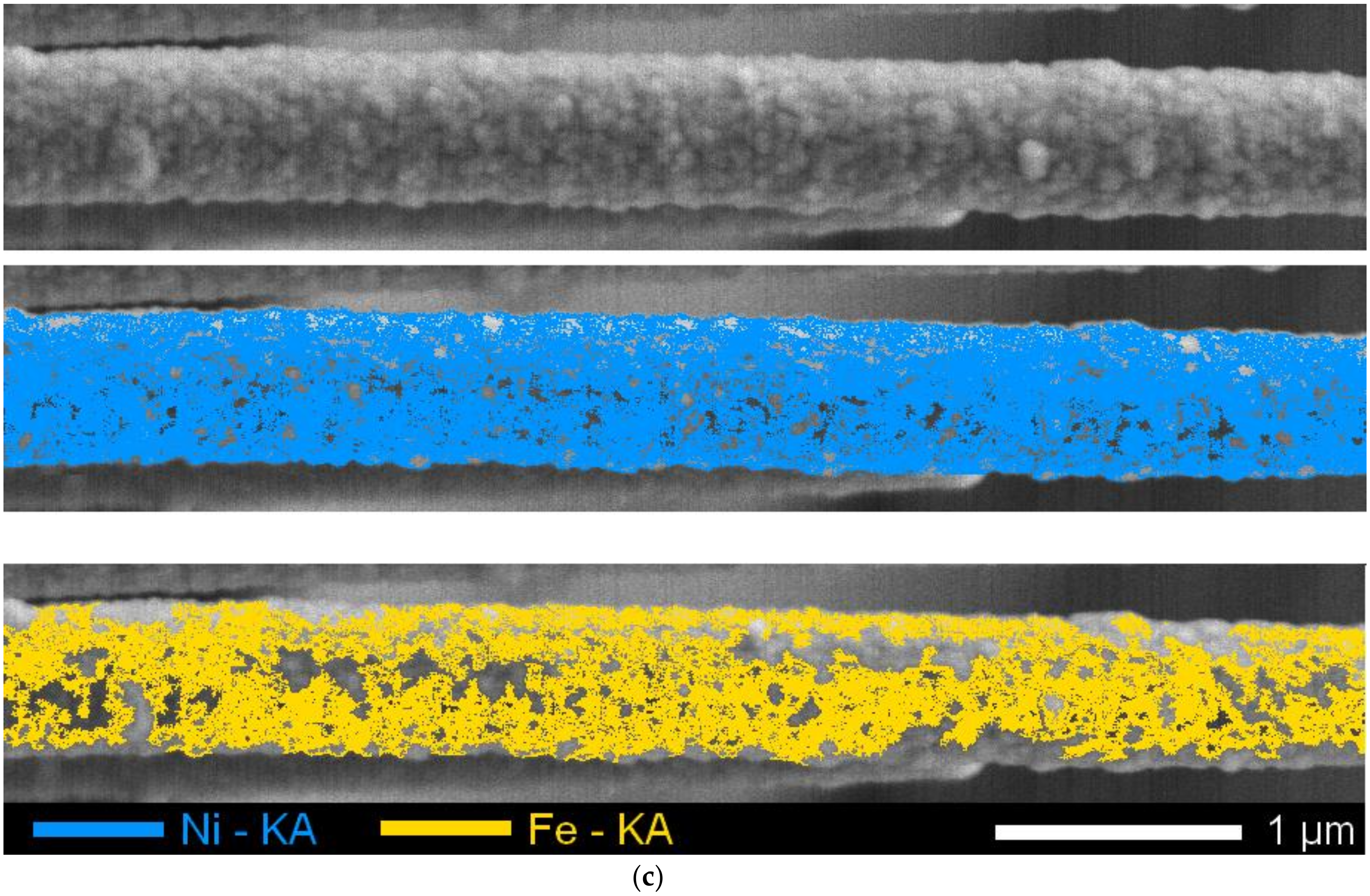
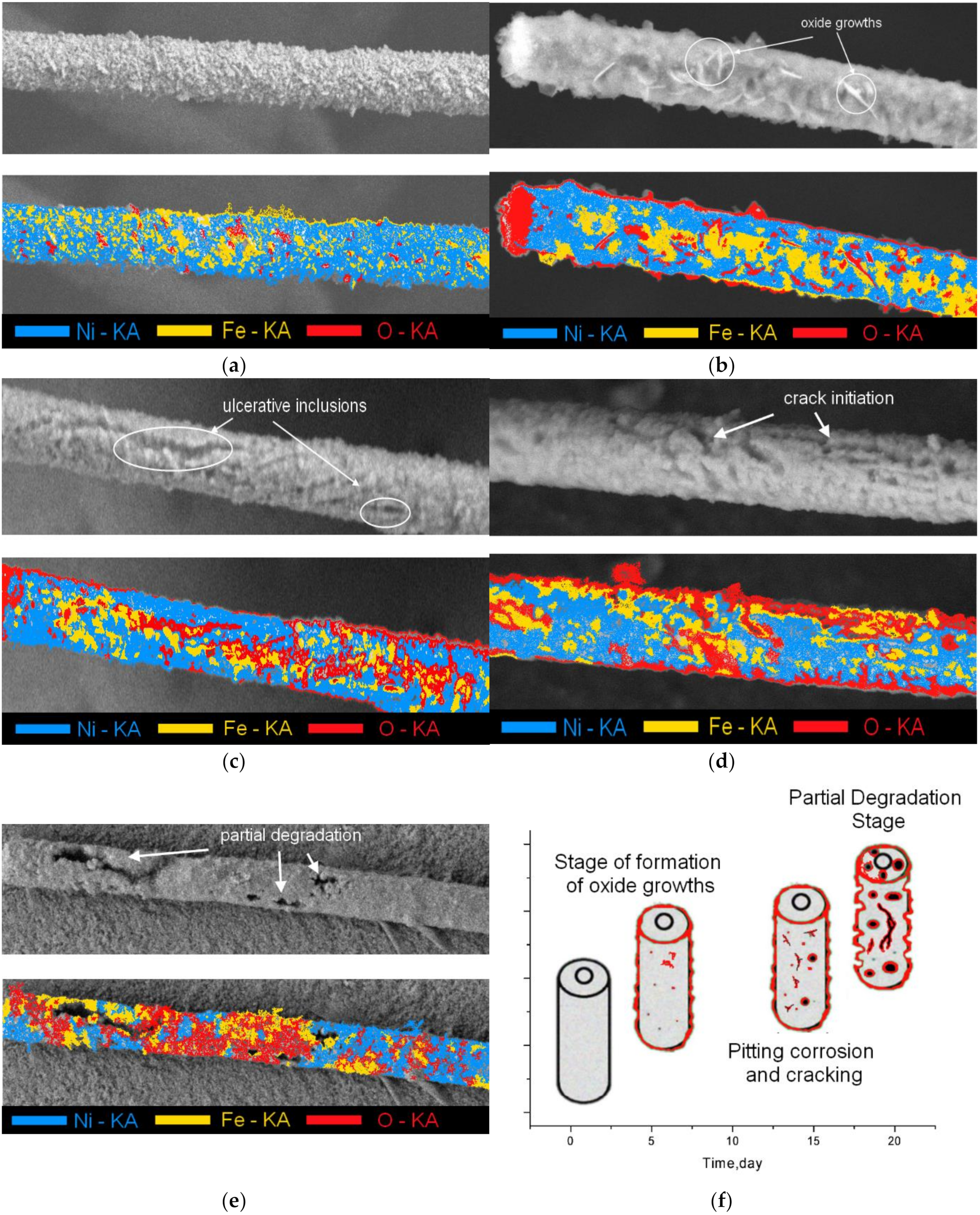
3.1. Study of Structural Changes Due to Irradiation
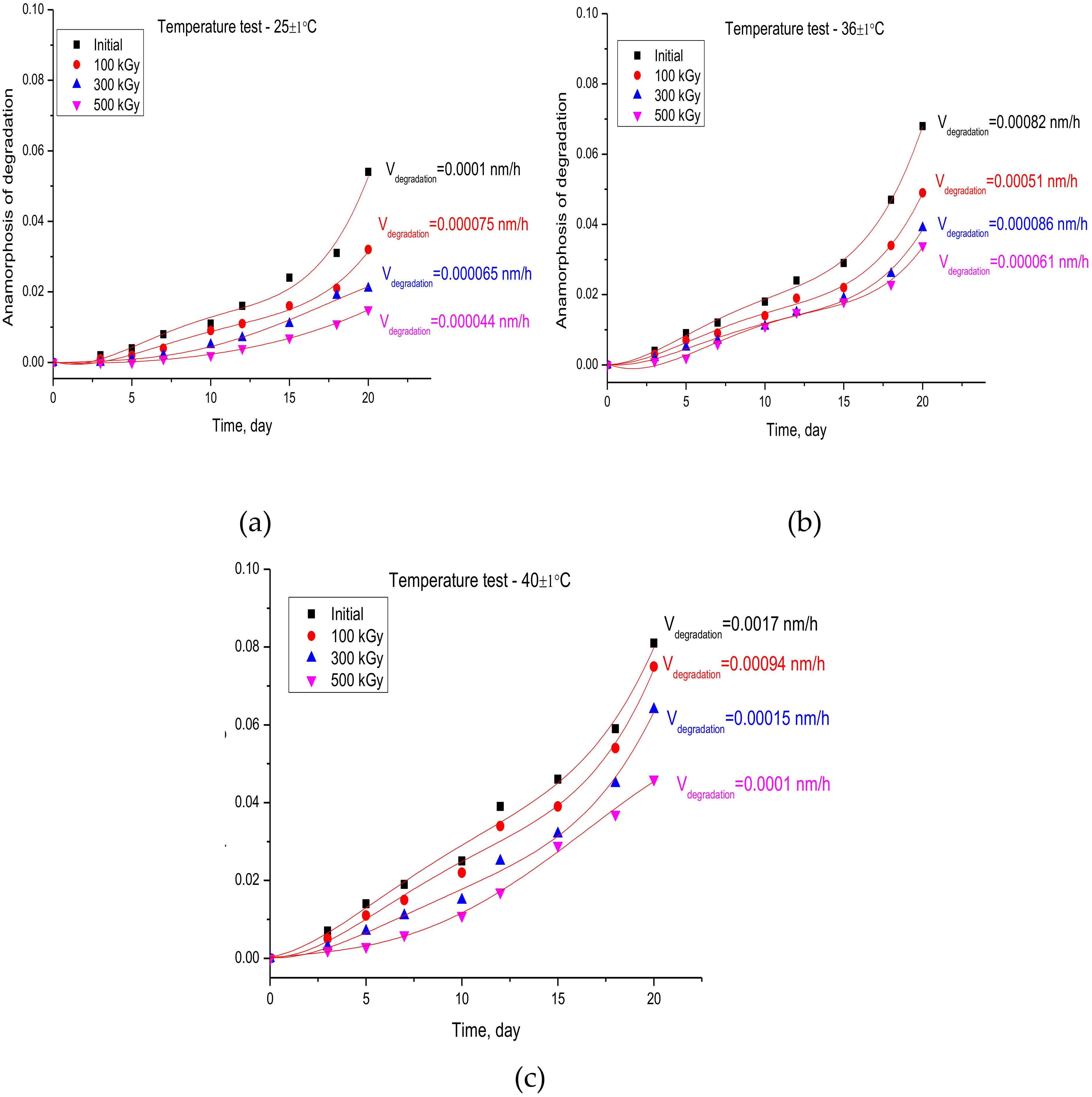
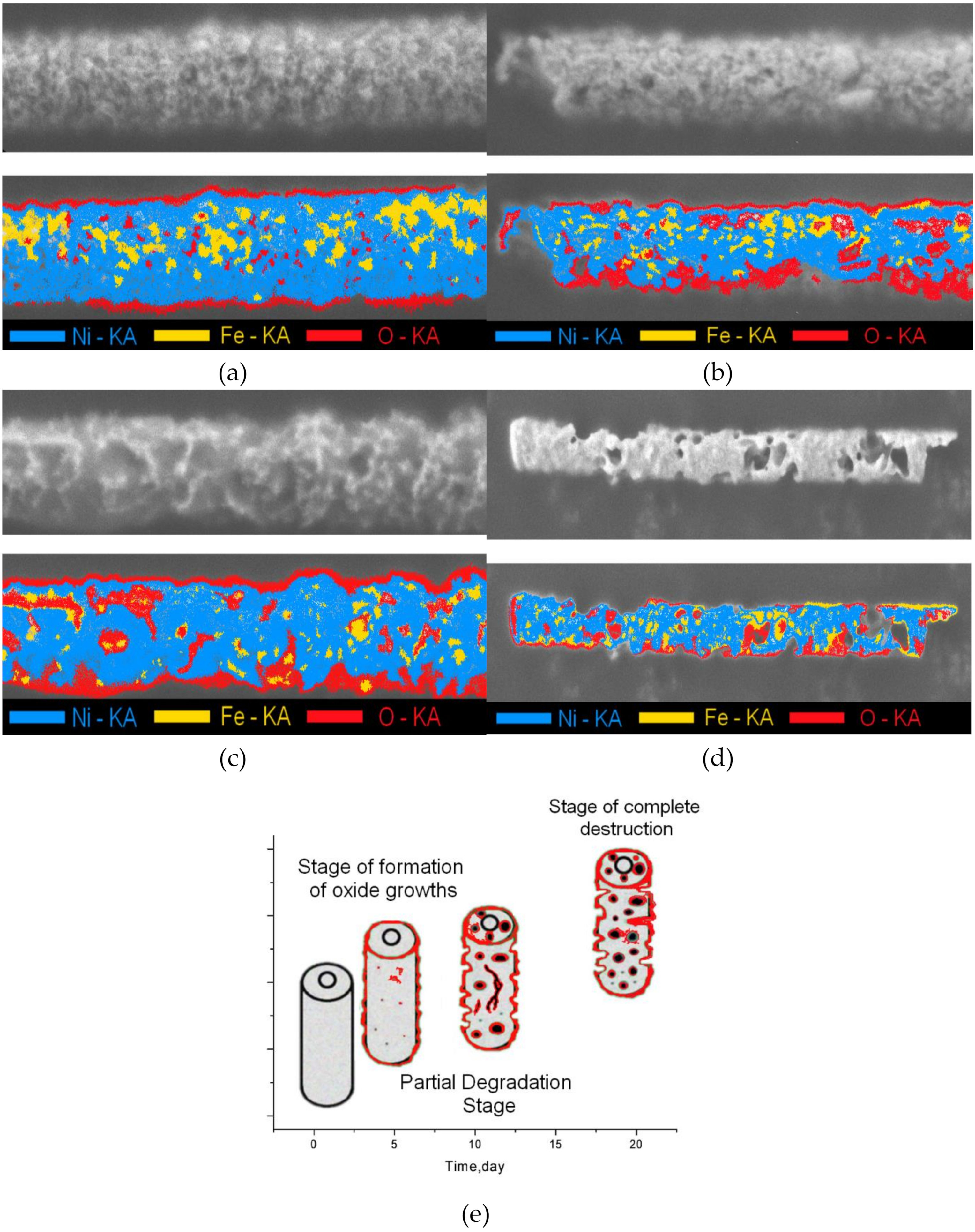
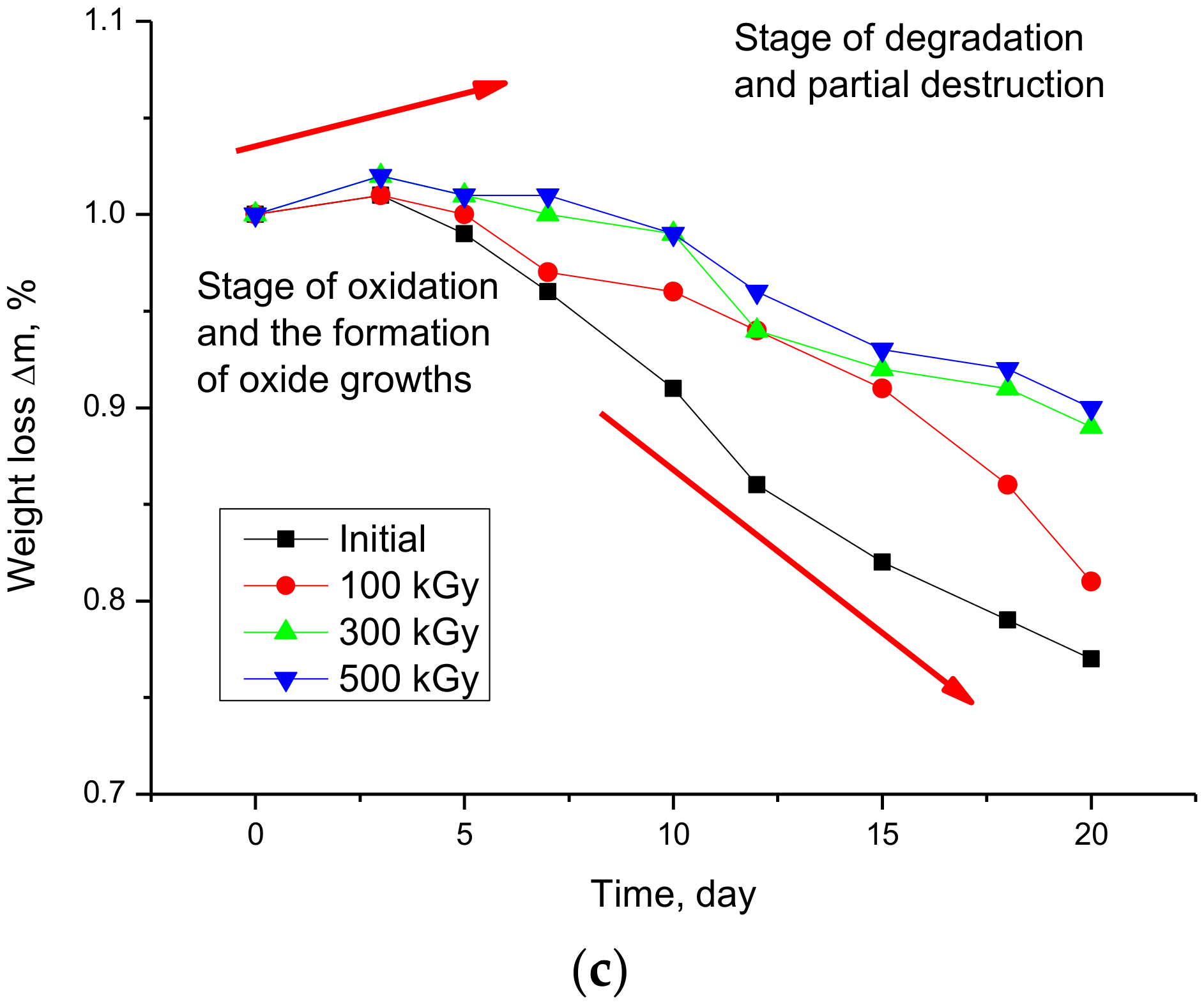
3.2. Studies of the Rate of Degradation of Nanostructures
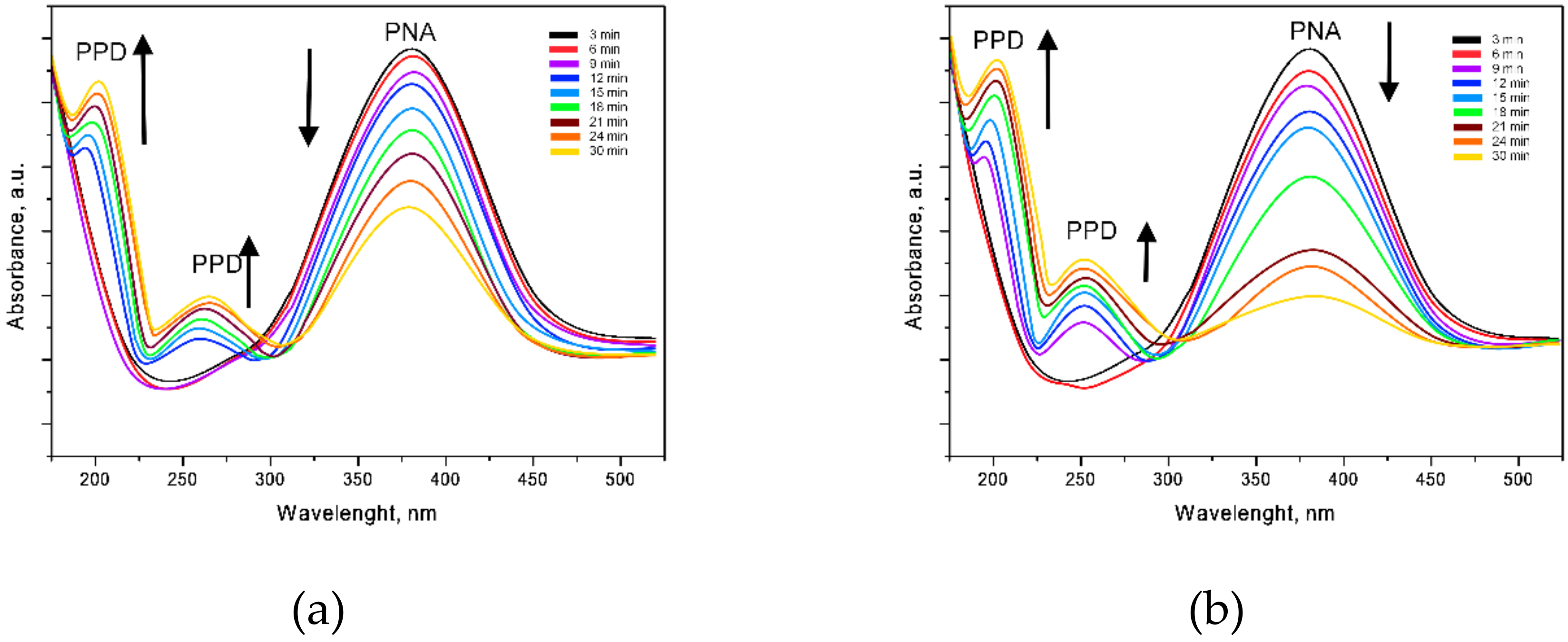
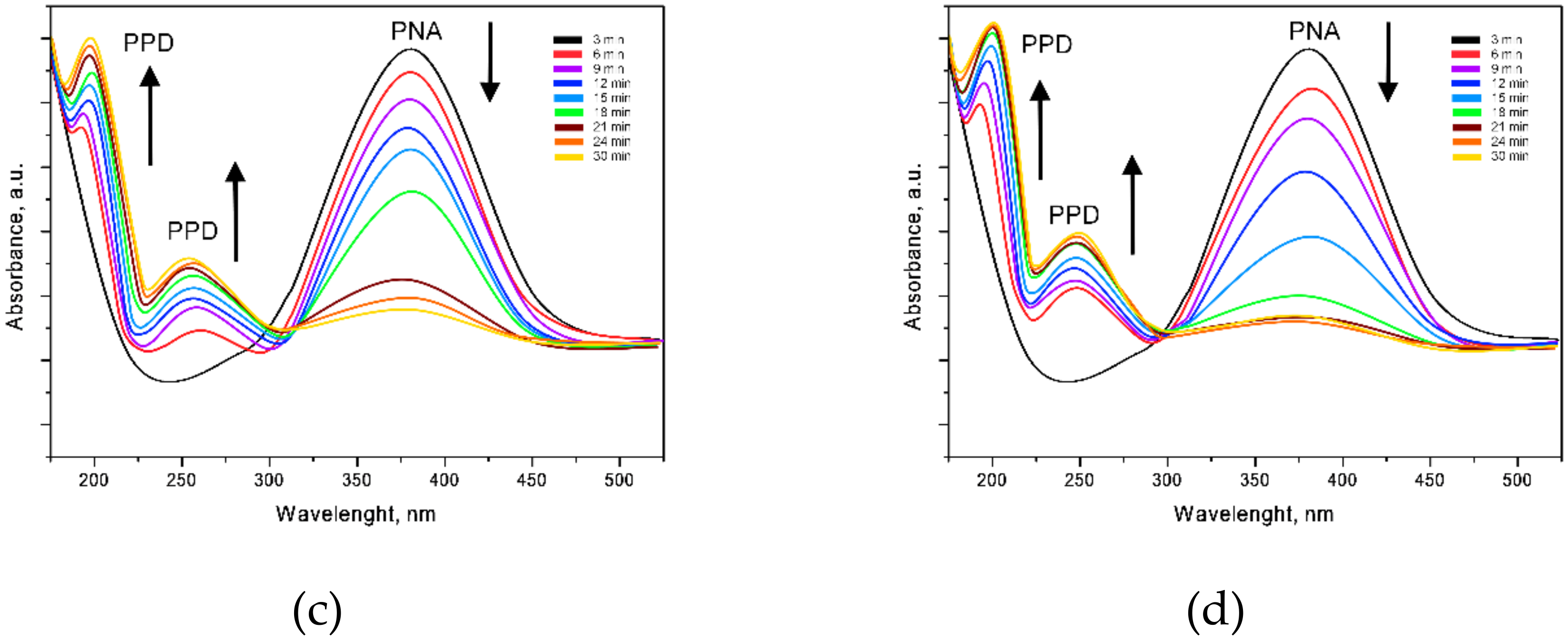
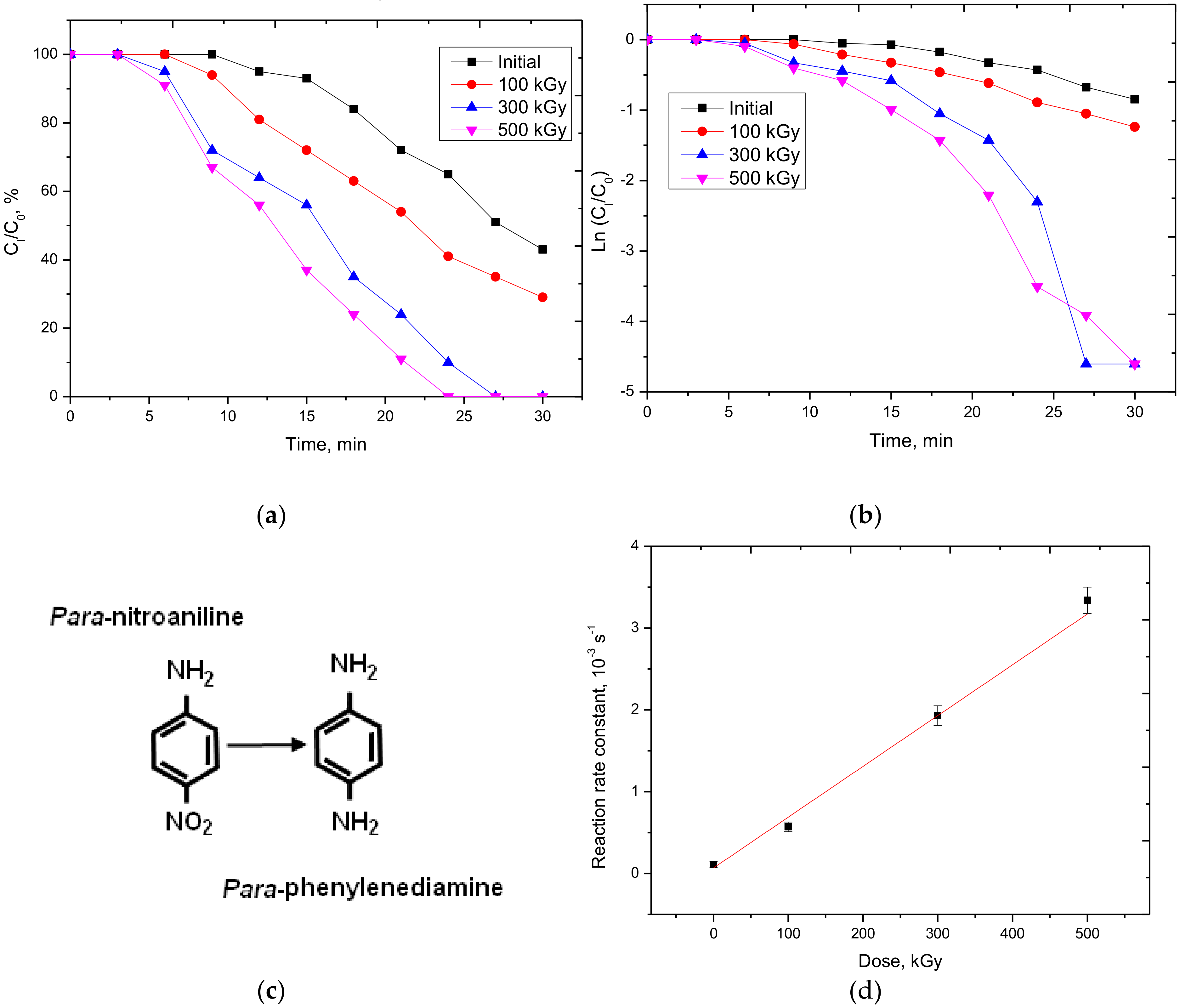
3.3. Study of the Catalytic Activity of Nanostructures
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Grozdanov, A.; Paunovic, P.; Vasilevska-Nikodinovska, V.; Dimitrov, A.; Yukhymchuk, V.; Stojkovski, I.; Arsova, Z. Structural analysis of X-ray irradiated carbon nanostructures. Mater. Sci. Eng. 2019, 3, 141–145. [Google Scholar] [CrossRef] [Green Version]
- Miller, K.J.; Sofman, M.; McNerny, K.; McHenry, M.E. Metastable γ-FeNi nanostructures with tunable Curie temperature. J. Appl. Phys. 2010, 107, 09A305. [Google Scholar] [CrossRef] [Green Version]
- Shumskaya, A.; Bundyukova, V.; Kozlovskiy, A.; Zdorovets, M.; Kadyrzhanov, K.; Kalkabay, G.; Kaniukov, E. Evolution of morphology, structure, and magnetic parameters of Ni nanotubes with growth in pores of a PET template. J. Magn. Magn. Mater. 2020, 497, 165913. [Google Scholar] [CrossRef]
- El-Said, A.; Wilhelm, R.; Heller, R.; Facsko, S. Creation of surface nanostructures in lanthanum fluoride single crystals by irradiation with slow highly charged ions. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2019, 460, 137–140. [Google Scholar] [CrossRef]
- Gong, M.; Ren, S. Phase transformation-driven surface reconstruction of FeNi nanostructures. Chem. Mater. 2015, 27, 7795–7800. [Google Scholar] [CrossRef]
- Volchkov, S.; Fernandez, E.; Arribas, A.G.; Barandiaran, J.M.; Lepalovskij, V.N.; Kurlyandskaya, G.V. Magnetic properties and giant magnetoimpedance of FeNi-based nanostructured multilayers with variable thickness of the central Cu lead. IEEE Trans. Magn. 2011, 47, 3328–3331. [Google Scholar] [CrossRef]
- Kholmanov, I.N.; Barborini, E.; Vinati, S.; Piseri, P.; Podesta’, A.; Ducati, C.; Lenardi, C.; Milani, P. The influence of the precursor clusters on the structural and morphological evolution of nanostructured TiO2 under thermal annealing. Nanotechnology 2003, 14, 1168. [Google Scholar] [CrossRef]
- Kozlovskiy, A.; Shlimas, D.; Kenzhina, I.; Zdorovets, M. The influence of thermal annealing on structural properties of Ni nanotubes. Vacuum 2018, 153, 254–261. [Google Scholar] [CrossRef]
- Zdorovets, M.; Kozlovskiy, A. Study of phase transformations in Co/CoCo2O4 nanowires. J. Alloy. Compd. 2020, 815, 152450. [Google Scholar] [CrossRef]
- Kang, M.; Goldman, R.S. Ion irradiation of III–V semiconductor surfaces: From self-assembled nanostructures to plasmonic crystals. Appl. Phys. Rev. 2019, 6, 041307. [Google Scholar] [CrossRef]
- Ramírez-Bon, R.; Espinoza-Beltrán, F.J.; Arizpe-Chávez, H.; Zelaya-Angel, O.; Sánchez-Sinencio, F. CdTe nanostructures prepared by thermal annealing. J. Appl. Phys. 1995, 77, 5461–5463. [Google Scholar]
- Zdorovets, M.V.; Kozlovskiy, A. Investigation of phase transformations and corrosion resistance in Co/CoCo2O4 nanowires and their potential use as a basis for lithium-ion batteries. Sci. Rep. 2019, 9, 16646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narayanasamy, A.; Sivakumar, N. Influence of mechanical milling and thermal annealing on electrical and magnetic properties of nanostructured Ni-Zn and cobalt ferrites. Bull. Mater. Sci. 2008, 31, 373–380. [Google Scholar] [CrossRef]
- Sarychev, V.; Nevskii, S.; Konovalov, S.; Granovskii, A.; Gromov, V. Thermocapillary model of formation of nanostructures on the surface irradiated by low-energy high-current electron beams. Mater. Res. Express 2019, 6, 076551. [Google Scholar] [CrossRef]
- El-Ghandour, A.; Awed, A.; Maksoud, M.I.A.A.; Nasher, M. 1, 2-Dihydroxyanthraquinone: Synthesis, and induced changes in the structural and optical properties of the nanostructured thin films due to γ-irradiation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 206, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Lemos, P.S.; Silva, G.S.; Roca, R.A.; Assis, M.; Torres-Mendieta, R.O.; Beltran-Mir, H.; Minguez-Vega, G.; Cordoncillo, E.; Andres, J.; Longo, E. Laser and electron beam-induced formation of Ag/Cr structures on Ag2CrO4. Phys. Chem. Chem. Phys. 2019, 21, 6101–6111. [Google Scholar]
- Antony, A.; Poornesh, P.; Kityk, I.; Myronchuk, G.; Sanjeev, G.; Petwal, V.C.; Verma, V.P.; Dwivedi, J. A study of 8 MeV e-beam on localized defect states in ZnO nanostructures and its role on photoluminescence and third harmonic generation. J. Lumin. 2019, 207, 321–332. [Google Scholar] [CrossRef]
- Kadyrzhanov, D.B.; Zdorovets, M.; Kozlovskiy, A.; E Kenzhina, I.; Petrov, A.V. Modification of structural and conductive properties of Zn nanotubes by irradiation with electrons with an energy of 5 MeV. Mater. Res. Express 2017, 4, 125023. [Google Scholar] [CrossRef]
- Ghorab, F.; Es’Haghi, Z.; Sheikh, N.; Akhavan, A. Gamma Irradiation Surface Modified Polypropylene-Based Hollow Fiber with Silver Nanoparticles and Its Impact on the Properties of Treated Membrane. Plasmonics 2019, 14, 1253–1260. [Google Scholar] [CrossRef]
- Sen, S.K.; Noor, M.; Al Mamun, M.A.; Manir, M.S.; Matin, M.A.; Hakim, M.A.; Nur, S.; Dutta, S. An investigation of 60Co gamma radiation-induced effects on the properties of nanostructured α-MoO3 for the application in optoelectronic and photonic devices. Opt. Quantum Electron. 2019, 51, 82. [Google Scholar] [CrossRef]
- Shumskaya, A.; Kaniukov, E.; Kutuzau, M.; Bundyukova, V.; Tulebayeva, D.; Kozlovskiy, A.; Borgekov, D.; Kenzhina, I.; Zdorovets, M. Influence of media with different acidity on structure of FeNi nanotubes. EPJ Web of Conferences. In EPJ Web of Conferences; EDP Sciences: Les Ulis, France, 2018; Volume 177, p. 01003. [Google Scholar]
- Kozlovskiy, A.; Kenzhina, I.; Zdorovets, M. Synthesis, phase composition and magnetic properties of double perovskites of A (FeM)O4‒x type (A = Ce; M = Ti). Ceram. Int. 2019, 45, 8669–8676. [Google Scholar] [CrossRef]
- Aziz, I.; Lee, J.G.; Duran, H.; Kirchhoff, K.; Baker, R.T.; Irvine, J.T.S.; Arshad, S.N. Nanostructured carbons containing FeNi/NiFe2O4 supported over N-doped carbon nanofibers for oxygen reduction and evolution reactions. RSC Adv. 2019, 9, 36586–36599. [Google Scholar] [CrossRef]
- Borgekov, D.; Zdorovets, M.; Shlimas, D.; Kozlovskiy, A.; Kadyrzhanov, K. The Study of the Applicability of Electron Irradiation for FeNi Microtubes Modification. Nanomaterials 2020, 10, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaniukov, E.; Shumskaya, A.; Yakimchuk, D.; Kozlovskiy, A.; Korolkov, I.; Ibragimova, M.; Zdorovets, M.; Kadyrzhanov, K.; Rusakov, V.; Fadeev, M.; et al. FeNi nanotubes: Perspective tool for targeted delivery. Appl. Nanosci. 2019, 9, 835–844. [Google Scholar] [CrossRef]
- Shumskaya, A.; Kozlovskiy, A.; Zdorovets, M.; Evstigneeva, S.; Trukhanov, A.; Trukhanov, S.; Vinnik, D.; Kaniukov, E.; Panina, L. Correlation between structural and magnetic properties of FeNi nanotubes with different lengths. J. Alloy. Compd. 2019, 810, 151874. [Google Scholar] [CrossRef]
- Wu, H.; Zeng, M.; Li, Z.; Zhu, X.; Tian, C.; Xia, C.; He, L.; Dai, S. Coupling FeNi alloys and hollow nitrogen-enriched carbon frameworks leads to high-performance oxygen electrocatalysts for rechargeable zinc–air batteries. Sustain. Energy Fuels 2019, 3, 136–141. [Google Scholar] [CrossRef]
- Kadyrzhanov, K.; Rusakov, V.; Fadeev, M.S.; Kiseleva, T.Y.; Kozlovskiy, A.; Kenzhina, I.; Zdorovets, M. Study of magnetic properties of Fe100‒xNix nanostructures using the mössbauer spectroscopy method. Nanomaterials 2019, 9, 757. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Yan, F.; Li, K.; Zhu, C.; Xie, Y.; Zhang, X.; Chen, Y. Ultrasmall FeNi3N particles with an exposed active (110) surface anchored on nitrogen-doped graphene for multifunctional electrocatalysts. J. Mater. Chem. A 2019, 7, 1083–1091. [Google Scholar] [CrossRef]
- Cui, X. Self-thermoregulating Ferromagnetic FeNi filled Carbon Nanotubes for Magnetic Hyperthermia Cancer Therapy. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019; Volume 310. [Google Scholar]
- Kurlyandskaya, G.V.; Shcherbinin, S.V.; Buznikov, N.A.; Chlenova, A.A.; Svalov, A.V. Magnetic Materials for Thin Film Based Magnetoimpedance Biosensing. Phys. Met. Metallogr. 2019, 120, 1243–1251. [Google Scholar] [CrossRef]
- Rusakov, V.; Kadyzhanov, K.; Kozlovskiy, A.; Fadeev, M.; Zdorovets, M. Phase transformations as a result of thermal annealing of nanocomposite Fe–Ni/Fe–Ni–O particles. Ceram. Int. 2020, 46, 1586–1595. [Google Scholar] [CrossRef]
- Rostami-Vartooni, A.; Moradi-Saadatmand, A. Green synthesis of magnetically recoverable Fe3O4/HZSM-5 and its Ag nanocomposite using Juglans regia L. leaf extract and their evaluation as catalysts for reduction of organic pollutants. IET Nanobiotechnol. 2019, 13, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Zubar, T.; Fedosyuk, V.M.; Trukhanov, A.V.; Kovaleva, N.N.; Astapovich, K.A.; Vinnik, D.; Trukhanova, E.L.; Kozlovskiy, A.L.; Zdorovets, M.V.; Solobai, A.A.; et al. Control of growth mechanism of electrodeposited nanocrystalline NiFe films. J. Electrochem. Soc. 2019, 166, D173–D180. [Google Scholar] [CrossRef]
- Zdorovets, M.; Kozlovskiy, A. Argon ion irradiation effect on Zn nanotubes. J. Mater. Sci. Mater. Electron. 2018, 29, 3621–3630. [Google Scholar] [CrossRef]
- Salati, A.; Ramazani, A.; Kashi, M.A. Tuning hyperthermia properties of FeNiCo ternary alloy nanoparticles by morphological and magnetic characteristics. J. Magn. Magn. Mater. 2020, 498, 166172. [Google Scholar] [CrossRef]
- Amin, P.; Patel, M. Magnetic nanoparticles-a promising tool for targeted drug delivery system. Asian J. Nanosci. Mater. 2020, 3, 24–37. [Google Scholar]
- Kaniukov, E.; Shumskaya, A.; Kutuzau, M.; Bundyukova, V.; Yakimchuk, D.; Borgekov, D.; Ibragimova, M.; Korolkov, I.; Giniyatova, S.; Kozlovskiy, A.; et al. Degradation mechanism and way of surface protection of nickel nanostructures. Mater. Chem. Phys. 2019, 223, 88–97. [Google Scholar] [CrossRef]
- Korolkov, I.; Kozlovskiy, A.; Gorin, Y.G.; Kazantsev, A.V.; Shlimas, D.I.; Zdorovets, M.V.; Ualieva, N.K.; Rusakov, V. Immobilization of carborane derivatives on Ni/Fe nanotubes for BNCT. J. Nanopart. Res. 2018, 20, 240. [Google Scholar] [CrossRef]
- Kozlovskiy, A.; Zhanbotin, A.; Zdorovets, M.; Manakova, I.; Ozernoy, A.; Kadyrzhanov, K.; Rusakov, V. Study of Ni/Fe nanotube properties. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2015, 365, 663–667. [Google Scholar] [CrossRef]
- Siwy, Z.S. Ion-current rectification in nanopores and nanotubes with broken symmetry. Adv. Funct. Mater. 2006, 16, 735–746. [Google Scholar] [CrossRef]
- Kaniukov, E.; Shumskaya, E.E.; Yakimchuk, D.; Kozlovskiy, A.; Ibragimova, M.A.; Zdorovets, M. Evolution of the polyethylene terephthalate track membranes parameters at the etching process. J. Contemp. Phys. Armen. Acad. Sci. 2017, 52, 155–160. [Google Scholar] [CrossRef]
- Puerta, J.; Martin, P. Three and four generalized Lorentzian approximations for the Voigt line shape. Appl. Opt. 1981, 20, 3923–3928. [Google Scholar] [CrossRef] [PubMed]
- Kozlovskiy, A.; Dukenbayev, K.; Ivanov, I.; Kozin, S.; Aleksandrenko, V.; Kurakhmedov, A.; Sambaev, E.; Kenzhina, I.; Tosi, D.; Loginov, V.; et al. Investigation of the influence of irradiation with Fe+7 ions on structural properties of AlN ceramics. Mater. Res. Express 2018, 5, 065502. [Google Scholar] [CrossRef]
- Mote, V.; Purushotham, Y.; Dole, B. Williamson-Hall analysis in estimation of lattice strain in nanometer-sized ZnO particles. J. Theor. Appl. Phys. 2012, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Shumskaya, A.E.; Kaniukov, E.Y.; Kozlovskiy, A.L.; Shlimas, D.I.; Zdorovets, M.V.; Ibragimova, M.A.; Rusakov, V.S.; Kadyrzhanov, K.K. Template synthesis and magnetic characterization of FeNi nanotubes. Prog. Electromagn. Res. 2017, 75, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Zhou, N.; Cai, L.-H.; Wen, F.-S.; Li, F.-S. Template synthesis and magnetic behavior of FeNi alloy nanotube arrays. Chin. J. Chem. Phys. 2007, 20, 821. [Google Scholar] [CrossRef]
- Kozlovskiy, A.L.; Kenzhina, I.E.; Zdorovets, M.V. FeCo–Fe2CoO4/Co3O4 nanocomposites: Phase transformations as a result of thermal annealing and practical application in catalysis. Ceram. Int. 2020. [Google Scholar] [CrossRef]
- Kozlovskiy, A.; Shlimas, D.; Zdorovets, M. Investigation of the effect of ionizing radiation on the structural and conductive characteristics of Ni nanostructures. Vacuum 2019, 163, 103–109. [Google Scholar] [CrossRef]
- Kozlovskiy, A.; Ermekova, A.; Korolkov, I.; Chudoba, D.; Jazdzewska, M.; Łudzik, K.; Nazarova, A.; Marciniak, B.; Kontek, R.; Shumskaya, A.; et al. Study of phase transformations, structural, corrosion properties and cytotoxicity of magnetite-based nanoparticles. Vacuum 2019, 163, 236–247. [Google Scholar] [CrossRef]
- Salah, N.; Habib, S.S.; Khan, Z.H.; Al-Hamedi, S.; Djouider, F. Functionalization of gold and carbon nanostructured materials using gamma-ray irradiation. Radiat. Phys. Chem. 2009, 78, 910–913. [Google Scholar] [CrossRef]
- Kozlovskiy, A.; Korolkov, I.; Ibragimova, M.A.; Zdorovets, M.V.; Kutuzau, M.; Nikolaevich, L.N.; Shumskaya, E.E.; Kaniukov, E. Magnetic nanostructured system for biomedical applications based on FeNi nanotubes. Nanotechnol. Russ. 2018, 13, 331–336. [Google Scholar] [CrossRef]
- Tartaj, P.; Morales, M.D.P.; Veintemillas-Verdaguer, S.; González-Carreño, T.; Serna, C. The preparation of magnetic nanoparticles for applications in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R182–R197. [Google Scholar] [CrossRef]
- Lu, X.; Wu, J.; Huo, G.; Sun, Q.; Huang, Y.; Han, Z.; Liang, G. Protein-passivated FeNi3 particles with low toxicity and high inductive heating efficiency for thermal therapy. Colloids Surf. A Physicochem. Eng. Asp. 2012, 414, 168–173. [Google Scholar] [CrossRef]
- Slaton, R.D.; Bae, I.-T.; Lutz, P.S.; Pathade, L.; Maye, M.M. The transformation of α-Fe nanoparticles into multi-domain FeNi–M3O4 (M = Fe, Ni) heterostructures by galvanic exchange. J. Mater. Chem. C 2015, 3, 6367–6375. [Google Scholar] [CrossRef]
- Pan, J.; Thierry, D.; Leygraf, C. Hydrogen peroxide toward enhanced oxide growth on titanium in PBS solution: Blue coloration and clinical relevance. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. 1996, 30, 393–402. [Google Scholar] [CrossRef]
- Wuang, S.C.; Neoh, K.G.; Kang, E.-T.; Pack, D.W.; Leckband, D.E. Synthesis and functionalization of polypyrrole-Fe3O4 nanoparticles for applications in biomedicine. J. Mater. Chem. 2007, 17, 3354–3362. [Google Scholar] [CrossRef]




| Sample | Lattice Parameter, Å | Interplanar Distance | Degree of Structural Ordering | Full Width at Half Maximum of Peak (111) (FWHM) | The Ratio of the 111 to the 200 Peak (Intensity (111)/Intensity (200)) |
|---|---|---|---|---|---|
| Initial | 3.5492 ± 0.0013 | 2.04781 | 86.4 | 1.494 | 6.11 |
| 100 kGy | 3.5415 ± 0.0016 | 2.04318 | 87.3 | 1.246 | 6.15 |
| 200 kGy | 3.5381 ± 0.0021 | 2.04087 | 91.1 | 0.917 | 6.21 |
| 300 kGy | 3.5367 ± 0.0015 | 2.04085 | 93.5 | 0.820 | 10.56 |
| 400 kGy | 3.5353 ± 0.0011 | 2.04071 | 94.1 | 0.812 | 16.32 |
| 500 kGy | 3.5221 ± 0.0017 | 2.03632 | 94.5 | 0.745 | 21.74 |
| TChkl | 111 | 200 | 220 |
|---|---|---|---|
| Initial | 1.875 * | 0.643 | 0.482 |
| 100 kGy | 1.942 | 0.743 | 0.315 |
| 200 kGy | 1.964 | 0.664 | 0.372 |
| 300 kGy | 2.353 | 0.364 | 0.283 |
| 400 kGy | 2.553 | 0.231 | 0.216 |
| 500 kGy | 2.734 | 0.153 | 0.113 |
| * Coefficients greater than one are shown in italics | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borgekov, D.B.; Zdorovets, M.V.; Shlimas, D.I.; Kozlovskiy, A.L. Investigation of the Structural Changes and Catalytic Properties of FeNi Nanostructures as a Result of Exposure to Gamma Radiation. Crystals 2020, 10, 254. https://doi.org/10.3390/cryst10040254
Borgekov DB, Zdorovets MV, Shlimas DI, Kozlovskiy AL. Investigation of the Structural Changes and Catalytic Properties of FeNi Nanostructures as a Result of Exposure to Gamma Radiation. Crystals. 2020; 10(4):254. https://doi.org/10.3390/cryst10040254
Chicago/Turabian StyleBorgekov, Daryn B., Maxim V. Zdorovets, Dmitriy I. Shlimas, and Artem L. Kozlovskiy. 2020. "Investigation of the Structural Changes and Catalytic Properties of FeNi Nanostructures as a Result of Exposure to Gamma Radiation" Crystals 10, no. 4: 254. https://doi.org/10.3390/cryst10040254





