Non Isothermal Crystallization Kinetics and Isothermal Decomposition of Poly(Ethylene-Co-Vinylalcohol/Poly(D,L-Lactic-Co-Glycolic Acid) Blend
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Blend Preparation
2.3. Analyses
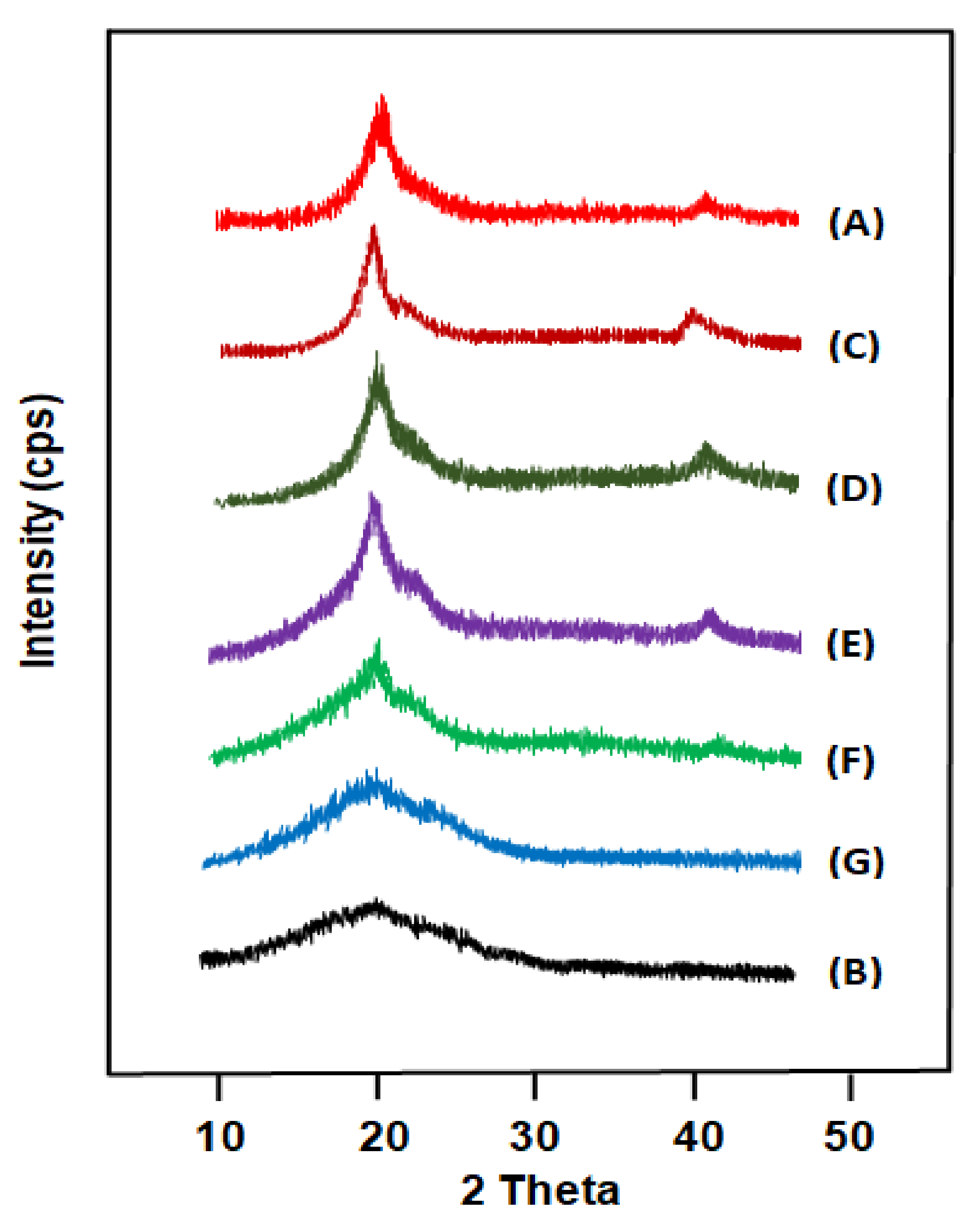
3. Results and Discussion
3.1. Viscosimetry Analysis
3.2. DSC Analysis
3.2.1. Miscibility of PE-VOL/PD,L-LGA Blend
3.2.2. Crystallization Behavior of PE-VOL/PD,L-LGA Blend
3.2.3. Non Isothermal Crystallization Kinetics of PE-VOL and PE-VOL/PD,L-LGA Blend
3.3. TGA Analysis
3.4. DART-ToF-MS Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, S.; McCarthy, S. Further investigations on the hydrolytic degradation of poly(DL-lactide). Biomaterials 1999, 201, 35–44. [Google Scholar] [CrossRef]
- Tsuji, H.; Miyauchi, S. Poly(L-lactide): VI effects of crystallinity on enzymatic hydrolysis of poly(L-lactide) without free amorphous region. Polym. Degrad. Stab. 2001, 71, 415–424. [Google Scholar] [CrossRef]
- Tsuji, H. In vitro hydrolysis of blends from enantiomeric poly(lactide)s. Biomaterials 2003, 24, 537–547. [Google Scholar] [CrossRef]
- Karst, D.; Yang, Y. Molecular modeling study of the resistance of PLA to hydrolysis based on the blending of PLLA and PDLA. Polymer 2006, 47, 4845–4850. [Google Scholar] [CrossRef]
- Mokwena, K.K.; Tang, J. Ethylene vinyl alcohol. Critical. Rev. Food Sci. Nutr. 2012, 52, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Isella, F.; Canellas, E.; Bosetti, O.; Nerin, C. Migration of non intentionally added substances from adhesives by UPLC–Q-TOF/MS and the role of EVOH to avoid migration in multilayer packaging materials. J. Mass Spectrom. 2013, 48, 430–437. [Google Scholar] [CrossRef]
- Tang, Y.; Guo, L.; Huang, L.; Liu, X. Mechanical and structural analysis of poly(vinyl alcohol)/poly(ethylene-co-vinyl alcohol) blend films. Polym. Mater. Sci. Eng. 2015, 31, 57–61. [Google Scholar]
- Guerra, N.; Barbani, L.; Lazzeri, L.; Lelli, L.; Palla, M.; Rizzo, C. The activation of human plasma prekallikrein as a hemocompatibility test for biomaterials. II. Contact activation by EVAL and EVAL– SMA copolymers. J. Biomater. Sci. 1995, 4, 643–652. [Google Scholar]
- Tomita, K.; Kojoh, K.; Suzuki, A. Isolation of thermophiles assimilating poly(ethylene-co-vinyl alcohol). J. Ferment. Bioengin. 1997, 84, 400–402. [Google Scholar] [CrossRef]
- Mejía, G.A.I.; López, O.B.L.; Sierra, L. Biodegradation of poly(vinylalcohol-co-ethylene) with the fungus Phanerochaete chrysosporium. Mater. Res. Innov. 2001, 4, 148–154. [Google Scholar] [CrossRef]
- Lopez, B.L.; Mejia, A.I.; Sierra, L. Biodegradability of poly(vinylalcohol). Polym. Eng. Sci. 1999, 39, 1346–1352. [Google Scholar]
- Mejía, G.A.I.; López, O.B.L.; Mulet, P.A. Biodegradation of poly(vinylalcohol) with enzymatic extracts of phanerochaete chrysosporium. Macromol. Symp. 1999, 148, 131–147. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Mukai, M.; Arihara, K.; Saito, T.; Kumagai, H. Ethylene-vinyl alcohol copolymer dialyzer membrane reduces protein oxidation in hemodialysis patients. Ren. Fail. 2011, 33, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Bonomini, M.; Pavone, B.; Sirolli, V.; Del Buono, F.; Di Cesare, M.; Del Boccio, P.; Amoroso, L.; Di Ilio, C.; Sacchetta, P.; Federici, G.; et al. Proteomics characterization of protein adsorption onto hemodialysis membranes. J. Proteome Res. 2006, 10, 2666–2674. [Google Scholar] [CrossRef]
- Ishida, M. Blood compatibility of ethylene-vinyl alcohol copolymer dialyzers. Cells 2005, 37, 30–34. [Google Scholar]
- Ito, S.; Suzuki, C.; Tsuji, T. Platelet activation through interaction with hemodialysis membranes induces neutrophils to produce reactive oxygen species. J. Biomed. Mater. Res. 2006, 77A, 294–303. [Google Scholar] [CrossRef]
- Sirolli, V.; Ballone, E.; Di Liberato, L.; Di Mascio, R.; Cappelli, P.; Albertazzi, A.; Bonomini, M. Leukocyte adhesion molecules and leukocyte- platelet interactions during hemo-dialysis: Effect of different synthetic membranes. Int. J. Artif. Org. 1999, 22, 536–542. [Google Scholar] [CrossRef]
- Young, T.H.; Lin, C.W.; Cheng, L.P.; Hsieh, C.C. Preparation of EVAL membranes with smooth and particulate morphologies for neuronal culture. Biomaterials 2001, 22, 1771–1777. [Google Scholar] [CrossRef]
- Fukuzaki, H.; Yoshida, M.; Asano, M.; Kumakura, M. Synthesis of copoly(d,l-lactic acid) with relatively low molecular weight and in vivo degradation. Eur. Polym. J. 1989, 25, 1019–1026. [Google Scholar] [CrossRef]
- Gilding, D.K.; Reed, A.M. Biodegradable polymers for use in surgery polyglycolid/poly(lactic acid) homo and copolymers. Polymer 1979, 20, 1459–1464. [Google Scholar] [CrossRef]
- Engineer, C.; Parikh, J.; Raval, A. Review on hydrolytic degradation behavior of biodegradable polymers from controlled drug delivery system. Trends Biomater. Artif. Organs. 2011, 25, 79–85. [Google Scholar]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Merkli, A.; Tabatabay, C.; Gurny, R.; Heller, J. Biodegradable polymers for the controlled release of ocular drugs. Progr. Polym. Sci. 1998, 23, 563–580. [Google Scholar] [CrossRef]
- Athanasiou, K.A.; Niederauer, G.G.; Agrawal, M. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials 1996, 17, 93–102. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Contr. Rel. 2012, 161, 505–522. [Google Scholar] [CrossRef]
- Ladewig, K. Drug delivery in soft tissue engineering. Expert. Opin. Drug Deliv. 2011, 8, 1175–1188. [Google Scholar] [CrossRef]
- Mikos, A.G.; Herring, S.W.; Ochareon, P.; Elisseeff, J.; Lu, H.H.; Kandel, R.; Schoen, F.J.; Toner, M.; Mooney, D.; Atala, A.; et al. Engineering complex tissues. Tissue Eng. 2006, 12, 3307–3339. [Google Scholar] [CrossRef]
- Yilgor, P.; Hasirci, N.; Hasirci, V. Sequential BMP-2/BMP-7 delivery from polyester nanocapsules. J. Biomed. Mater. Res. A 2010, 93, 528–536. [Google Scholar] [CrossRef]
- Tuli, R.; Li, W.J.m.; Tuan, R.S. Current state of cartilage tissue engineering. Arthritis Res. Ther. 2003, 5, 235–238. [Google Scholar] [CrossRef]
- Atala, A.; Robert, P.L. (Eds.) Methods of Tissue Engineering; Academic Press: Cambridge, MA, USA, 2002. [Google Scholar]
- Attawia, M.A.; Herbert, K.M.; Laurencin, C.T. Osteoblast like cell adherence and migration through 3-dimensional porous polymer matrices. Biochem. Biophys. Res. Commun. 1995, 213, 639–644. [Google Scholar] [CrossRef]
- Bendix, D. Chemical synthesis of polylactide and its copolymers for medical applications. Polym. Degrad. Stab. 1998, 59, 129–135. [Google Scholar] [CrossRef]
- Wu, X.S.; Wang, N. Synthesis, characterization, biodegradation, and drug delivery application of biodegradable lactic/glycolic acid polymers. Part II: Biodegradation. J. Biomater. Sci. Polym. Ed. 2001, 12, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Kenawy, E.; Laymana, E.J.M.; Watkinsa, J.R.; Bowlinb, G.L.; Matthewsb, J.A.; Simpsonc, D.G.; Wnek, G.E. Electrospinning of poly(ethylene-co-vinyl alcohol) fibers. Biomaterials 2003, 24, 907–913. [Google Scholar] [CrossRef]
- Kit, K.M.; Schultz, J.M.; Gohil, R.M. Morphology and barrier properties of blends of poly(ethylene terephthalate) and poly(ethylene 2,6 naphthalate) with poly(ethylene-co-vinyl alcohol). Polym. Eng. Sci. 1995, 35, 680–692. [Google Scholar] [CrossRef]
- Matsumura, K.; Hyon, S.H.; Nakajima, N.; Iwata, H.; Watazu, A.; Tsutsumi, S. Surface modification of poly(ethylene-co-vinyl alcohol): Hydroxyaptite immobilization and control of periodontal ligament cells differentiation. Biomaterials 2004, 25, 4817–4824. [Google Scholar] [CrossRef]
- Wang, B.; Chao, X.; Li, Y.; Reid, S.R. Tensile strength of electrospun poly(vinylalcohol-co-ethylene) nanofibre sheets. Key Eng. Mater. 2013, 535–536, 215–218. [Google Scholar] [CrossRef]
- Luo, D.-J.; Shao, H.-J.; Wei, F.-J.; Zhang, K.-Z.; Cui, Z.-Y.; Yu, J.; Qui, S.-H. Morphology and isothermal crystallization kinetics of polypropylene/poly(ethylene-co-vinylalcohol) blends. Inter. Polym. Process. 2019, 34, 195–208. [Google Scholar] [CrossRef]
- Saeed, W.S.; Al-Odayni, A.-B.; Alghamdi, A.A.; Al-Owais, A.A.; Semlali, A.; Aouak, T. Miscibility of poly(ethylene-co-vinylalcohol)/poly(δ-valerolactone) blend and tissue engineering scaffold fabrication using naphtalene as porogen. Polym. Plast. Technol. Engin. 2019, 58, 1–23. [Google Scholar]
- Zhu, B.-D.; Zhang, J.-Y.; Lin, C.-H.; Chen, H.-L.; Wang, J. Nonisothermal crystallization kinetics of ethylene vinyl alcohol copolymer with poly(oxypropylene)diamine intercalated montmorrilonite. J. Macromol. Sci. Part B 2018, 57, 333–347. [Google Scholar] [CrossRef]
- Soria, V.; Gomez, C.M.; Falo, M.; Abad, C.; Campos, A. Relative strength of H-bonding groups on biodegradable polymer-based blends in solution. J. Appl. Polym. Sci. 2006, 100, 900–910. [Google Scholar] [CrossRef]
- Huggins, M.L. The viscosity of dilute solutions of long-chain molecules. IV. Dependence on concentration. J. Am. Chem. Soc. 1942, 64, 2716–2720. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, W.; Fung, Z. Criterion of polymer-polymer miscibility determined by viscometry. Eur. Polym. J. 1992, 28, 1259–1261. [Google Scholar] [CrossRef]
- Zhang, Y.; Shams, T.; Harker, A.H.; Parhizkar, M.; Edirisinghe, M. Effect of copolymer composition on particle morphology and release behavior in vitro using progesterone. Mater. Des. 2018, 159, 57–67. [Google Scholar] [CrossRef]
- Takahashi, M.; Tashiro, K.; Amiya, S. Crystal structure of ethylene vinyl alcohol copolymers. Macromolecules 1999, 32, 5860–5871. [Google Scholar] [CrossRef]
- Cerrada, M.L.; Perez, E.; Perena, J.M.; Benavente, R. Wide angle X-ray diffraction study of the phase behavior of vinyl alcohol-ethylene copolymers. Macromolecules 1998, 31, 2559–2564. [Google Scholar] [CrossRef]
- Weng, M.; Qiu, Z. Crystallization kinetics and morphology of novel miscible crystalline/amorphous polymer blends of biodegradable poly(butylene succinate-co-butylene carbonate) and poly(vinyl phenol). Ind. Eng. Chem. Res. 2013, 52, 10198–10205. [Google Scholar] [CrossRef]
- Chou, H.-J. Poly(trimethylene terephthalate)/amorphous poly(ethylene terephthalate) blends. Polym. Eng. Sci. 2007, 47, 2005–2011. [Google Scholar] [CrossRef]
- Freire, E.; Bianchi, O.; Martins, J.N.; Montteiro, E.E.C.; Forte, M.M.C. Non-isothermal crystallization of PVDF/PMMA blends processed in low and high shear mixers. J. Non Cryst. Solid. 2012, 358, 2674–2681. [Google Scholar] [CrossRef]
- Hoffman, J.D.; Weeks, J. Melting process and the equilibrium melting temperature of polychlorotrifluoroethylene. J. Res. Natl. Bur. Stand. A Phys. Chem. 1962, 66A, 13–28. [Google Scholar] [CrossRef]
- Alvarez, V.A.; Kenny, J.M.; Vázquez, A. Isothermal crystallization of poly(vinyl alcohol–co–ethylene). J. Appl. Polym. Sci. 2003, 89, 1071–1077. [Google Scholar] [CrossRef]
- Nishi, T.; Wang, T.T. Melting point depression and kinetic effects of cooling on crystallization in poly(vinylidene fluoride)-poly(methyl methacrylate) mixtures. Macromolecules 1975, 8, 909–915. [Google Scholar] [CrossRef]
- Young, T.H.; Lai, J.Y.; You, W.M.; Cheng, L.P. Equilibrium phase behavior of the membrane forming water–DMSO–EVAL system. J. Membr. Sci. 1997, 128, 55–65. [Google Scholar] [CrossRef]
- Akiba, I.; Akiyama, S. Phase behavior of poly(ethylene-co-vinyl alcohol)/nylon 6—12 blends. Polym. J. 1994, 26, 873–979. [Google Scholar] [CrossRef][Green Version]
- Kuo, S.W.; Chang, F.C. The study of miscibility and hydrogen bonding in blends of phenolics with poly(ε-caprolactone. Macromol. Chem. Phys. 2001, 202, 3112–3119. [Google Scholar] [CrossRef]
- Aouak, T.; AlArifi, A.S.; Ouladsmane, M. Miscibility and crystallization behavior of poly(ethylene-co-vinylalcohol)/poly(maleic anhydride-alt-ethylene) blend. J. Appl. Polym. Sci. 2012, 125, 2262–2270. [Google Scholar] [CrossRef]
- Guo, Q.; Groeninckx, G. Crystallization kinetics of poly(ε-caprolactone) in miscible thermosetting polymer blends of epoxy resin and poly(ε-caprolactone). Polymer 2001, 42, 8647–8655. [Google Scholar] [CrossRef]
- Ozawa, T. Kinetics of non-isothermal crystallization. Polymer 1971, 12, 150–158. [Google Scholar] [CrossRef]
- Liu, X.H.; Wu, Q.J.; Berglund, L.A.; Qi, Z.N. Investigation on unusaual crystallization behavior in polyamide 6/montmorillonite nanocomposites. Macromol. Mater. Eng. 2002, 287, 515–522. [Google Scholar] [CrossRef]
- Chen, Z.; Yao, C.; Yang, G. Non isothermal crystallization behavior, and morphology of poly(trimethylene terephthalate)/polyethylene glycol copolymers. Polym. Test. 2012, 31, 393–403. [Google Scholar] [CrossRef]
- Run, M.; Song, A.; Wang, Y.; Yao, C. Melting, crystallization behaviors, and nonisothermal crystallization kinetics of PET/PTT/PBT ternary blends. J. Appl. Polym. Sci. 2007, 104, 3459–3468. [Google Scholar] [CrossRef]
- Dhandapani, S.; Nayak, S.K.; Mohanty, S. Non-isothermal crystallization kinetics and activation energy of bio-based poly(trimethylene terephthalate)/poly(butylene adipate-co-terephthalate) polyester blend. Polym. Sci. Ser. A 2015, 57, 628–634. [Google Scholar] [CrossRef]
- Hammami, A.; Spruiell, J.E.; Mohrotra, A.K. Quiescent non isothermal crystallization kinetics of isotactic polypropylenes. Polym. Eng. Sci. 1995, 35, 797–804. [Google Scholar] [CrossRef]
- Jeziorny, A. Parameters characterizing the kinetics of the non-isothermal crystallization of poly(ethylene terephtalate) determined by DSC. Polymer 1978, 19, 1142–1144. [Google Scholar] [CrossRef]
- Ziabicki, A. Theoretical analysis of oriented and nonisothermal crystallization I. Phenomenological considerations, isothermal crystallization accompanied by simultaneous orientation or disorientation. Colloid. Polym. Sci. 1974, 252, 207–221. [Google Scholar] [CrossRef]
- Ziabicki, A. Theoretical analysis of oriented and non-isothermal cyrstallization II. Extension of the Kolmogoroff-Avrami-Evans theory onto processes with variable rates and mechanisms. Colloid Polym. Sci. 1974, 252, 433–447. [Google Scholar] [CrossRef]
- Liu, T.; Mo, Z.; Wang, S.; Zhang, H. Non-isothermal melt and cold crystallization kinetics of poly(aryl ether ether ketone ketone). Polym. Eng. Sci. 1997, 37, 568–575. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change I general theory. J. Chem. Phys. 1939, 7, 1103–1112. [Google Scholar] [CrossRef]
- Alvarez, V.A.; Stephani, P.M.; Vazquez, A. Non-isothermal crystallization of polyvinylalcohol-co-ethylene. J. Therm. Anal. Cal. 2005, 79, 187–193. [Google Scholar] [CrossRef]
- Pratt, C.F.; Hobbs, S.Y. Comparative study of crystallization rates by DSC and depolarization microscopy. Polymer 1976, 17, 12–16. [Google Scholar] [CrossRef]
- Gupta, A.K.; Rana, S.K.; Deopura, B.L. Crystallization kinetics of high-density polyethylene/linear low-density polyethylene blend. J. Appl. Polym. Sci. 1994, 51, 231–239. [Google Scholar] [CrossRef]
- Kissinger, H.E. Variation of peak temperature with heating rate in differential thermal analysis. J. Res. Natl. Bur. Stand. 1956, 57, 217–221. [Google Scholar] [CrossRef]
- Alvarez, V.A.; Ruseckaite, V.A.; Vázquez, A. Kinetic analysis of thermal degradation in poly(ethylene-vinyl alcohol) copolymers. J. Appl. Polym. Sci. 2003, 90, 3157–3163. [Google Scholar] [CrossRef]
- Park, J.S.; Kang, S.K. A study on surface, thermal and mechanical properties of absorbable PLGA plate. Int. J. Control. Automat. 2013, 6, 73–82. [Google Scholar] [CrossRef]
- Holland, B.J.; Hay, J.N. The thermal degradation of poly(vinyl alcohol). Polymer 2001, 42, 6775–6783. [Google Scholar] [CrossRef]
- Sivalingam, G.; Madras, G. Thermal degradation of binary physical mixtures and copolymers of poly(ε-caprolactone), poly( d, l-lactide), poly(glycolide). Polym. Degrad. Stab. 2004, 84, 393–398. [Google Scholar] [CrossRef]
- McNeill, I.C.; Leiper, H.A. Degradation studies of some polyesters and polycarbonates—2. Polylactide: Degradation under isothermal conditions, thermal degradation mechanism and photolysis of the polymer. Polym. Degrad. Stab. 1985, 11, 309–326. [Google Scholar] [CrossRef]
- McNeill, I.C.; Leiper, H.A. Degradation studies of some polyesters and polycarbonates: 3-polyglycolide. Polym. Degrad. Stab. 1985, 12, 373–385. [Google Scholar] [CrossRef]












| System | PE–VOL | PD,L–LGA | DMF | Blend Appearance | ||
|---|---|---|---|---|---|---|
| (g) | (wt%) | (g) | (wt%) | (mL) | ||
| PE-VOL/PD,L-LGA10 | 0.50 | 10 | 4.50 | 90 | 20 | Single phase |
| PE-VOL/PD,L-LGA25 | 1.25 | 25 | 3.75 | 75 | 20 | Single phase |
| PE-VOL/PD,L-LGA50 | 2.50 | 50 | 2.50 | 50 | 20 | Single phase |
| PE-VOL/PD,L-LGA75 | 3.75 | 75 | 1.25 | 25 | 20 | Single phase |
| PE-VOL/PD,L-LGA90 | 4.50 | 90 | 0.50 | 10 | 20 | Single phase |
| System | [η] (dL/g) | k | α |
|---|---|---|---|
| PE-co-VAL | 0.62 | 1.626 | - |
| PE-VOL/PD,L-LGA10 | 0.58 | 8.87 | 3.12 |
| PE-VOL/PD,L-LGA25 | 0.52 | 6.67 | 3.42 |
| PE-VOL/PD,L-LGA50 | 0.40 | 6.04 | 4.80 |
| PE-VOL/PD,L-LGA75 | 0.38 | 5.44 | 4.50 |
| PE-VOL/PD,L-LGA90 | 0.32 | 3.92 | 3.20 |
| PD,L-LGA | 0.27 | 0.548 | - |
| System PE-VOL/PD,L-LGA | Volume Fraction, φ1 | Tg (°C) | Tm (°C) |
|---|---|---|---|
| 0/100 | 1.00 | 37 | - |
| 10/90 | 0.90 | 38 | 156 |
| 25/75 | 0.75 | 39 | 159 |
| 50/50 | 0.50 | 40 | 165 |
| 75/25 | 0.25 | 41 | 170 |
| 90/10 | 0.10 | 51 | 177 |
| 100/0 | 0.0 | 56 | 188 |
| PE-VOL/PD,L-LGA | Tc (°C) | η | |
|---|---|---|---|
| 100:0 | 154 | 184.9 | 0.09 |
| 90:10 | 149 | 181.8 | 0.12 |
| 75:25 | 143 | 175.5 | 0.18 |
| 50:50 | 142 | 171.5 | 0.22 |
| 25:75 | 137 | 168.1 | 0.26 |
| 10:90 | 135 | 166.8 | 0.34 |
| System | β (°C min−1) | ΔHc(J g−1) | X (%) |
|---|---|---|---|
| PE-VOL | 10.0 | 46.98 | 29.77 |
| 12.5 | 47.11 | 30.00 | |
| 15.0 | 56.00 | 35.48 | |
| 17.5 | 54.00 | 34.22 | |
| 20.0 | 57.00 | 36.12 | |
| PE-VOL/PD,L-LGA90 | 10.0 | 35.22 | 24.78 |
| 12.5 | 40.17 | 28.78 | |
| 15.0 | 46.03 | 32.41 | |
| 17.5 | 48.86 | 34.44 | |
| 20.0 | 52.47 | 36.95 | |
| PE-VOL/PD,L-LGA75 | 10.0 | 27.00 | 22.81 |
| 12.5 | 30.32 | 25.62 | |
| 15.0 | 32.04 | 27.07 | |
| 17.5 | 36.57 | 30.90 | |
| 20.0 | 41.21 | 34.82 | |
| PE-VOL/PD,L-LGA50 | 10.0 | 13.36 | 16.93 |
| 12.5 | 17.46 | 22.13 | |
| 15.0 | 21.2 | 24.12 | |
| 17.5 | 25.81 | 32.71 | |
| 20.0 | 28.19 | 35.73 | |
| PE-VOL/PD,L-LGA25 | 10.0 | - | - |
| 12.5 | - | - | |
| 15.0 | 2.18 | 5.52 | |
| 17.5 | 4.32 | 10.95 | |
| 20.0 | 6.12 | 15.51 |
| System | PE-VOL/PD,L-LGA Composition (wt %) | T (°C) | m | kT |
|---|---|---|---|---|
| PE-VOL | 100/0 | 145 | 1.30 | 85.62 |
| 150 | 1.60 | 112.17 | ||
| 152.5 | 1.94 | 172.43 | ||
| 155 | 2.40 | 1299.80 | ||
| PE-VOL/PD,L-LGA90 | 90:10:00 | 130 | 2.70 | 340.36 |
| 135 | 2.40 | 107.77 | ||
| 140 | 2.24 | 30.88 | ||
| 145 | 2.80 | 33.11 | ||
| PE-VOL/PD,L-LGA75 | 75:25:00 | 130 | 1.70 | 235.10 |
| 135 | 1.90 | 190.57 | ||
| 140 | 2.00 | 167.33 | ||
| 145 | 2.32 | 138.38 | ||
| PE-VOL/PD,L-LGA50 | 50:50:00 | 130 | 2.00 | 115.58 |
| 135 | 2.10 | 76.69 | ||
| 140 | 2.00 | 46.99 | ||
| 145 | 2.00 | 18.73 | ||
| PE-VOL/PD,L-LGA25 | 25:75 | 130 | 1.40 | 51.94 |
| 135 | 1.64 | 60.34 | ||
| 140 | 2.00 | 86.49 | ||
| 145 | 2.60 | 170.72 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouladsmane, M.; Saeed, W.S.; Al-Odayni, A.-B.; Ahmed, A.Y.B.H.; Alghamdi, A.A.; Al-Kahtani, A.; Aouak, T. Non Isothermal Crystallization Kinetics and Isothermal Decomposition of Poly(Ethylene-Co-Vinylalcohol/Poly(D,L-Lactic-Co-Glycolic Acid) Blend. Crystals 2020, 10, 425. https://doi.org/10.3390/cryst10060425
Ouladsmane M, Saeed WS, Al-Odayni A-B, Ahmed AYBH, Alghamdi AA, Al-Kahtani A, Aouak T. Non Isothermal Crystallization Kinetics and Isothermal Decomposition of Poly(Ethylene-Co-Vinylalcohol/Poly(D,L-Lactic-Co-Glycolic Acid) Blend. Crystals. 2020; 10(6):425. https://doi.org/10.3390/cryst10060425
Chicago/Turabian StyleOuladsmane, Mohamed, Waseem Sharaf Saeed, Abdel-Basit Al-Odayni, Ahmed Yacine Badjah Hadj Ahmed, Abdulaziz Ali Alghamdi, Abdullah Al-Kahtani, and Taieb Aouak. 2020. "Non Isothermal Crystallization Kinetics and Isothermal Decomposition of Poly(Ethylene-Co-Vinylalcohol/Poly(D,L-Lactic-Co-Glycolic Acid) Blend" Crystals 10, no. 6: 425. https://doi.org/10.3390/cryst10060425
APA StyleOuladsmane, M., Saeed, W. S., Al-Odayni, A.-B., Ahmed, A. Y. B. H., Alghamdi, A. A., Al-Kahtani, A., & Aouak, T. (2020). Non Isothermal Crystallization Kinetics and Isothermal Decomposition of Poly(Ethylene-Co-Vinylalcohol/Poly(D,L-Lactic-Co-Glycolic Acid) Blend. Crystals, 10(6), 425. https://doi.org/10.3390/cryst10060425








