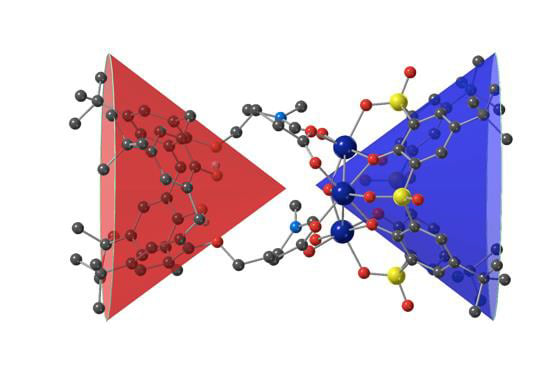
Formation of Unsymmetrical Trinuclear Metallamacrocycles Based on Two Different Cone Calix[4]arene Macrocyclic Rings
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Co3(H2O)(DMF)2-(2-2H)-3
2.3. Synthesis of Ni3(H2O)(DMF)2-(2-2H)-3
2.4. Synthesis of Zn3(H2O)(DMF)2-(2-2H)-3
2.5. Physical Measurements
2.6. Single Crystal X-Ray Diffraction Studies
2.7. X-Ray Diffraction on Powder
3. Results and Discussion
3.1. Synthesis of the Trinuclear Complexes
3.2. Description of the Strutcure of the Trinuclear Complexes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, H.-B. Monograph in Supramolecular Chemistry: Metallomacrocycles: From Structures to Applications; The Royal Society of Chemistry: Cambridge, UK, 2017. [Google Scholar]
- Saalfrank, R.W.; Stark, A.; Peters, K.; von Schnering, H.-G. The First “Adamantoid” Alkaline Earth Metal Chelate Complex: Synthesis, Structure, and Reactivity. Angew. Chem. Int. Ed. 1988, 27, 851–853. [Google Scholar] [CrossRef]
- Guerriero, P.; Tamburini, S.; Vigato, P.A. From mononuclear to polynuclear macrocyclic or macroacyclic complexes. Coord. Chem. Rev. 1995, 139, 17–243. [Google Scholar] [CrossRef]
- Leininger, S.; Olenyuk, B.; Stang, P.J. Self-Assembly of Discrete Cyclic Nanostructures Mediated by Transition Metals. Chem. Rev. 2000, 100, 853–907. [Google Scholar] [CrossRef]
- Fujita, M. Self-Assembly of [2]Catenanes Containing Metals in Their Backbones. Acc. Chem. Res. 1999, 32, 53–61. [Google Scholar] [CrossRef]
- Rojo, J.; Romero-Salguero, F.J.; Lehn, J.-M.; Baum, G.; Fenske, D. Self-Assembly, Structure, and Physical Properties of Tetranuclear ZnII and CoII Complexes of [2 × 2] Grid-Type. Eur. J. Inorg. Chem. 1999, 1999, 1421–1428. [Google Scholar] [CrossRef]
- Song, J.; Moon, D.; Lah, M.S. Manganese Metallamacrocycles with Various Coordination Solvents. Bull. Korean Chem. Soc. 2002, 23, 708–714. [Google Scholar]
- Lee, S.J.; Lin, W.B. Chiral Metallocycles: Rational Synthesis and Novel Applications. Acc. Chem. Res. 2008, 41, 521–537. [Google Scholar] [CrossRef]
- El-Sayed, M.; Yuan, D. Metal-Organic Cages (MOCs): From Discrete to Cage-based Extended Architectures. Chem. Lett. 2020, 49, 28–53. [Google Scholar] [CrossRef]
- Deiters, E.; Bulach, V.; Hosseini, M.W. Porphyrin based metallamacrocycles. New J. Chem. 2006, 30, 1289–1294. [Google Scholar] [CrossRef]
- Durot, S.; Flamigni, L.; Taesch, J.; Dang, T.T.; Heitz, V.; Ventura, B. Synthesis and Solution Studies of Silver(I)-Assembled Porphyrin Coordination Cages. Chem. Eur. J. 2014, 20, 9979–9990. [Google Scholar] [CrossRef]
- Gutsche, C.D. Calixarenes Revised: Monographs in Supramolecular Chemistry; The Royal Society of Chemistry: Cambridge, UK, 1998; Volume 6. [Google Scholar]
- Ikeda, A.; Shinkai, S. Novel Cavity Design Using Calix[n]arene Skeletons: Toward Molecular Recognition and Metal Binding. Chem. Rev. 1997, 97, 1713–1734. [Google Scholar] [CrossRef]
- Kajiwara, T.; Iki, N.; Yamashita, M. Transition metal and lanthanide cluster complexes constructed with thiacalix[n]arene and its derivatives. Coord. Chem. Rev. 2007, 251, 1734–1746. [Google Scholar] [CrossRef]
- Ovsyannikov, A.; Solovieva, S.; Antipin, I.; Ferlay, S. Coordination Polymers based on calixarene derivatives: Structures and properties. Coord. Chem. Rev. 2017, 352, 151–186. [Google Scholar] [CrossRef]
- Taylor, S.M.; Karotsis, G.; McIntosh, R.D.; Kennedy, S.; Teat, S.J.; Beavers, C.M.; Wernsdorfer, W.; Piligkos, S.; Dalgarno, S.J.; Brechin, E.K. A Family of Calix[4]arene-Supported [MnIII2MnII2] Clusters. Chem. Eur. J. 2011, 17, 7521–7530. [Google Scholar] [CrossRef]
- Kajiwara, T.; Kobashi, T.; Shinagawa, R.; Ito, T.; Takaishi, S.; Yamashita, M.; Iki, N. Highly Symmetrical Tetranuclear Cluster Complexes Supported by p-tert-Butylsulfonylcalix[4]arene as a Cluster-Forming Ligand. Eur. J. Inorg. Chem. 2006, 2006, 1765–1770. [Google Scholar] [CrossRef]
- Iki, N.; Kumagai, H.; Morohashi, N.; Ejima, K.; Hasegawa, M.; Miyanari, S.; Miyano, S. Selective oxidation of thiacalix[4]arenes to the sulfinyl- and sulfonylcalix[4]arenes and their coordination ability to metal ions. Tetrahedron Lett. 1998, 39, 7559–7562. [Google Scholar] [CrossRef]
- Mislin, G.; Graf, E.; Hosseini, M.W.; De Cian, A. Sulfone-calixarenes: A new class of molecular building block. J. Chem. Soc. Chem. Commun. 1998, 1345–1346. [Google Scholar] [CrossRef]
- Bi, Y.; Du, S.; Liao, W. Thiacalixarene-based nanoscale polyhedral coordination cages. Coord. Chem. Rev. 2014, 276, 61–72. [Google Scholar] [CrossRef]
- Lamouchi, M.; Jeanneau, E.; Novitchi, G.; Luneau, G.; Brioude, A.; Desroches, C. Polynuclear Complex Family of Cobalt(II)/Sulfonylcalixarene: One- Pot Synthesis of Cluster Salt [Co14II]+[Co4II]− and Field-Induced Slow Magnetic Relaxation in a Six-Coordinate Dinuclear Cobalt(II)/Sulfonylcalixarene Complex. Inorg. Chem. 2014, 53, 63–72. [Google Scholar] [CrossRef]
- Du, S.; Hu, C.; Xiao, J.-C.; Tana, H.; Liao, W. A giant coordination cage based on sulfonylcalix[4]arenes. Chem. Commun. 2012, 48, 9177–9179. [Google Scholar] [CrossRef]
- Xiong, K.; Jiang, F.; Gai, Y.; He, Z.; Yuan, D.; Chen, L.; Su, K.; Hong, M. Self-Assembly of Thiacalix[4]arene-Supported Nickel(II)/Cobalt(II) Complexes Sustained by in Situ Generated 5-Methyltetrazolate Ligand. Cryst. Growth. Des. 2012, 12, 3335–3341. [Google Scholar] [CrossRef]
- Geng, D.; Han, X.; Bi, Y.; Qin, Y.; Li, Q.; Huang, L.; Zhou, K.; Song, L.; Zheng, Z. Merohedral icosahedral M48 (M ¼ CoII, NiII) cage clusters supported by thiacalix[4]arene. Chem. Sci. 2018, 9, 8535–8541. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Chen, M.; Han, X.; Bi, Y.; Huang, L.; Zhou, K.; Zheng, Z. Thiacalix[4]arene-supported tetradecanuclear cobalt nanocage cluster as precursor to synthesize CoO/Co9S8@CN composite for supercapacitor Application. Inorg. Chem. Front. 2018, 5, 1329–1335. [Google Scholar] [CrossRef]
- Bi, Y.; Xu, G.; Liao, W.; Du, S.; Wang, X.; Deng, R.; Zhang, H.; Gao, S. Making a [Co24] metallamacrocycle from the shuttlecock-like tetranuclear cobalt-calixarene building blocks. Chem. Commun. 2010, 46, 6362–6364. [Google Scholar] [CrossRef]
- Klein, C.; Graf, E.; Hosseini, M.W.; De Cian, A.; Kyritsakas, N. Design and Structural Analysis of Metallamacrocycles Based on Zinc Halides and a V-Shaped Bismonodentate Ligand of the Cyclophane Type. Eur. J. Inorg. Chem. 2003, 7, 1299–1302. [Google Scholar] [CrossRef]
- Ehrhart, J.; Planeix, J.-M.; Kyritsakas-Gruber, N.; Hosseini, M.W. Synthesis and structural studies of metallamacrotricycles based on a metacyclophane in 1,3-alternate conformation bearing four imidazolyl units. Dalton Trans. 2009, 14, 2552–2557. [Google Scholar] [CrossRef]
- Ehrhart, J.; Planeix, J.-M.; Kyritsakas-Gruber, N.; Hosseini, M.W. Molecular tectonics: Formation and structural studies on a 2-D directional coordination network based on a non-centric metacyclophane based tecton and zinc cation. Dalton Trans. 2010, 39, 2137–2146. [Google Scholar] [CrossRef]
- Chernova, E.F.; Ovsyannikov, A.S.; Ferlay, S.; Solovieva, S.E.; Antipin, I.S.; Konovalov, A.I.; Kyritsakas, N.; Hosseini, M.W. Molecular tectonics: From a binuclear metallamacrocycle to a 1D isostructural coordination network based on tetracyanomethyl[1.1.1.1]metacyclophane and a silver cation. Mendeleev Commun. 2017, 27, 260–262. [Google Scholar] [CrossRef]
- Lesińska, U.; Bocheńska, M. Lower-Rim-Substituted tert-Butylcalix[4]arenes; Part IX: One-Pot Synthesis of Calix[4]arene-Hydroxamates and Calix[4]arene-Amides. Synthesis 2006, 16, 2671–2676. [Google Scholar]
- Hallale, O.; Bourne, S.A.; Koch, K.R. Metallamacrocyclic complexes of Ni(ii) with 3,3,3′,3′-tetraalkyl-1,1′-aroylbis(thioureas): Crystal and molecular structures of a 2:2 metallamacrocycle and a pyridine adduct of the analogous 3:3 complex. CrystEngComm 2005, 7, 161–166. [Google Scholar] [CrossRef]
- Ferrer, M.; Gutierrez, A.; Mounir, M.; Rossell, O.; Ruiz, E.; Rang, A.; Engeser, M. Self-Assembly Reactions between the Cis-Protected Metal Corners (N−N)MII (N−N = Ethylenediamine, 4,4‘-Substituted 2,2‘-Bipyridine; M = Pd, Pt) and the Fluorinated Edge 1,4-Bis(4-pyridyl)tetrafluorobenzene. Inorg. Chem. 2007, 46, 3395–3406. [Google Scholar] [CrossRef] [PubMed]
- Burkill, H.A.; Robertson, N.; Vilar, R.; White, A.J.P.; Williams, D.J. Synthesis, Structural Characterization, and Magnetic Studies of Polynuclear Iron Complexes with a New Disubstituted Pyridine Ligand. Inorg. Chem. 2005, 44, 3337–3346. [Google Scholar] [CrossRef] [PubMed]
- Granzhan, A.; Schouwey, C.; Riis-Johannessen, T.; Scopelliti, R.; Severin, K. Connection of Metallamacrocycles via Dynamic Covalent Chemistry: A Versatile Method for the Synthesis of Molecular Cages. J. Am. Chem. Soc. 2011, 133, 7106–7115. [Google Scholar] [CrossRef] [PubMed]
- Shan, N.; Vickers, S.J.; Adams, H.; Ward, M.D.; Thomas, J.A. Switchable electron-transfer processes in a mixed-valence, kinetically locked, trinuclear Ru(II) metallamacrocycle. Angew. Chem. Int. Ed. 2004, 43, 3938–3941. [Google Scholar] [CrossRef] [PubMed]
- Rouge, P.; Silva Pires, V.; Gaboriau, F.; Dassonville-Klimpt, A.; Guillon, J.; Da Nascimento, S.; Leger, J.-M.; Lescoat, G.; Sonnet, P. Antiproliferative effect on HepaRG cell cultures of new calix[4]arenes. J. Enz. Inhib. Med. Chem. 2010, 25, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Taylor, S.; Accorsi, G.; Armaroli, N.; Reed, C.A.; Boyd, P.D.W. Calix[4]arene-Linked Bisporphyrin Hosts for Fullerenes: Binding Strength, Solvation Effects, and Porphyrin-Fullerene Charge Transfer Bands. J. Am. Chem. Soc. 2006, 128, 15903–15913. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H.J. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT: Integrating space group determination and structure solution. Acta Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. 2007, 64, 112–122. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; Van De Streek, J. Visualization and analysis of crystal structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Guzei, I.A. An idealized molecular geometry library for refinement of poorly behaved molecular fragments with constraints. J. Appl. Crystallogr. 2014, 47, 806–809. [Google Scholar] [CrossRef]
- Available online: https://www.ccdc.cam.ac.uk/structures/ (accessed on 27 March 2020).
- Wang, S.; Hang, X.; Zhu, X.; Han, H.; Zhang, G.; Liao, W. 1D morning glory-like calixarene-based coordination polymers as a support for Au/Ag nanoparticles. Polyhedron 2017, 130, 75–80. [Google Scholar] [CrossRef]
- Katsenis, A.D.; Kessler, V.G.; Papaefstathiou, G.S. High-spin Ni(II) clusters: Triangles and planar tetranuclear complexes. Dalton Trans. 2011, 40, 4590–4598. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, S.; van Leusen, J.; Izarova, N.V.; Bourone, S.D.M.; Ellern, A.; Kögerler, P.; Monakhov, K.Y. Triangular {Ni3} coordination cluster with a ferromagnetically coupled metal-ligand core. Polyhedron 2018, 144, 144–151. [Google Scholar] [CrossRef]






| Co3(H2O)(DMF)2-(2-2H)-3 | Ni3(H2O)(DMF)2-(2-2H)-3 | Zn3(H2O)(DMF)2-(2-2H)-3 | |
|---|---|---|---|
| Formula | C98H125Co3N2O23S4, 6.5(C3H7NO) | C98H126N2Ni3O23S4, 6.5(C3H7NO) | C98H126N2O23S4Zn3, 6.5(C3H7NO) 0.5(H2O) |
| Molecular weight | 2479.14 | 2479.49 | 2508.48 |
| Crystal System | Triclinic | Triclinic | Triclinic |
| Space Group | P | P | P |
| a (Å) | 13.0582(2) | 13.1486(3) | 13.12500(10) |
| b (Å) | 22.3247(3) | 22.2435(5) | 22.4394(2) |
| c (Å) | 22.6890(3) | 22.6199(3) | 22.5845(2) |
| α (˚) | 76.5080(10) | 76.6746(14) | 76.5670(10) |
| β (˚) | 79.8350(10) | 79.6798(13) | 79.7540(10) |
| γ (˚) | 78.3650(10) | 77.9782(17) | 78.0450(10) |
| V (Å3) | 6241.17(16) | 6236.3(2) | 6270.11(10) |
| Z | 2 | 2 | 2 |
| Color | Pink | Pale green | Colorless |
| Crystal dim (mm3) | 0.258 × 0.186 × 0.084 | 0.426 × 0.393 × 0.117 | 0.426 × 0.393 × 0.117 |
| D (g/cm3) | 1.319 | 1.320 | 1.329 |
| F(000) | 2632 | 2640 | 2662 |
| µ (mm−1) | 4.332 | 1.773 | 1.914 |
| Wavelength (Å) | 1.54184 | 1.54184 | 1.54184 |
| Number of data meas. | 198262 | 75586 | 213361 |
| Number of data with I > 2σ(I) | 25040 | 5524 [R(int) = 0.0347] | 8993 [R(int) = 0.0297] |
| R | 0.0867 | 0.0714 | 0.0731 |
| Rw | 0.0950 | 0.0864 | 0.0793 |
| GOF | 1.086 | 1.022 | 1.016 |
| Largest peak in final difference (eÅ−3) | −1.335 and 1.833 | −0.940 and 1.533 | −1.077 and 0.107 |
| Co3(H2O)(DMF)2-(2-2H)-3 | Ni3(H2O)(DMF)2-(2-2H)-3 | Zn3(H2O)(DMF)2-(2-2H)-3 | |
|---|---|---|---|
| M-O | 2.0097 (30) 2.0236 (36) 2.0623 (34) 2.0755 (30) 2.0775 (29) 2.0844 (29) 2.0924 (32) 2.0999 (27) 2.1077 (30) 2.1081 (31) 2.1159 (29) 2.1446 (29) 2.1832 (29) | 1.9917 (546) 1.9956 (272) 1.9994 (29) 2.0227 (28) 2.0129(515) 2.0341 (30) 2.0395 (240) 2.0479 (31) 2.0534 (29) 2.0689 (30) 2.0729 (29) 2.0759 (29) 2.0881 (27) 2.1039 (29) 2.1280 (31) | 1.9538 (313) 2.0064 (29) 2.0358 (271) 2.0376 (258) 2.0543 (28) 2.0712 (276) 2.0891 (29) 2.1058 (30) 2.1065 (32) 2.1090 (28) 2.1411 (29) 2.1458 (31) 2.1733 (32) 2.1733 (26) 2.2480 (30) |
| M-Oμ3water | 2.0172 (28) 2.0332 (28) 2.1201 (28) | 2.0127 (28) 2.0237 (26) 2.0469 (28) | 2.0169(28) 2.0324 (30) 2.0712 (27) |
| M-ODMF | 2.0335 (57) 2.0597 (31) | 1.9964 (106) 2.0204 (154) 2.0223 (31) | 1.9954 (87) 2.0352 (31) 2.0471(153) |
| M-M | 2.9890 (19) 2.9919 (22) 3.7839 (18) | 2.9380 (24) 2.9436 (30) 3.7823 (23) | 2.9886 (18) 2.9914 (21) 3.8214 (16) |
| MMM Angle (°) | 50.720 50.787 78.493 | 49.913 50.044 80.044 | 50.249 50.313 79.439 |
| C-O (carboxylate) | (1.2517 (44) +1.2704 (47)) (1.2578 (64) +1.2602 (56)) | (1.2115 (550) +1.3617 (327)) (1.2413 (289) +1.3037 (540)) (1.2608 (44) + 1.2646 (43)) | (1.2697 (486) +1.2918 (412)) (1.2609 (432) +1.3050 (325)) (1.2622 (47) +1.2646 (45)) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kniazeva, M.V.; Ovsyannikov, A.S.; Islamov, D.R.; Samigullina, A.I.; Gubaidullin, A.T.; Solovieva, S.E.; Antipin, I.S.; Ferlay, S. Formation of Unsymmetrical Trinuclear Metallamacrocycles Based on Two Different Cone Calix[4]arene Macrocyclic Rings. Crystals 2020, 10, 364. https://doi.org/10.3390/cryst10050364
Kniazeva MV, Ovsyannikov AS, Islamov DR, Samigullina AI, Gubaidullin AT, Solovieva SE, Antipin IS, Ferlay S. Formation of Unsymmetrical Trinuclear Metallamacrocycles Based on Two Different Cone Calix[4]arene Macrocyclic Rings. Crystals. 2020; 10(5):364. https://doi.org/10.3390/cryst10050364
Chicago/Turabian StyleKniazeva, Mariia V., Alexander S. Ovsyannikov, Daut R. Islamov, Aida I. Samigullina, Aidar T. Gubaidullin, Svetlana E. Solovieva, Igor S. Antipin, and Sylvie Ferlay. 2020. "Formation of Unsymmetrical Trinuclear Metallamacrocycles Based on Two Different Cone Calix[4]arene Macrocyclic Rings" Crystals 10, no. 5: 364. https://doi.org/10.3390/cryst10050364
APA StyleKniazeva, M. V., Ovsyannikov, A. S., Islamov, D. R., Samigullina, A. I., Gubaidullin, A. T., Solovieva, S. E., Antipin, I. S., & Ferlay, S. (2020). Formation of Unsymmetrical Trinuclear Metallamacrocycles Based on Two Different Cone Calix[4]arene Macrocyclic Rings. Crystals, 10(5), 364. https://doi.org/10.3390/cryst10050364