Pump-Probe Time-Resolved Serial Femtosecond Crystallography at X-Ray Free Electron Lasers
Abstract
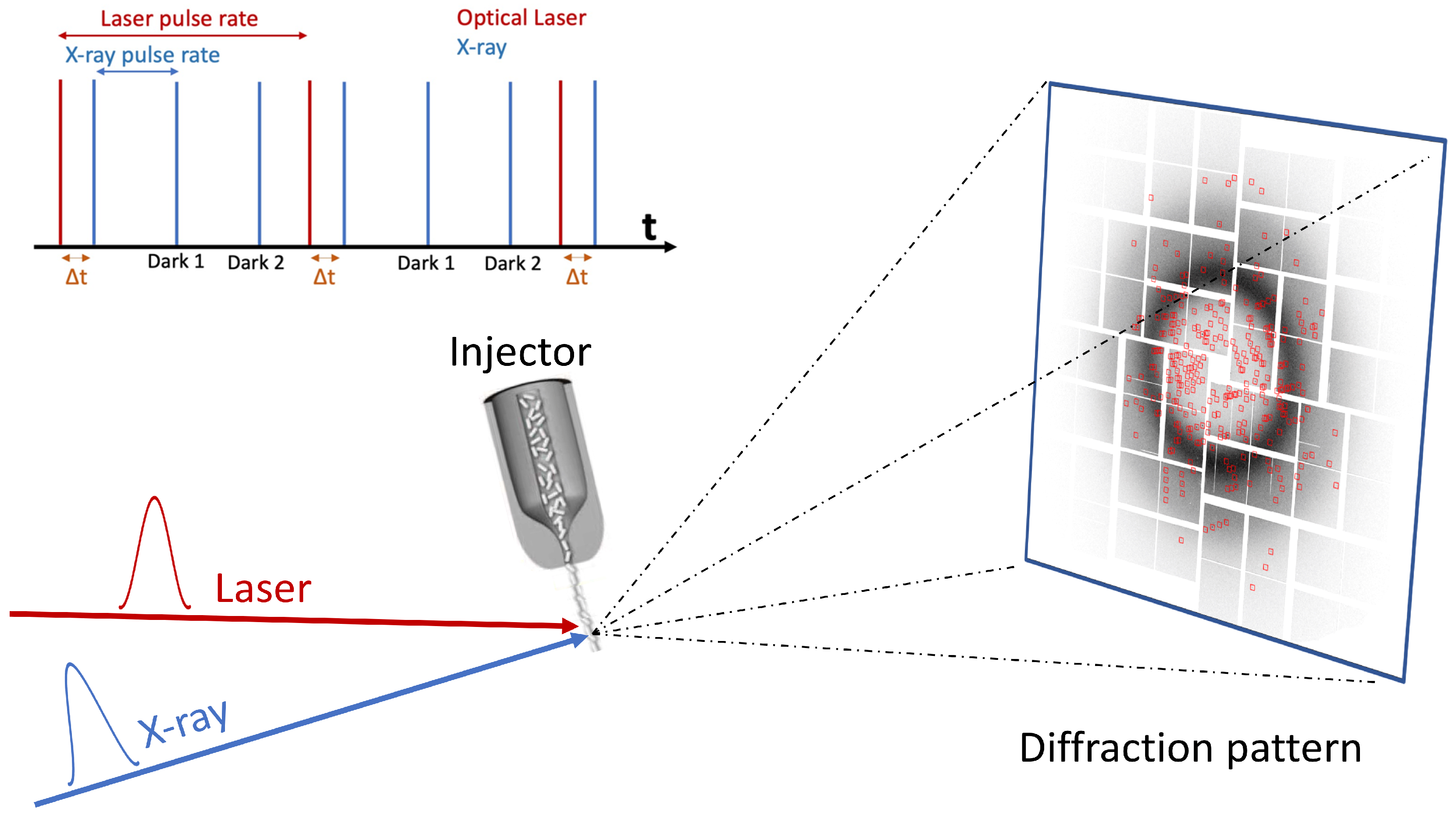
:1. Introduction
2. Time-Resolved Crystallography at Synchrotron Sources
3. Free-Electron Lasers
3.1. Injector Systems
3.2. Detectors
4. Time-Resolved Serial Femtosecond Crystallography (TR-SFX)
5. Designing Pump-Probe TR-SFX Experiments.
6. Data Processing: From Diffraction Patterns to Structure Determination in TRX
6.1. Indexing and Three-Dimensional Merging of Diffraction Patterns
6.2. Difference Maps and Structure Determination
7. TR-SFX on Photoactive Yellow Protein
8. Biological Relevance of Pump-Probe TR-SFX
9. Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moffat, K. Time-resolved biochemical crystallography: A mechanistic perspective. Chem. Rev. 2001, 101, 1569–1582. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M. Time-Resolved Crystallography at X-ray Free Electron Lasers and Synchrotron Light Sources. Synchrotron Radiat. News 2015, 28, 25–30. [Google Scholar] [CrossRef]
- Bourgeois, D.; Weik, M. Kinetic protein crystallography: A tool to watch proteins in action. Crystallogr. Rev. 2009, 15, 87–118. [Google Scholar] [CrossRef]
- Moffat, K.; Henderson, R. Freeze trapping of reaction intermediates. Curr. Opin. Struct. Boil. 1995, 5, 656–663. [Google Scholar] [CrossRef]
- Schmidt, M. Structure Based Kinetics by Time-Resolved X-ray Crystallography. In Ultrashort Laser Pulses in Biology and Medicine; Braun, M., Gilch, P.Z.W., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 201–241. [Google Scholar] [CrossRef]
- Schmidt, M.; Rajagopal, S.; Ren, Z.; Moffat, K. Application of Singular Value Decomposition to the Analysis of Time-Resolved Macromolecular X-ray Data. Biophys. J. 2003, 84, 2112–2129. [Google Scholar] [CrossRef] [Green Version]
- Ihee, H.; Rajagopal, S.; Srajer, V.; Pahl, R.; Anderson, S.; Schmidt, M.; Schotte, F.; Anfinrud, P.A.; Wulff, M.; Moffat, K. Visualizing reaction pathways in photoactive yellow protein from nanoseconds to seconds. Proc. Natl. Acad. Sci. USA 2005, 102, 7145–7150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, M.; Pahl, R.; Srajer, V.; Anderson, S.; Ren, Z.; Ihee, H.; Rajagopal, S.; Moffat, K. Protein kinetics: Structures of intermediates and reaction mechanism from time-resolved x-ray data. Proc. Natl. Acad. Sci. USA 2004, 101, 4799–4804. [Google Scholar] [CrossRef] [Green Version]
- Pande, K.; Hutchison, C.D.M.; Groenhof, G.; Aquila, A.; Robinson, J.S.; Tenboer, J.; Basu, S.; Boutet, S.; DePonte, D.P.; Liang, M.; et al. Femtosecond structural dynamics drives the trans/cis isomerization in photoactive yellow protein. Science 2016, 352, 725–729. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, M. Mix and Inject: Reaction Initiation by Diffusion for Time-Resolved Macromolecular Crystallography. Adv. Condens. Matter Phys. 2013, 2013, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Calvey, G.D.; Katz, A.M.; Schaffer, C.; Pollack, L. Mixing injector enables time-resolved crystallography with high hit rate at X-ray free electron lasers. Struct. Dyn. 2016, 3, 054301. [Google Scholar] [CrossRef] [Green Version]
- Bourgeois, D.; Weik, M. Kinetic protein crystallography using caged compounds. Protein Sci. Encycl. 2008. [Google Scholar] [CrossRef]
- Hekstra, D.; White, K.I.; Socolich, M.A.; Henning, R.W.; Srajer, V.; Ranganathan, R. Electric-field-stimulated protein mechanics. Nature 2016, 540, 400–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kupitz, C.; Olmos, J.L.; Holl, M.; Tremblay, L.; Pande, K.; Pandey, S.; Oberthür, M.; Hunter, M.; Liang, M.; Aquila, A.; et al. Structural enzymology using X-ray free electron lasers. Struct. Dyn. 2016, 4, 044003. [Google Scholar] [CrossRef]
- Olmos, J.L.; Pandey, S.; Martin-Garcia, J.M.; Calvey, G.; Katz, A.; Knoška, J.; Kupitz, C.; Hunter, M.S.; Liang, M.; Oberthuer, D.; et al. Enzyme intermediates captured “on the fly” by mix-and-inject serial crystallography. BMC Boil. 2018, 16, 59. [Google Scholar] [CrossRef] [Green Version]
- Stagno, J.R.; Liu, Y.; Bhandari, Y.R.; Conrad, C.E.; Panja, S.; Swain, M.; Fan, L.; Nelson, G.; Li, C.; Wendel, D.R.; et al. Structures of riboswitch RNA reaction states by mix-and-inject XFEL serial crystallography. Nature 2016, 541, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M. Time-Resolved Macromolecular Crystallography at Pulsed X-ray Sources. Int. J. Mol. Sci. 2019, 20, 1401. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, M. Reaction Initiation in Enzyme Crystals by Diffusion of Substrate. Crystals 2020, 10, 116. [Google Scholar] [CrossRef] [Green Version]
- Ren, Z.; Bourgeois, M.; Helliwell, J.R.; Moffat, K.; Stoddard, B.L.; Srajer, V. Laue crystallography: Coming of age. J. Synchrotron Radiat. 1999, 6, 891–917. [Google Scholar] [CrossRef]
- Srajer, V.; Crosson, S.; Schmidt, M.; Key, J.; Schotte, F.; Anderson, S.; Perman, B.; Ren, Z.; Teng, T.; Bourgeois, D.; et al. Extraction of accurate structure-factor amplitudes from Laue data: Wavelength normalization with wiggler and undulator X-ray sources. J. Synchrotron Radiat. 2000, 7, 236–244. [Google Scholar] [CrossRef] [Green Version]
- Genick, U.K.; Borgstahl, G.E.O.; Ng, K.; Ren, Z.; Pradervand, C.; Burke, P.M. Structure of a protein photocycle intermediate by millisecond time-resolved crystallography. Science 1997, 275, 1471–1475. [Google Scholar] [CrossRef]
- Srajer, V.; Teng, T.-Y.; Ursby, T.; Pradervand, C.; Ren, Z.; Adachi, S.-I.; Schildkamp, W.; Bourgeois, D.; Wulff, M.; Moffat, K. Photolysis of the Carbon Monoxide Complex of Myoglobin: Nanosecond Time-Resolved Crystallography. Science 1996, 274, 1726–1729. [Google Scholar] [CrossRef]
- Schotte, F.; Cho, H.S.; Kaila, V.R.I.; Kamikubo, H.; Dashdorj, N.; Henry, E.R.; Graber, T.J.; Henning, R.; Wulff, M.; Hummer, G.; et al. Watching a signaling protein function in real time via 100-ps time-resolved Laue crystallography. Proc. Natl. Acad. Sci. USA 2012, 109, 19256–19261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, Y.O.; Lee, J.H.; Kim, J.; Schmidt, M.; Moffat, K.; Šrajer, V.; Ihee, H. Volume-conserving trans–cis isomerization pathways in photoactive yellow protein visualized by picosecond X-ray crystallography. Nat. Chem. 2013, 5, 212–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coppens, P.; Vorontsov, I.I.; Graber, T.; Gembicky, M.; Kovalevsky, A. The structure of short-lived excited states of molecular complexes by time-resolved X-ray diffraction. Acta Crystallogr. Sect. A Found. Crystallogr. 2005, 61, 162–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripathi, S.; Šrajer, V.; Purwar, N.; Henning, R.; Schmidt, M. pH Dependence of the Photoactive Yellow Protein Photocycle Investigated by Time-Resolved Crystallography. Biophys. J. 2012, 102, 325–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Šrajer, V.; Ren, Z.; Teng, T.-Y.; Schmidt, M.; Ursby, T.; Bourgeois, M.; Pradervand, C.; Schildkamp, W.; Wulff, M.; Moffat, K. Protein Conformational Relaxation and Ligand Migration in Myoglobin: A Nanosecond to Millisecond Molecular Movie from Time-Resolved Laue X-ray Diffraction. Biochemistry 2001, 40, 13802–13815. [Google Scholar] [CrossRef] [PubMed]
- Srajer, V.; Schmidt, M. Watching proteins function with time-resolved x-ray crystallography. J. Phys. D Appl. Phys. 2017, 50, 373001. [Google Scholar] [CrossRef]
- Haldrup, K.; Harlang, T.C.B.; Christensen, M.; Dohn, A.O.; Van Driel, T.B.; Kjær, K.S.; Harrit, N.; Vibenholt, J.; Guérin, L.; Wulff, M.; et al. Bond Shortening (1.4 Å) in the Singlet and Triplet Excited States of [Ir2(dimen)4]2+in Solution Determined by Time-Resolved X-ray Scattering. Inorg. Chem. 2011, 50, 9329–9336. [Google Scholar] [CrossRef] [PubMed]
- Oang, K.Y.; Kim, K.H.; Jo, J.; Kim, Y.; Kim, J.G.; Kim, J.; Jun, S.; Kim, J.; Ihee, H. Sub-100-ps structural dynamics of horse heart myoglobin probed by time-resolved X-ray solution scattering. Chem. Phys. 2014, 442, 137–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neutze, R.; Wouts, R.; van der Spoel, D.; Weckert, E.; Haidu, J. Potential for biomolecular imaging with femtoscond X-ray pulses. Nature 2000, 406, 752–757. [Google Scholar] [CrossRef] [PubMed]
- Gaffney, K.J.; Chapman, H.N. Imaging Atomic Structure and Dynamics with Ultrafast X-ray Scattering. Science 2007, 316, 1444–1448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DePonte, D.; Weierstall, U.; Schmidt, K.; Warner, J.; Starodub, D.; Spence, J.C.H.; Doak, R.B. Gas dynamic virtual nozzle for generation of microscopic droplet streams. J. Phys. D Appl. Phys. 2008, 41, 195505. [Google Scholar] [CrossRef] [Green Version]
- Nogly, P.; Panneels, V.; Nelson, G.; Gati, C.; Kimura, T.; Milne, C.; Milathianaki, D.; Kubo, M.; Wu, W.; Conrad, C.; et al. Lipidic cubic phase injector is a viable crystal delivery system for time-resolved serial crystallography. Acta Crystallogr. Sect. A Found. Adv. 2016, 72, s41–s42. [Google Scholar] [CrossRef]
- Sugahara, M.; Nakane, T.; Masuda, T.; Suzuki, M.; Inoue, S.; Song, C.; Tanaka, R.; Nakatsu, T.; Mizohata, E.; Yumoto, F.; et al. Hydroxyethyl cellulose matrix applied to serial crystallography. Sci. Rep. 2017, 7, 703. [Google Scholar] [CrossRef]
- Weierstall, U.; James, D.; Wang, C.; White, T.A.; Wang, D.; Liu, W.; Spence, J.C.H.; Doak, R.B.; Nelson, G.; Fromme, P.; et al. Lipidic cubic phase injector facilitates membrane protein serial femtosecond crystallography. Nat. Commun. 2014, 5, 3309. [Google Scholar] [CrossRef]
- Liu, W.; Wacker, D.; Gati, C.; Han, G.W.; James, D.; Wang, D.; Nelson, G.; Weierstall, U.; Katritch, V.; Barty, A.; et al. Serial Femtosecond Crystallography of G Protein-Coupled Receptors. Science 2013, 342, 1521–1524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunter, M.S.; Segelke, B.; Messerschmidt, M.; Williams, G.J.; Zatsepin, N.A.; Barty, A.; Benner, W.H.; Carlson, D.B.; Coleman, M.; Graf, A.; et al. Fixed-target protein serial microcrystallography with an X-ray free electron laser. Sci. Rep. 2014, 4, srep06026. [Google Scholar] [CrossRef] [Green Version]
- Sierra, R.G.; Laksmono, H.; Kern, J.; Tran, R.; Hattne, J.; Alonso-Mori, R.; Lassalle-Kaiser, B.; Glöckner, C.; Hellmich, J.; Schafer, N.W.; et al. Nanoflow electrospinning serial femtosecond crystallography. Acta Crystallogr. Sect. D Boil. Crystallogr. 2012, 68, 1584–1587. [Google Scholar] [CrossRef]
- Liu, P.; Ziemann, P.J.; Kittelson, D.B.; McMurry, P.H. Generating Particle Beams of Controlled Dimensions and Divergence: I. Theory of Particle Motion in Aerodynamic Lenses and Nozzle Expansions. Aerosol Sci. Technol. 1995, 22, 293–313. [Google Scholar] [CrossRef] [Green Version]
- Oberthuer, D.; Knoška, J.; Wiedorn, M.O.; Beyerlein, K.R.; Bushnell, D.A.; Kovaleva, E.G.; Heymann, M.; Gumprecht, L.; Kirian, R.A.; Barty, A.; et al. Double-flow focused liquid injector for efficient serial femtosecond crystallography. Sci. Rep. 2017, 7, 44628. [Google Scholar] [CrossRef]
- Nelson, G.; Kirian, R.A.; Weierstall, U.; Zatsepin, N.; Faragó, T.; Baumbach, T.; Wilde, F.; Niesler, F.B.P.; Zimmer, B.; Ishigami, I.; et al. Three-dimensional-printed gas dynamic virtual nozzles for X-ray laser sample delivery. Opt. Express 2016, 24, 11515–11530. [Google Scholar] [CrossRef] [PubMed]
- Au, A.K.; Huynh, W.; Horowitz, L.F.; Folch, A. 3D-Printed Microfluidics. Angew. Chem. Int. Ed. 2016, 55, 3862–3881. [Google Scholar] [CrossRef] [PubMed]
- Echelmeier, A.; Kim, D.; Villarreal, J.C.; Coe, J.; Quintana, S.; Brehm, G.; Egatz-Gomez, A.; Nazari, R.; Sierra, R.G.; Koglin, J.E.; et al. 3D printed droplet generation devices for serial femtosecond crystallography enabled by surface coating. J. Appl. Crystallogr. 2019, 52, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.U.; Basaran, O.A. A new method for significantly reducing drop radius without reducing nozzle radius in drop-on-demand drop production. Phys. Fluids 2002, 14. [Google Scholar] [CrossRef]
- Hart, P.; Boutet, S.; Carini, G.; Dubrovin, M.; Duda, B.; Fritz, D.; Haller, G.; Herbst, R.; Herrmann, S.; Kenney, C.; et al. The CSPAD megapixel x-ray camera at LCLS. In Proceedings of the Spie Optical Engineering + Applications, San Diego, CA, USA, 12–16 August 2012. [Google Scholar] [CrossRef]
- Allahgholi, A.; Becker, J.; Delfs, A.; DiNapoli, R.; Goettlicher, P.; Greiffenberg, D.; Henrich, B.; Hirsemann, H.; Kuhn, M.; Klanner, R.; et al. The Adaptive Gain Integrating Pixel Detector at the European XFEL. J. Synchrotron Radiat. 2019, 26, 74–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blaj, G.; Caragiulo, P.; Carini, G.; Carron, S.; Dragone, A.; Freytag, D.; Haller, G.; Hart, P.; Hasi, J.; Herbst, R.; et al. X-ray detectors at the Linac Coherent Light Source. J. Synchrotron Radiat. 2015, 22, 577–583. [Google Scholar] [CrossRef]
- Aquila, A.; Hunter, M.S.; Doak, R.B.; Kirian, R.; Fromme, P.; White, T.A.; Andreasson, J.; Arnlund, D.; Bajt, S.; Barends, T.R.M.; et al. Time-resolved protein nanocrystallography using an X-ray free-electron laser. Opt. Express 2012, 20, 2706–2716. [Google Scholar] [CrossRef]
- Tenboer, J.; Basu, S.; Zatsepin, N.; Pande, K.; Milathianaki, D.; Frank, M.; Hunter, M.; Boutet, S.; Williams, G.J.; Koglin, J.E.; et al. Time-resolved serial crystallography captures high-resolution intermediates of photoactive yellow protein. Science 2014, 346, 1242–1246. [Google Scholar] [CrossRef] [Green Version]
- Barends, T.R.; Foucar, L.; Ardevol, A.; Nass, K.; Aquila, A.; Botha, S.; Doak, R.B.; Falahati, K.; Hartmann, E.; Hilpert, M.; et al. Direct observation of ultrafast collective motions in CO myoglobin upon ligand dissociation. Science 2015, 350, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Nango, E.; Royant, A.; Kubo, M.; Nakane, T.; Wickstrand, C.; Kimura, T.; Tanaka, T.; Tono, K.; Song, C.; Tanaka, R.; et al. A three-dimensional movie of structural changes in bacteriorhodopsin. Science 2016, 354, 1552–1557. [Google Scholar] [CrossRef]
- Nogly, P.; Weinert, T.; James, D.; Carbajo, S.; Ozerov, D.; Furrer, A.; Gashi, D.; Borin, V.; Skopintsev, P.; Jaeger, K.; et al. Retinal isomerization in bacteriorhodopsin captured by a femtosecond X-ray laser. Science 2018, 361, eaat0094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kern, J.; Alonso-Mori, R.; Tran, R.; Hattne, J.; Gildea, R.; Echols, N.; Glöckner, C.; Hellmich, J.; Laksmono, H.; Sierra, R.G.; et al. Simultaneous Femtosecond X-ray Spectroscopy and Diffraction of Photosystem II at Room Temperature. Science 2013, 340, 491–495. [Google Scholar] [CrossRef] [Green Version]
- Kupitz, C.; Basu, S.; Grotjohann, I.; Fromme, R.; Zatsepin, N.A.; Rendek, K.N.; Hunter, M.S.; Shoeman, R.L.; White, T.A.; Wang, D.; et al. Serial time-resolved crystallography of photosystem II using a femtosecond X-ray laser. Nature 2014, 513, 261–265. [Google Scholar] [CrossRef]
- Young, I.D.; Ibrahim, M.; Chatterjee, R.; Gul, S.; Fuller, F.D.; Koroidov, S.; Brewster, A.S.; Tran, R.; Alonso-Mori, R.; Kröll, T.; et al. Structure of photosystem II and substrate binding at room temperature. Nature 2016, 540, 453–457. [Google Scholar] [CrossRef] [Green Version]
- Michihiro, S.; Fusamichi, A.; Michihiro, S.; Minoru, K.; Yoshiki, N.; Takanori, N. Light-induced structural changes and the site of O=O bond formation in PSII caught by XFEL. Nature 2017. [Google Scholar] [CrossRef]
- Claesson, E.; Wahlgren, W.Y.; Takala, H.; Pandey, S.; Castillon, L.; Kuznetsova, V.; Henry, L.; Panman, M.R.; Carrillo, M.; Kübel, J.; et al. The primary structural photoresponse of phytochrome proteins captured by a femtosecond X-ray laser. eLife 2020, 9. [Google Scholar] [CrossRef]
- Hutchison, C.D.M.; Kaucikas, M.; Tenboer, J.; Kupitz, C.; Moffat, K.; Schmidt, M.; Van Thor, J.J. Photocycle populations with femtosecond excitation of crystalline photoactive yellow protein. Chem. Phys. Lett. 2016, 654, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Glownia, J.M.; Cryan, J.; Andreasson, J.; Belkacem, A.; Berrah, N.; Blaga, C.I.; Bostedt, C.; Bozek, J.; DiMauro, L.F.; Fang, L.; et al. Time-resolved pump-probe experiments at the LCLS. Opt. Express 2010, 18, 17620–17630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foucar, L.; Barty, A.; Coppola, N.; Hartmann, R.; Holl, P.; Hoppe, U.; Kassemeyer, S.; Kimmel, N.; Küpper, J.; Scholz, M.; et al. CASS—CFEL-ASG software suite. Comput. Phys. Commun. 2012, 183, 2207–2213. [Google Scholar] [CrossRef]
- Yoon, C.H. Psocake: GUI for Making Data Analysis a Piece of Cake. In Handbook on Big Data and Machine Learning in the Physical Sciences; World Scientific: Singapore, 2020; pp. 169–178. [Google Scholar]
- Shin, H.; Kim, S.; Yoon, C.H. Data Analysis using Psocake at PAL-XFEL. J. Korean Phys. Soc. 2018, 73, 16–20. [Google Scholar] [CrossRef]
- Barty, A.; Kirian, R.A.; Maia, F.R.; Hantke, M.; Yoon, C.H.; White, T.A.; Chapman, H.N. Cheetah: Software for high-throughput reduction and analysis of serial femtosecond X-ray diffraction data. J. Appl. Crystallogr. 2014, 47, 1118–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadian-Jazi, M.; Messerschmidt, M.; Darmanin, C.; Giewekemeyer, K.; Mancuso, A.P.; Abbey, B. A peak-finding algorithm based on robust statistical analysis in serial crystallography. J. Appl. Crystallogr. 2017, 50, 1705–1715. [Google Scholar] [CrossRef]
- Hattne, J.; Echols, N.; Tran, R.; Kern, J.; Gildea, R.; Brewster, A.S.; Alonso-Mori, R.; Glöckner, C.; Hellmich, J.; Laksmono, H.; et al. Accurate macromolecular structures using minimal measurements from X-ray free-electron lasers. Nat. Methods 2014, 11, 545–548. [Google Scholar] [CrossRef] [PubMed]
- White, T.A.; Kirian, R.; Martin, A.; Aquila, A.; Nass, K.; Barty, A.; Chapman, H.N. CrystFEL: A software suite for snapshot serial crystallography. J. Appl. Crystallogr. 2012, 45, 335–341. [Google Scholar] [CrossRef] [Green Version]
- White, T.A.; Mariani, V.; Brehm, W.; Yefanov, O.; Barty, A.; Beyerlein, K.R.; Chervinskii, F.; Galli, L.; Gati, C.; Nakane, T.; et al. Recent developments in CrystFEL. J. Appl. Crystallogr. 2016, 49, 680–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, T.A. Processing serial crystallography data with CrystFEL: A step-by-step guide. Acta Crystallogr. Sect. D Struct. Boil. 2019, 75, 219–233. [Google Scholar] [CrossRef] [Green Version]
- Leslie, A.G.W. The integration of macromolecular diffraction data. Acta Crystallogr. Sect. D Boil. Crystallogr. 2005, 62, 48–57. [Google Scholar] [CrossRef]
- Duisenberg, A.J.M. Indexing in single-crystal diffractometry with an obstinate list of reflections. J. Appl. Crystallogr. 1992, 25, 92–96. [Google Scholar] [CrossRef]
- Kabsch, W. Integration, scaling, space-group assignment and post-refinement. Acta Crystallogr. Sect. D Boil. Crystallogr. 2010, 66, 133–144. [Google Scholar] [CrossRef] [Green Version]
- Gevorkov, Y.; Yefanov, O.; Barty, A.; White, T.A.; Mariani, V.; Brehm, W.; Tolstikova, A.; Grigat, R.-R.; Chapman, H.N. XGANDALF—Extended gradient descent algorithm for lattice finding. Acta Crystallogr. Sect. A Found. Adv. 2019, 75, 694–704. [Google Scholar] [CrossRef] [Green Version]
- Brehm, W.; Diederichs, K. Breaking the indexing ambiguity in serial crystallography. Acta Crystallogr. Sect. D Boil. Crystallogr. 2013, 70, 101–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winn, M.; Ballard, C.C.; Cowtan, K.D.; Dodson, E.J.; Emsley, P.; Evans, P.R.; Keegan, R.M.; Krissinel, E.B.; Leslie, A.G.W.; McCoy, A.; et al. Overview of theCCP4 suite and current developments. Acta Crystallogr. Sect. D Boil. Crystallogr. 2011, 67, 235–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drenth, J.; Mesters, J. Principles of Protein X-ray Crystallography, 3rd ed.; Springer Science & Business Media: Berlin, Germany, 2007. [Google Scholar] [CrossRef]
- Schmidt, M.; Šrajer, V.; Henning, R.; Ihee, H.; Purwar, N.; Tenboer, J.; Tripathi, S. Protein energy landscapes determined by five-dimensional crystallography. Acta Crystallogr. Sect. D Boil. Crystallogr. 2013, 69, 2534–2542. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Bean, R.; Sato, T.; Poudyal, I.; Bielecki, J.; Villarreal, J.C.; Yefanov, O.; Mariani, V.; White, T.; Kupitz, C.; et al. Time-resolved serial femtosecond crystallography at the European XFEL. Nat. Methods 2019, 17, 73–78. [Google Scholar] [CrossRef]
- Moffat, K. Time-resolved crystallography and protein design: Signalling photoreceptors and optogenetics. Philos. Trans. R. Soc. B Boil. Sci. 2014, 369, 20130568. [Google Scholar] [CrossRef] [Green Version]
- Kostov, K.S.; Moffat, K. Cluster Analysis of Time-Dependent Crystallographic Data: Direct Identification of Time-Independent Structural Intermediates. Biophys. J. 2011, 100, 440–449. [Google Scholar] [CrossRef] [Green Version]
- Rajagopal, S.; Anderson, S.; Šrajer, V.; Schmidt, M.; Pahl, R.; Moffat, K. A Structural Pathway for Signaling in the E46Q Mutant of Photoactive Yellow Protein. Structure 2005, 13, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Meyer, T. Isolation and characterization of soluble cytochromes, ferredoxins and other chromophoric proteins from the halophilic phototrophic bacterium Ectothiorhodospira halophila. Biochim. Biophys. Acta Bioenerg. 1985, 806, 175–183. [Google Scholar] [CrossRef]
- Purwar, N.; Tenboer, J.; Tripathi, S.; Schmidt, M. Spectroscopic Studies of Model Photo-Receptors: Validation of a Nanosecond Time-Resolved Micro-Spectrophotometer Design Using Photoactive Yellow Protein and α-Phycoerythrocyanin. Int. J. Mol. Sci. 2013, 14, 18881–18898. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, M. A short history of structure based research on the photocycle of photoactive yellow protein. Struct. Dyn. 2017, 4, 032201. [Google Scholar] [CrossRef] [Green Version]
- Polli, D.; Altoe, P.; Weingart, O.; Spillane, K.M.; Manzoni, C.; Brida, D.; Tomasello, G.; Orlandi, G.; Kukura, P.; A Mathies, R.; et al. Conical intersection dynamics of the primary photoisomerization event in vision. Nature 2010, 467, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Kono, M.; Goletz, P.W.; Crouch, R.K. 11-cis- and All-trans-Retinols Can Activate Rod Opsin: Rational Design of the Visual Cycle. Biochemistry 2008, 47, 7567–7571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edlund, P.; Takala, H.; Claesson, E.; Henry, L.; Dods, R.; Lehtivuori, H.; Panman, M.; Pande, K.; White, T.; Nakane, T.; et al. The room temperature crystal structure of a bacterial phytochrome determined by serial femtosecond crystallography. Sci. Rep. 2016, 6, 35279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez, J.; Carrillo, M.; Pandey, S.; Noda, M.; Aldama, L.; Feliz, D.; Claesson, E.; Wahlgren, W.Y.; Tracy, G.; Duong, P.; et al. High-resolution crystal structures of a myxobacterial phytochrome at cryo and room temperatures. Struct. Dyn. 2019, 6, 054701. [Google Scholar] [CrossRef] [Green Version]
- Hellingwerf, K.; Hendriks, J.; Gensch, T. On the Configurational and Conformational Changes in Photoactive Yellow Protein that Leads to Signal Generation in Ectothiorhodospira halophila. J. Boil. Phys. 2002, 28, 395–412. [Google Scholar] [CrossRef]
- Wiedorn, M.O.; Oberthür, M.; Bean, R.; Schubert, R.; Werner, N.; Abbey, B.; Aepfelbacher, M.; Adriano, L.; Allahgholi, A.; Al-Qudami, N.; et al. Megahertz serial crystallography. Nat. Commun. 2018, 9, 4025. [Google Scholar] [CrossRef] [Green Version]
- Fenno, L.; Yizhar, O.; Deisseroth, K. The development and application of optogenetics. Annu. Rev. Neurosci. 2011, 34, 389–412. [Google Scholar] [CrossRef]
- Ali, A.M.; Reis, J.M.; Xia, Y.; Rashid, A.J.; Mercaldo, V.; Walters, B.J.; Brechun, K.E.; Borisenko, V.; Josselyn, S.A.; Karanicolas, J.; et al. Optogenetic Inhibitor of the Transcription Factor CREB. Chem. Boil. 2015, 22, 1531–1539. [Google Scholar] [CrossRef] [Green Version]
- Hori, Y.; Norinobu, T.; Sato, M.; Arita, K.; Shirakawa, M.; Kikuchi, K. Development of Fluorogenic Probes for Quick No-Wash Live-Cell Imaging of Intracellular Proteins. J. Am. Chem. Soc. 2013, 135, 12360–12365. [Google Scholar] [CrossRef]
- Haupts, U.; Tittor, J.; Oesterhelt, D. CLOSING IN ON BACTERIORHODOPSIN: Progress in Understanding the Molecule. Annu. Rev. Biophys. Biomol. Struct. 1999, 28, 367–399. [Google Scholar] [CrossRef] [Green Version]
- Bae, G.; Choi, G. Decoding of Light Signals by Plant Phytochromes and Their Interacting Proteins. Annu. Rev. Plant Boil. 2008, 59, 281–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, J. Phytochrome Cytoplasmic Signaling. Annu. Rev. Plant Boil. 2013, 64, 377–402. [Google Scholar] [CrossRef]
- Molecular Devices and Machines: Concepts and Perspectives for the Nanoworld. Nano Today 2008, 3, 47. [CrossRef]
- Gegear, R.J.; Casselman, A.; Waddell, S.; Reppert, S.M. Cryptochrome mediates light-dependent magnetosensitivity in Drosophila. Nature 2008, 454, 1014–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajagopal, S.; Schmidt, M.; Anderson, S.; Ihee, H.; Moffat, K. Analysis of experimental time-resolved crystallographic data by singular value decomposition. Acta Crystallogr. Sect. D Boil. Crystallogr. 2004, 60, 860–871. [Google Scholar] [CrossRef]
- Burgie, E.S.; Zhang, J.; Vierstra, R.D. Crystal Structure of Deinococcus Phytochrome in the Photoactivated State Reveals a Cascade of Structural Rearrangements during Photoconversion. Structure 2016, 24, 448–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woitowich, N.C.; Halavaty, A.S.; Waltz, P.; Kupitz, C.; Valera, J.; Tracy, G.; Gallagher, K.D.; Claesson, E.; Nakane, T.; Pandey, S.; et al. Structural basis for light control of cell development revealed by crystal structures of a myxobacterial phytochrome. IUCrJ 2018, 5, 619–634. [Google Scholar] [CrossRef] [Green Version]
- Kruschel, D.; Zagrovic, B. Conformational averaging in structural biology: Issues, challenges and computational solutions. Mol. BioSyst. 2009, 5, 1606–1616. [Google Scholar] [CrossRef]
- Hosseinizadeh, A.; Mashayekhi, G.; Copperman, J.; Schwander, P.; Dashti, A.; Sepehr, R.; Fung, R.; Schmidt, M.; Yoon, C.H.; Hogue, B.G.; et al. Conformational landscape of a virus by single-particle X-ray scattering. Nat. Methods 2017, 14, 877–881. [Google Scholar] [CrossRef]
- Poudyal, I.; Schmidt, M.; Schwander, P. Single-particle imaging by x-ray free-electron lasers—How many snapshots are needed? Struct. Dyn. 2020, 7, 024102. [Google Scholar] [CrossRef]
- Botha, S.; Nass, K.; Barends, T.R.M.; Kabsch, W.; Latz, B.; Dworkowski, F.; Foucar, L.; Panepucci, E.; Wang, M.; Shoeman, R.L.; et al. Room-temperature serial crystallography at synchrotron X-ray sources using slowly flowing free-standing high-viscosity microstreams. Acta Crystallogr. Sect. D Boil. Crystallogr. 2015, 71, 387–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nogly, P.; James, D.; Wang, D.; White, T.A.; Zatsepin, N.; Shilova, A.; Nelson, G.; Liu, H.; Johansson, L.; Heymann, M.; et al. Lipidic cubic phase serial millisecond crystallography using synchrotron radiation. IUCrJ 2015, 2, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Martin-Garcia, J.M.; Conrad, C.E.; Nelson, G.; Stander, N.; Zatsepin, N.; Zook, J.; Zhu, L.; Geiger, J.; Chun, E.; Kissick, D.; et al. Serial millisecond crystallography of membrane and soluble protein microcrystals using synchrotron radiation. IUCrJ 2017, 4, 439–454. [Google Scholar] [CrossRef]
- Weinert, T.; Olieric, N.; Cheng, R.; Brünle, S.; James, D.; Ozerov, D.; Gashi, D.; Vera, L.; Marsh, M.; Jaeger, K.; et al. Serial millisecond crystallography for routine room-temperature structure determination at synchrotrons. Nat. Commun. 2017, 8, 542. [Google Scholar] [CrossRef] [PubMed]
- Berntsen, P.; Jazi, M.H.; Kusel, M.; Martin, A.V.; Ericsson, T.; Call, M.E.; Trenker, R.; Roque, F.G.; Darmanin, C.; Abbey, B. The serial millisecond crystallography instrument at the Australian Synchrotron incorporating the “Lipidico” injector. Rev. Sci. Instrum. 2019, 90, 085110. [Google Scholar] [CrossRef] [PubMed]
- Meents, A.; Wiedorn, M.O.; Srajer, V.; Henning, R.; Sarrou, I.; Bergtholdt, J.; Barthelmess, M.; Reinke, P.Y.A.; Dierksmeyer, D.; Tolstikova, A.; et al. Pink-beam serial crystallography. Nat. Commun. 2017, 8, 1281. [Google Scholar] [CrossRef] [Green Version]
- Martin-Garcia, J.M.; Zhu, L.; Mendez, D.; Lee, M.-Y.; Chun, E.; Li, C.; Hu, H.; Subramanian, G.; Kissick, D.; Ogata, C.; et al. High-viscosity injector-based pink-beam serial crystallography of microcrystals at a synchrotron radiation source. IUCrJ 2019, 6, 412–425. [Google Scholar] [CrossRef] [Green Version]
- Schulz, E.C.; Mehrabi, P.; Müller-Werkmeister, H.M.; Tellkamp, F.; Jha, A.; Stuart, W.; Persch, E.; De Gasparo, R.; Diederich, F.; Pai, E.F.; et al. The hit-and-return system enables efficient time-resolved serial synchrotron crystallography. Nat. Methods 2018, 15, 901–904. [Google Scholar] [CrossRef] [Green Version]
- Mehrabi, P.; Schulz, E.C.; Agthe, M.; Horrell, S.; Bourenkov, G.; Von Stetten, D.; Leimkohl, J.-P.; Schikora, H.; Schneider, T.R.; Pearson, A.R.; et al. Liquid application method for time-resolved analyses by serial synchrotron crystallography. Nat. Methods 2019, 16, 979–982. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandey, S.; Poudyal, I.; Malla, T.N. Pump-Probe Time-Resolved Serial Femtosecond Crystallography at X-Ray Free Electron Lasers. Crystals 2020, 10, 628. https://doi.org/10.3390/cryst10070628
Pandey S, Poudyal I, Malla TN. Pump-Probe Time-Resolved Serial Femtosecond Crystallography at X-Ray Free Electron Lasers. Crystals. 2020; 10(7):628. https://doi.org/10.3390/cryst10070628
Chicago/Turabian StylePandey, Suraj, Ishwor Poudyal, and Tek Narsingh Malla. 2020. "Pump-Probe Time-Resolved Serial Femtosecond Crystallography at X-Ray Free Electron Lasers" Crystals 10, no. 7: 628. https://doi.org/10.3390/cryst10070628
APA StylePandey, S., Poudyal, I., & Malla, T. N. (2020). Pump-Probe Time-Resolved Serial Femtosecond Crystallography at X-Ray Free Electron Lasers. Crystals, 10(7), 628. https://doi.org/10.3390/cryst10070628





