Microstructures and Wear Resistance of CoCrFeNi2V0.5Tix High-Entropy Alloy Coatings Prepared by Laser Cladding
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
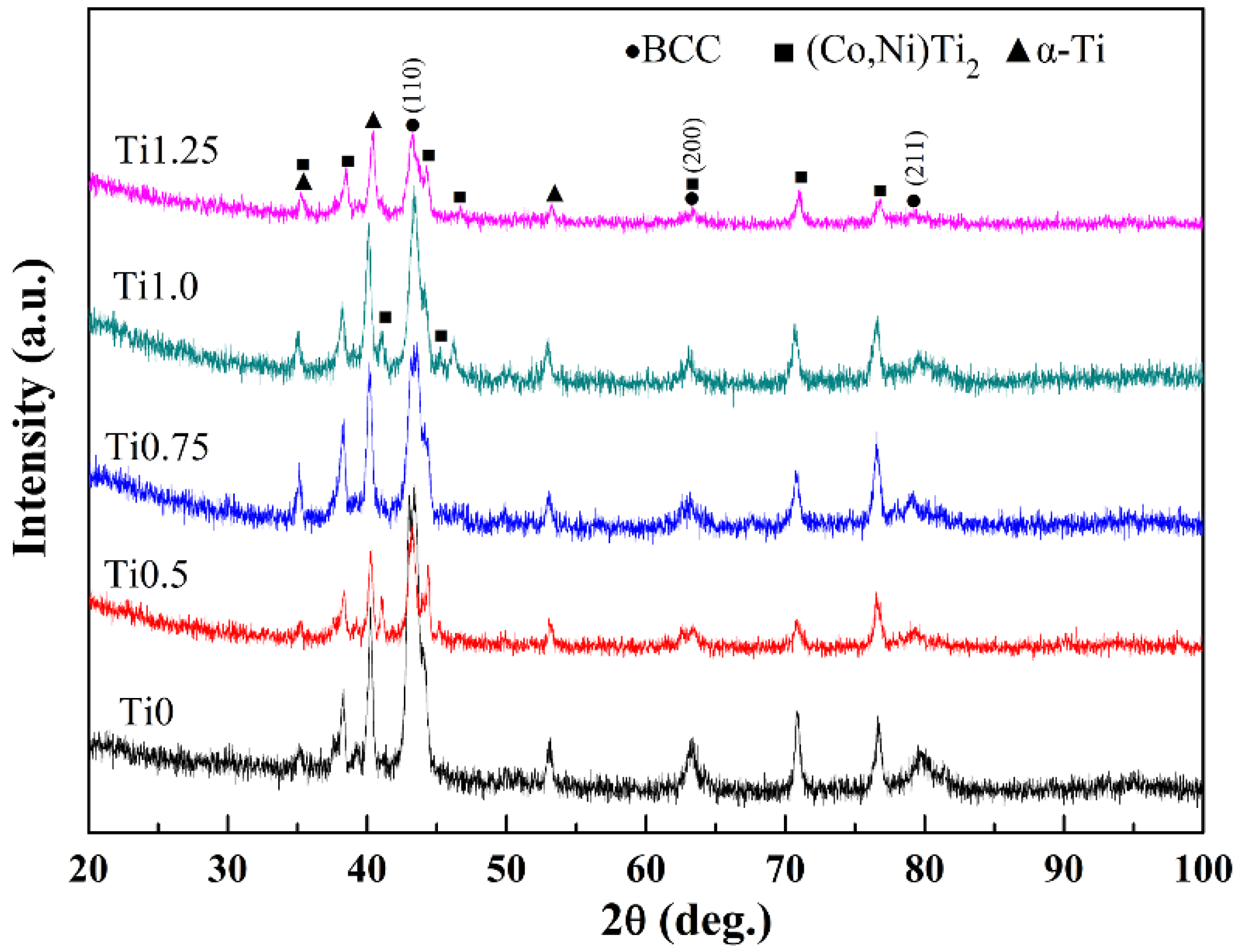
3.1. Phase Structures
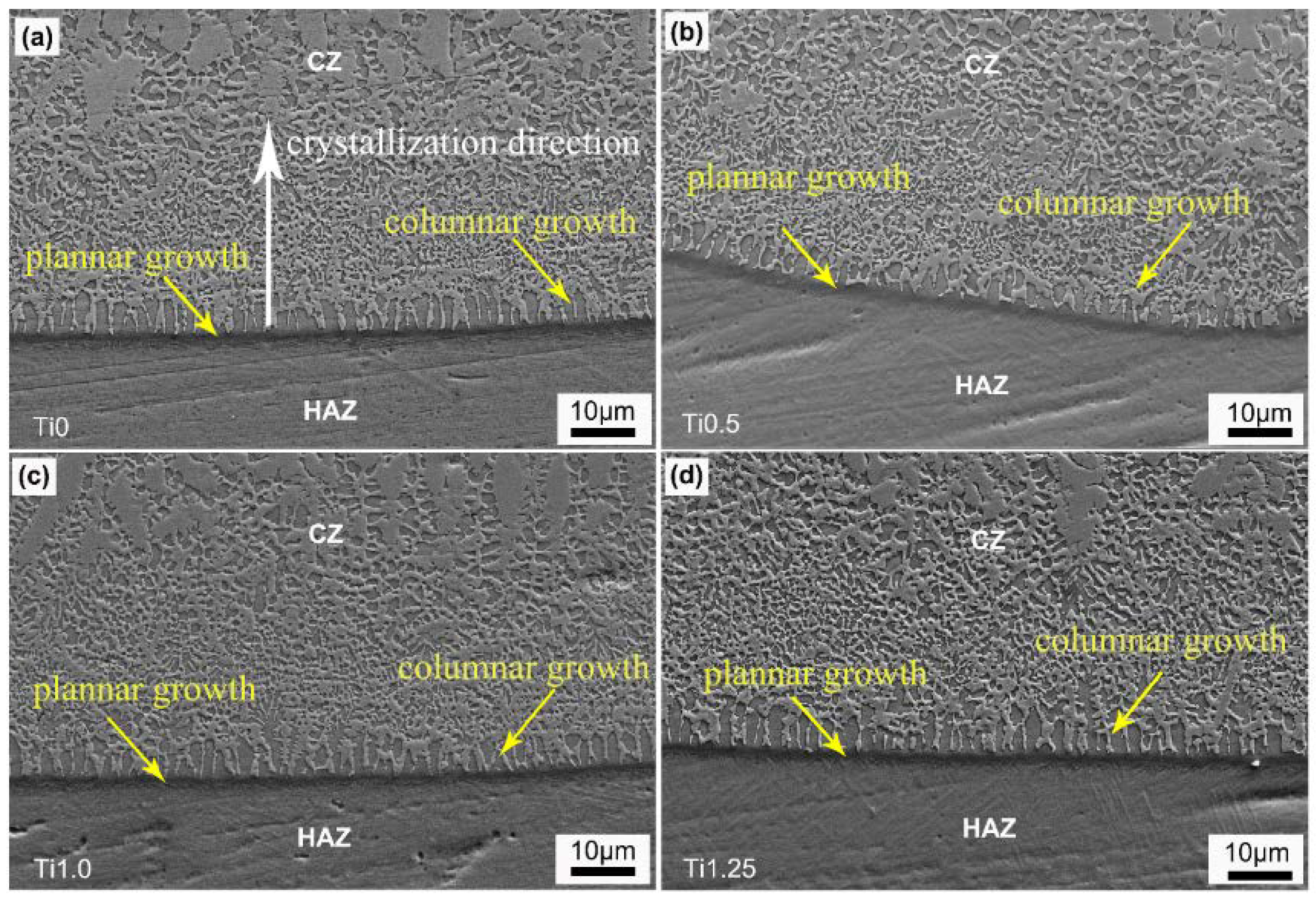
3.2. Microstructures
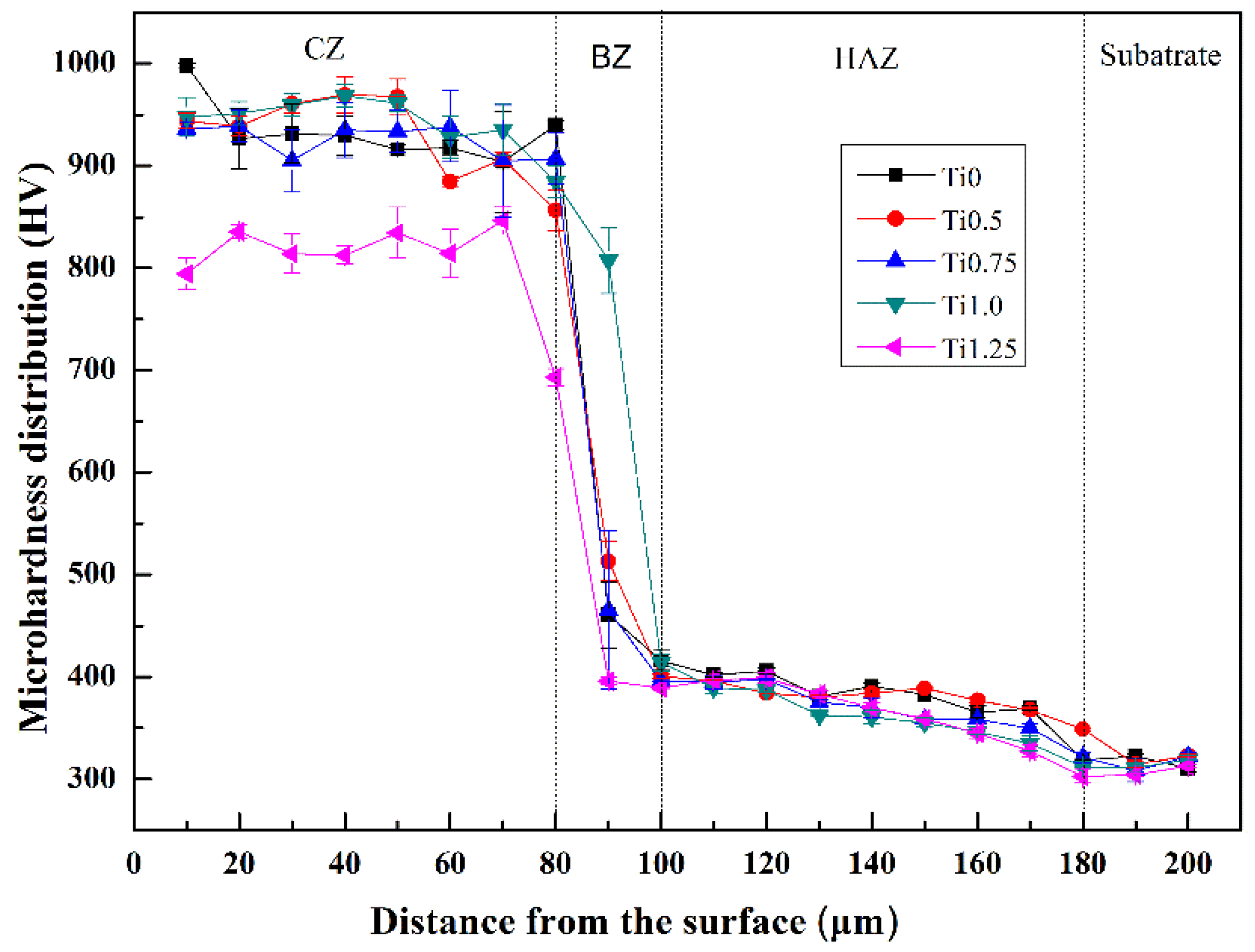
3.3. Microhardness
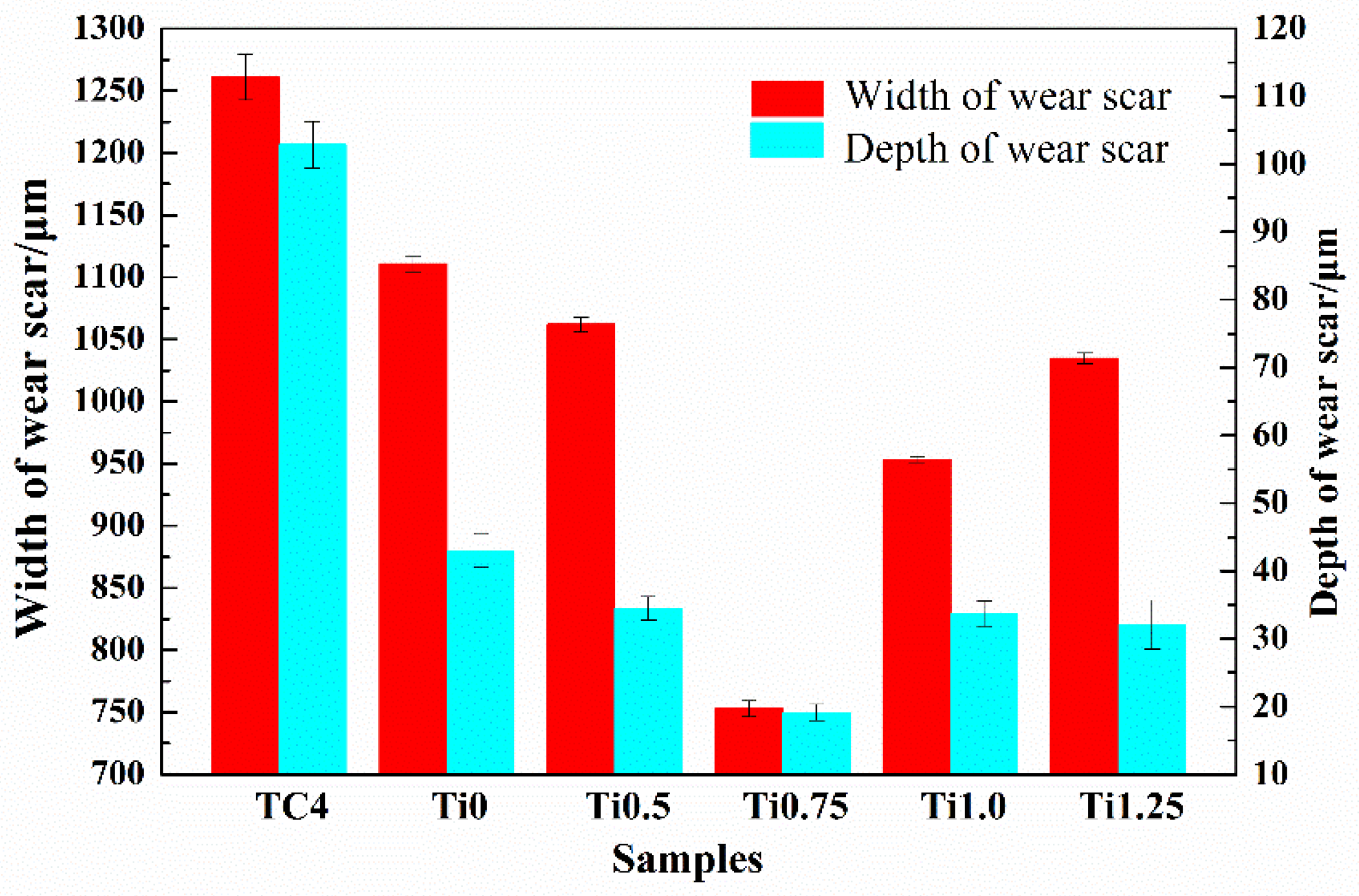
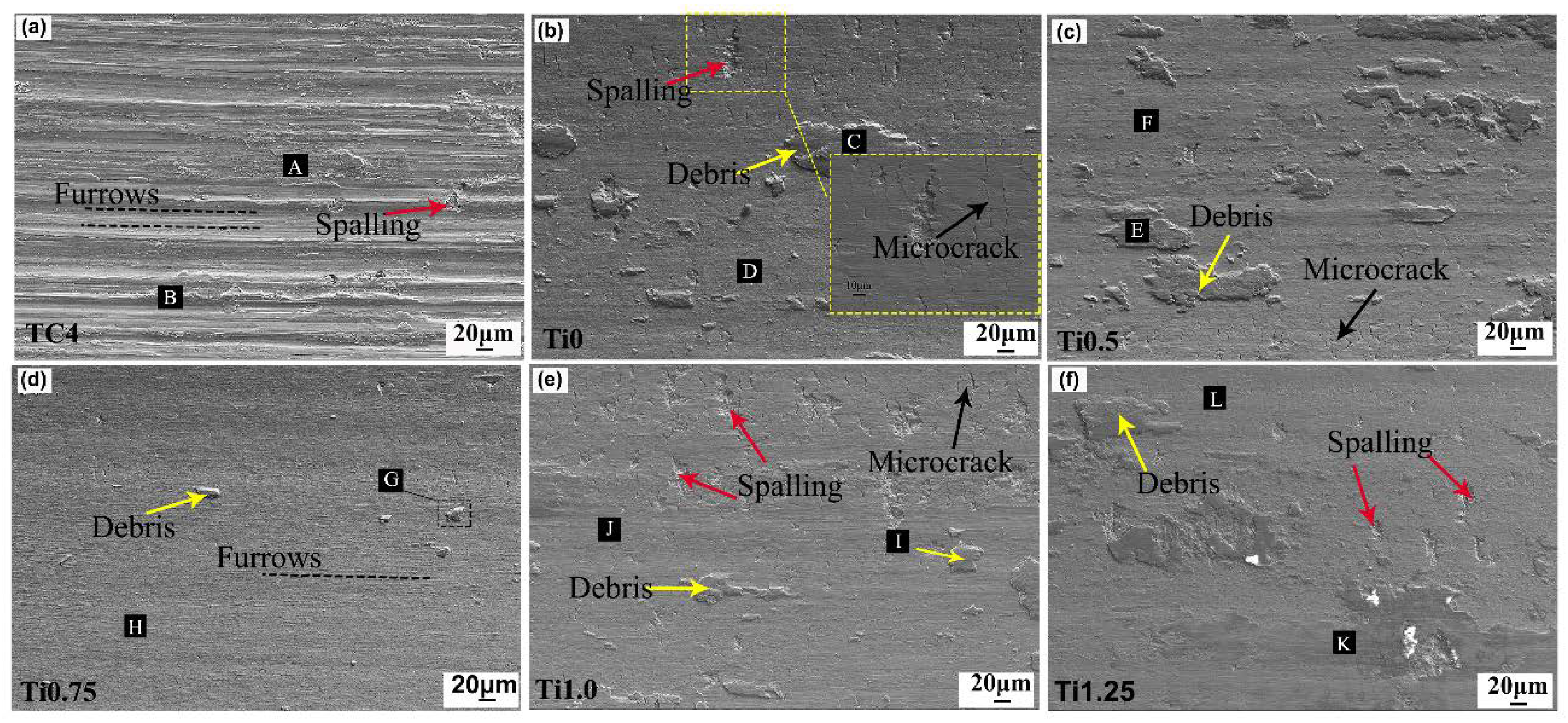
3.4. Wear Resistance
4. Conclusions
- (1)
- All of the CoCrFeNi2V0.5Tix alloy coatings are composed of Fe,Cr-BCC solid solution phase, (Co,Ni)Ti2 phase, and α-Ti phase. With the increase of Ti content, the volume fraction of the (Co,Ni)2Ti phase increases.
- (2)
- The microhardness of the CoCrFeNi2V0.5Tix alloy coating is significantly higher than that of the TC4 substrate, even 2.6–3.0 times higher than the substrate. The improvement is mainly attributed to second phase strengthening and solid solution strengthening.
- (3)
- The CoCrFeNi2V0.5Ti0.75 alloy coating exhibits the best wear resistance, and its wear rate is about 4.426 × 10−5 mm3/(N·m). The wear mechanism of all of the CoCrFeNi2V0.5Tix HEA coatings is mainly adhesive wear and oxidation wear, while the wear mechanism of TC4 substrate is mainly abrasive wear and oxidative wear.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, X.S.; Chen, Y.J.; Hu, J.L. Recent advances in the development of aerospace materials. Prog. Aerosp. Sci. 2018, 97, 22–34. [Google Scholar] [CrossRef]
- Budinski, K.G. Tribological properties of titanium alloys. Wear 1991, 151, 203–217. [Google Scholar] [CrossRef]
- Tian, Y.S.; Zhang, Q.Y.; Wang, D.Y. Study on the microstructures and properties of the boride layers laser fabricated on Ti-6Al-4V alloy. J. Mater. Process. Technol. 2009, 209, 2887–2891. [Google Scholar] [CrossRef]
- Lazurenko, D.V.; Bataev, I.A.; Laptev, I.S.; Ruktuev, A.A.; Maliutina, I.N.; Golkovsky, M.G.; Bataev, A.A. Formation of Ti-Al intermetallics on a surface of titanium by non-vacuum electron beam treatment. Mater. Charact. 2017, 134, 202–212. [Google Scholar] [CrossRef]
- Qiao, H.C.; Zhao, J.B.; Gao, Y. Experimental investigation of laser peening on TiAl alloy microstructure and properties. Chin. J. Aeronaut. 2015, 28, 609–616. [Google Scholar] [CrossRef] [Green Version]
- Li, J.F.; He, X.J.; Zhang, G.N.; Hang, R.Q.; Huang, X.B.; Tang, B.; Zhang, X.Y. Electrochemical corrosion, wear and cell behavior of ZrO2/TiO2 alloyed layer on Ti-6Al-4V. Bioelectrochemistry 2018, 121, 105–114. [Google Scholar] [CrossRef]
- Zhang, K.M.; Zou, J.X.; Li, J.; Yu, Z.S.; Wang, H.P. Synthesis of Y2O3 particle enhanced Ni/TiC composite on TC4 Ti alloy by laser cladding. Trans. Nonferrous Met. Soc. China 2012, 22, 1817–1823. [Google Scholar] [CrossRef]
- Chen, W.; Wang, K.; Liu, X.; He, N.; Xin, H.; Hao, W.H. Investigation of the friction and wear characteristics of Si3N4-hBN ceramic composites under marine atmospheric environment. Int. J. Refract. Met. Hard Mater. 2019, 81, 345–357. [Google Scholar] [CrossRef]
- Zhao, L.; Cui, C.X.; Liu, S.J.; Zhao, L.C.; Li, N. Microstructure and mechanical properties of TC4 alloy modified and reinforced by TiB + TiN/Ti inoculants ribbons. Mater. Sci. Eng. A. 2016, 663, 8–16. [Google Scholar] [CrossRef]
- Dey, D.; Bal, K.S.; Singh, A.K.; Choudhury, A.R. Hardness and wear behavior of multiple component coating on Ti-6Al-4V substrate by laser application. Optik 2020, 202, 163225. [Google Scholar] [CrossRef]
- Cai, X.J.; Gao, Y.; Cai, F.; Zhang, L.; Zhang, S.H. Effects of multi-layer structure on microstructure, wear and erosion performance of the Cr/CrN films on Ti alloy substrate. Appl. Surf. Sci. 2019, 483, 661–669. [Google Scholar] [CrossRef]
- Tsai, K.Y.; Tsai, M.H.; Yeh, J.W. Sluggish diffusion in Co–Cr–Fe–Mn–Ni high-entropy alloys. Acta Mater. 2013, 61, 4887–4897. [Google Scholar] [CrossRef]
- Tang, Q.H.; Huang, Y.; Huang, Y.Y.; Liao, X.Z.; Langdon, T.G.; Dai, P.Q. Hardening of an Al0.3CoCrFeNi high entropy alloy via high-pressure torsion and thermal annealing. Mater. Lett. 2015, 151, 126–129. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.J.; Zhang, Y.; Wang, Y.L.; Chen, G.L. Solid solution alloys of AlCoCrFeNiTix with excellent room-temperature mechanical properties. Appl. Phys. Lett. 2007, 90, 181904. [Google Scholar] [CrossRef]
- Jiang, Y.Q.; Li, J.; Juan, Y.F.; Lu, Z.J.; Jia, W.L. Evolution in microstructure and corrosion behavior of AlCoCrxFeNi high-entropy alloy coatings fabricated by laser cladding. J. Alloys Compd. 2019, 775, 1–14. [Google Scholar] [CrossRef]
- Xiang, C.; Zhang, Z.M.; Fu, H.M.; Han, E.H.; Wang, J.Q.; Zhang, H.F.; Hu, G.D. Microstructure, mechanical properties, and corrosion behavior of MoNbFeCrV, MoNbFeCrTi, and MoNbFeVTi high-entropy alloys. Acta Metall. Sin. (Engl. Lett.) 2019, 32, 1053–1064. [Google Scholar] [CrossRef] [Green Version]
- Yeh, J.W.; Lin, S.J. Breakthrough applications of high-entropy materials. J. Mater. Res. 2018, 33, 3129–3137. [Google Scholar] [CrossRef]
- Du, L.M.; Lan, L.W.; Zhu, S.; Yang, H.J.; Shi, X.H.; Liaw, P.K.; Qiao, J.W. Effects of temperature on the tribological behavior of Al0.25CoCrFeNi high-entropy alloy. J. Mater. Sci. Technol. 2019, 35, 917–925. [Google Scholar] [CrossRef]
- Chuang, M.H.; Tsai, M.H.; Wang, W.R.; Lin, S.J.; Yeh, J.W. Microstructure and wear behavior of AlxCo1.5CrFeNi1.5Tiy high-entropy alloys. Acta Mater. 2011, 59, 6308–6317. [Google Scholar] [CrossRef]
- Chang, Y.J.; Yeh, A.C. The evolution of microstructures and high temperature properties of AlxCo1.5CrFeNi1.5Tiy high entropy alloys. J. Alloys Compd. 2015, 653, 379–385. [Google Scholar] [CrossRef]
- Khan, N.A.; Akhavan, B.; Zhou, H.; Chang, L.; Wang, Y.; Sun, L.; Bilek, M.M.; Liu, Z. High entropy alloy thin films of AlCoCrCu0.5FeNi with controlled microstructure. Appl. Surf. Sci. 2019, 495, 143560. [Google Scholar] [CrossRef]
- Tang, H.; Zhang, H.; Chen, L.; Guo, S. Novel laser rapidly solidified medium-entropy high speed steel coatings with enhanced hot wear resistance. J. Alloys Compd. 2019, 772, 719–727. [Google Scholar] [CrossRef]
- Jiang, H.; Han, K.M.; Li, D.Y.; Cao, Z.Q. Synthesis and characterization of AlCoCrFeNiNbx high-entropy alloy coatings by laser cladding. Crystals 2016, 9, 56. [Google Scholar] [CrossRef] [Green Version]
- Yin, S.; Li, W.; Song, B.; Yan, X.C.; Kuang, M.; Xu, Y.X.; Wen, K.; Lupoi, R. Deposition of FeCoNiCrMn high entropy alloy (HEA) coating via cold spraying. J. Mater. Sci. Technol. 2019, 35, 1003–1007. [Google Scholar] [CrossRef]
- Shkodich, N.F.; Spasova, M.; Farle, M.; Kovalev, D.Y.; Nepapushev, A.A.; Kuskov, K.V.; Vergunova, Y.S.; Scheck, Y.B.; Rogachev, A.S. Structural evolution and magnetic properties of high-entropy CuCrFeTiNi alloys prepared by high-energy ball milling and spark plasma sintering. J. Alloys Compd. 2020, 816, 152611. [Google Scholar] [CrossRef]
- Ni, C.; Shi, Y.; Liu, J.; Huang, G. Characterization of Al0.5FeCu0.7NiCoCr high-entropy alloy coating on aluminum alloy by laser cladding. Opt. Laser Technol. 2018, 105, 257–263. [Google Scholar] [CrossRef]
- Qiu, X.W.; Liu, C.G. Microstructure and properties of Al2CrFeCoCuTiNix high-entropy alloys prepared by laser cladding. J. Alloys Compd. 2013, 553, 216–220. [Google Scholar] [CrossRef]
- Liang, H.; Yao, H.W.; Qiao, D.X.; Nie, S.; Lu, Y.P.; Deng, D.W.; Cao, Z.Q.; Wang, T.M. Microstructures and wear resistance of AlCrFeNi2W0.2Nbx high-entropy alloy coatings prepared by laser cladding. J. Therm. Spray Technol. 2019, 28, 1318–1329. [Google Scholar] [CrossRef]
- Juan, Y.F.; Li, J.; Jiang, Y.Q.; Jia, W.L.; Lu, Z.J. Modified criterions for phase prediction in the multi-component laser-clad coatings and investigations into microstructural evolution/wear resistance of FeCrCoNiAlMox laser-clad coatings. Appl. Surf. Sci. 2019, 465, 700–714. [Google Scholar] [CrossRef]
- Zhang, H.X.; Dai, J.J.; Sun, C.X.; Li, S.Y. Microstructure and wear resistance of TiAlNiSiV high-entropy laser cladding coating on Ti-6Al-4V. J. Mater. Process. Technol. 2020, 282, 116671. [Google Scholar] [CrossRef]
- Jiang, L.; Lu, Y.P.; Dong, Y.; Wang, T.M.; Cao, Z.Q.; Li, T.J. Effects of Nb addition on structural evolution and properties of the CoFeNi2V0.5 high-entropy alloy. Appl. Phys. A. 2015, 119, 291–297. [Google Scholar] [CrossRef]
- Dong, H. Tribological properties of titanium-based alloys. In Surface Engineering of Light Alloys; Woodhead Publishing: Florida, America, 2010; pp. 58–80. [Google Scholar]
- Wu, C.L.; Zhang, S.; Zhang, C.H.; Zhang, H.; Dong, S.Y. Phase evolution and properties in laser surface alloying of FeCoCrAlCuNix high-entropy alloy on copper substrate. Surf. Coat. Technol. 2017, 315, 368–376. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.; Dahmen, K.; Liaw, P.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Takeuchi, A.; Akihisa, I. Classification of bulk metallic glasses by atomic size difference, heat of mixing and period of constituent elements and its application to characterization of the main alloying element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef] [Green Version]
- Cai, Z.B.; Jin, G.; Cui, X.F.; Liu, Z.; Zheng, W.; Li, Y.; Wang, L.Q. Synthesis and microstructure characterization of Ni-Cr-Co-Ti-V-Al high entropy alloy coating on Ti-6Al-4V substrate by laser surface alloying. Mater. Charact. 2016, 120, 229–233. [Google Scholar] [CrossRef]
- Dragan, T.; Jelena, B.C.; Vasil, K. Ab initio studies of the structural, elastic, electronic and thermal properties of NiTi2 intermetallic. J. Phys. Chem. Solids 2015, 85, 197–205. [Google Scholar]
- Chao, Q.; Guo, T.T.; Jarvis, T.; Wu, X.H.; Hodgson, P.; Fabijanic, D. Direct laser deposition cladding of Alx CoCrFeNi high entropy alloys on a high-temperature stainless steel. Surf. Coat. Technol. 2017, 332, 440–451. [Google Scholar] [CrossRef]
- Cai, Y.C.; Chen, Y.; Luo, Z.; Gao, F.; Li, L. Manufacturing of FeCoCrNiCux medium-entropy alloy coating using laser cladding technology. Mater. Des. 2017, 133, 91–108. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, P.; He, Y.Z. Synthesis and characterization of FeCoNiCrCu high-entropy alloy coating by laser cladding. Mater. Des. 2011, 32, 1910–1915. [Google Scholar] [CrossRef]
- Jiang, L.; Wu, W.; Cao, Z.Q.; Deng, D.W.; Li, T.J. Microstructure evolution and wear behavior of the laser cladded CoFeNi2V0.5Nb0.75 and CoFeNi2V0.5Nb high-entropy alloy coatings. J. Therm. Spray Technol. 2016, 25, 806–814. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, C.L.; Zhang, C.H.; Guan, M.; Tan, J.Z. Laser surface alloying of FeCoCrAlNi high-entropy alloy on 304 stainless steel to enhance corrosion and cavitation erosion resistance. Opt. Laser Technol. 2016, 84, 23–31. [Google Scholar] [CrossRef]
- Cai, Z.B.; Cui, X.F.; Liu, Z.; Li, Y.; Dong, M.L.; Jin, G. Microstructure and wear resistance of laser cladded Ni-Cr-Co-Ti-V high-entropy alloy coating after laser remelting processing. Opt. Laser Technol. 2018, 99, 276–281. [Google Scholar] [CrossRef]
- Xiang, K.; Chen, L.Y.; Chai, L.J.; Guo, N.; Wang, H. Microstructural characteristics and properties of CoCrFeNiNbx high-entropy alloy coatings on pure titanium substrate by pulsed laser cladding. Appl. Surf. Sci. 2020, 517, 146216. [Google Scholar] [CrossRef]
- Shang, C.Y.; Axinte, E.; Sun, J.; Li, X.T.; Li, P.; Du, J.W.; Qiao, P.C.; Wang, Y. CoCrFeNi(W1−xMox) high-entropy alloy coatings with excellent mechanical properties and corrosion resistance prepared by mechanical alloying and hot pressing sintering. Mater. Des. 2017, 117, 193–202. [Google Scholar] [CrossRef]
- Liang, J.; Yin, X.Y.; Lin, Z.Y.; Chen, S.Y.; Liu, C.S.; Wang, C. Microstructure and wear behaviors of laser cladding in-situ synthetic (TiBx + TiC)/(Ti2Ni + TiNi) gradient composite coatings. Vacuum. 2020, 176, 109305. [Google Scholar] [CrossRef]
- Xu, X.P.; Yu, Y.P.; Huang, H. Mechanisms of abrasive wear in the grinding of titanium (TC4) and nickel (K417) alloys. Wear 2003, 255, 1421–1426. [Google Scholar] [CrossRef]
- Wu, W.; Jiang, L.; Jiang, H.; Pan, X.; Cao, Z.Q.; Deng, D.W.; Wang, T.M.; Li, T.J. Phase evolution and properties of Al2CrFeNiMox high-entropy alloys coatings by laser cladding. J. Therm. Spray Technol. 2015, 24, 1333–1340. [Google Scholar] [CrossRef]
- Miao, J.W.; Guo, T.M.; Ren, J.F.; Zhang, A.J.; Su, B.; Meng, J.H. Optimization of mechanical and tribological properties of FCC CrCoNi multi-principal element alloy with Mo addition. Vacuum 2018, 149, 324–330. [Google Scholar] [CrossRef]




| Element | Al | V | Fe | O | Si | C | N | H | else | Ti |
|---|---|---|---|---|---|---|---|---|---|---|
| Content | 5.5–6.8 | 3.5–4.5 | 0.3 | 0.2 | 0.15 | 0.1 | 0.05 | 0.01 | 0.5 | Balance |
| △H | Co | Cr | Fe | Ni | V | Ti |
|---|---|---|---|---|---|---|
| Co | −4 | −1 | 0 | −14 | −28 | |
| Cr | −1 | −7 | −2 | −7 | ||
| Fe | −2 | −7 | −17 | |||
| Ni | −18 | −35 | ||||
| V | −2 | |||||
| Ti |
| Sample | Region | Co | Cr | Fe | Ni | V | Ti |
|---|---|---|---|---|---|---|---|
| Ti0 | Nominal | 18.18 | 18.18 | 18.18 | 36.36 | 9.1 | 0 |
| A | 12.44 | 29.16 | 18.81 | 17.33 | 12.39 | 9.87 | |
| B | 14.27 | 13.86 | 15.06 | 29.91 | 8.41 | 18.49 | |
| Ti0.5 | Nominal | 16.67 | 16.67 | 16.67 | 33.33 | 8.33 | 8.33 |
| A | 10.51 | 14.72 | 16.89 | 24.99 | 7.34 | 25.55 | |
| B | 14.56 | 8.37 | 11.79 | 26.20 | 6.17 | 32.91 | |
| Ti0.75 | Nominal | 16.00 | 16.00 | 16.00 | 32.00 | 8.00 | 12.00 |
| A | 10.19 | 16.03 | 18.14 | 20.92 | 7.21 | 27.51 | |
| B | 14.43 | 8.25 | 11.18 | 24.99 | 6.06 | 35.09 | |
| Ti1.0 | Nominal | 15.38 | 15.38 | 15.38 | 30.76 | 7.69 | 15.41 |
| A | 8.75 | 19.33 | 16.61 | 17.89 | 8.50 | 29.22 | |
| B | 13.44 | 7.11 | 10.96 | 23.67 | 5.27 | 39.54 | |
| Ti1.25 | Nominal | 14.81 | 14.81 | 14.81 | 29.63 | 7.41 | 15.83 |
| A | 7.09 | 20.25 | 14.11 | 16.05 | 9.20 | 33.29 | |
| B | 13.72 | 6.83 | 11.03 | 23.20 | 4.57 | 40.00 |
| Sample | Co | Cr | Fe | Ni | V | Ti | O | Al | |
|---|---|---|---|---|---|---|---|---|---|
| TC4 | A | 2.52 | 53.54 | 37.68 | 6.26 | ||||
| B | 2.85 | 65.13 | 23.13 | 8.88 | |||||
| Ti0 | C | 6.89 | 7.44 | 7.50 | 14.03 | 4.41 | 16.88 | 42.85 | |
| D | 11.75 | 8.83 | 13.40 | 27.60 | 6.61 | 26.40 | 5.41 | ||
| Ti0.5 | E | 4.12 | 4.90 | 4.78 | 9.37 | 3.00 | 3.53 | 60.33 | |
| F | 10.24 | 7.88 | 10.00 | 19.83 | 5.56 | 29.12 | 17.36 | ||
| Ti0.75 | G | 8.39 | 8.97 | 8.90 | 18.08 | 4.97 | 12.12 | 38.57 | |
| H | 10.53 | 13.75 | 12.85 | 25.47 | 7.34 | 19.73 | 10.32 | ||
| Ti1.0 | I | 6.74 | 7.37 | 7.72 | 14.65 | 4.38 | 16.65 | 42.49 | |
| J | 10.64 | 11.25 | 12.68 | 21.45 | 6.02 | 21.41 | 16.54 | ||
| Ti1.25 | K | 3.07 | 3.51 | 3.39 | 6.55 | 2.19 | 11.82 | 69.47 | |
| L | 11.71 | 9.42 | 11.71 | 2130 | 5.71 | 33.26 | 6.88 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Liang, H.; Nie, Q.; Qi, Z.; Deng, D.; Jiang, H.; Cao, Z. Microstructures and Wear Resistance of CoCrFeNi2V0.5Tix High-Entropy Alloy Coatings Prepared by Laser Cladding. Crystals 2020, 10, 352. https://doi.org/10.3390/cryst10050352
Li Y, Liang H, Nie Q, Qi Z, Deng D, Jiang H, Cao Z. Microstructures and Wear Resistance of CoCrFeNi2V0.5Tix High-Entropy Alloy Coatings Prepared by Laser Cladding. Crystals. 2020; 10(5):352. https://doi.org/10.3390/cryst10050352
Chicago/Turabian StyleLi, Yaning, Hui Liang, Qiuxin Nie, Zhaoxin Qi, Dewei Deng, Hui Jiang, and Zhiqiang Cao. 2020. "Microstructures and Wear Resistance of CoCrFeNi2V0.5Tix High-Entropy Alloy Coatings Prepared by Laser Cladding" Crystals 10, no. 5: 352. https://doi.org/10.3390/cryst10050352





