Controlled Synthesis of Hydroxyapatite Nanomaterials Regulated by Different Phosphorus Sources
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
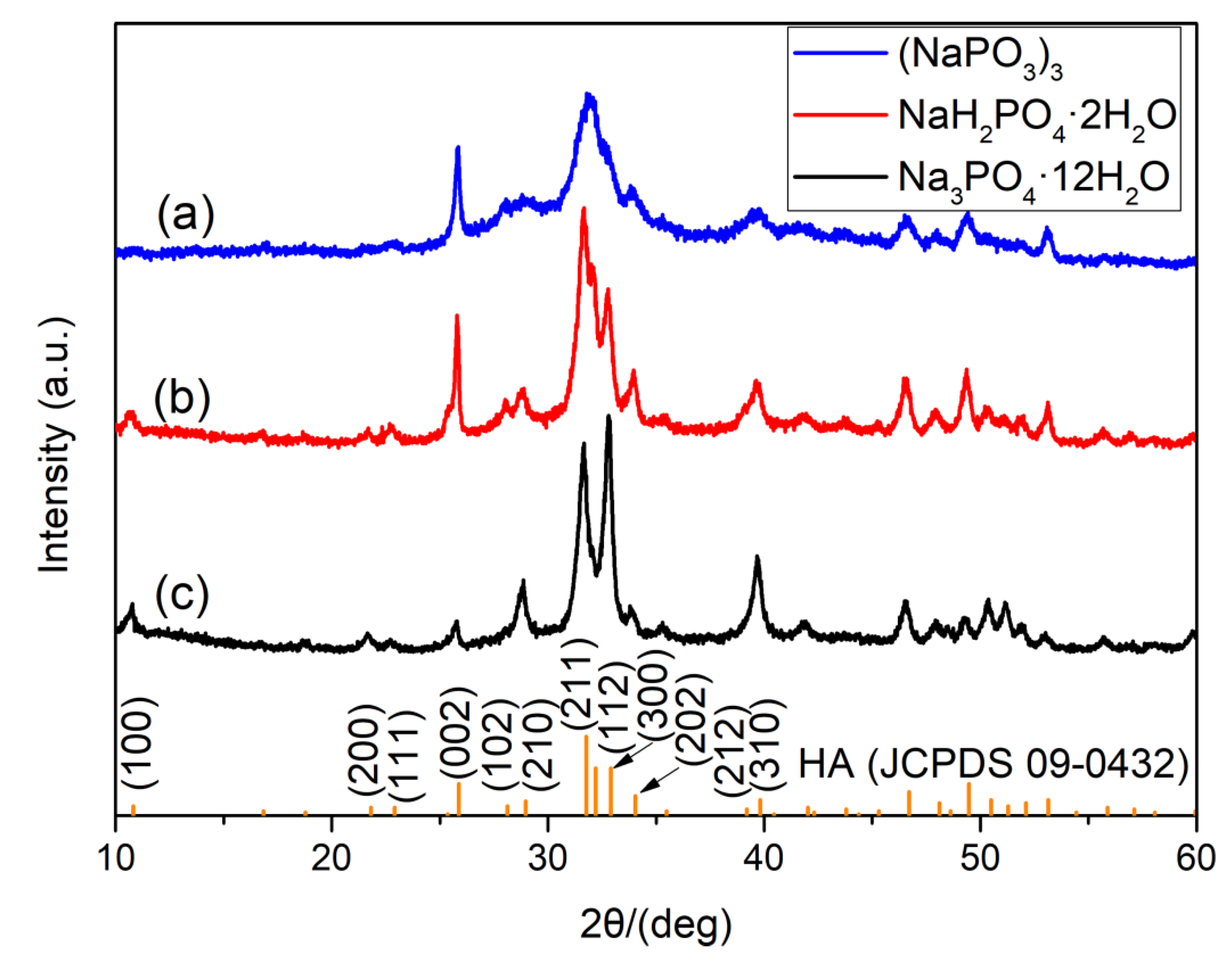
3.1. Phase Analysis
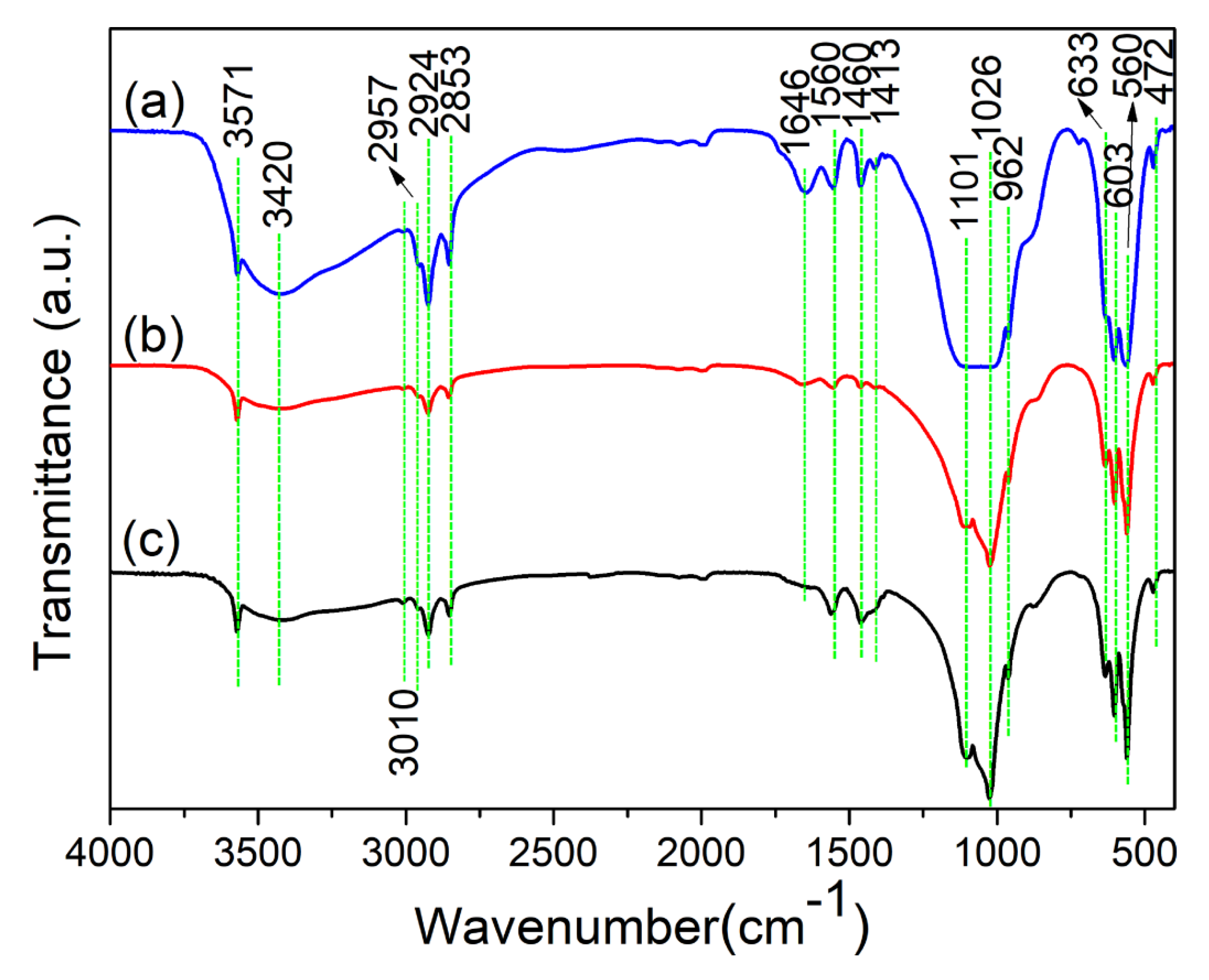
3.2. Functional Group Analysis
3.3. Microstructural Characterization
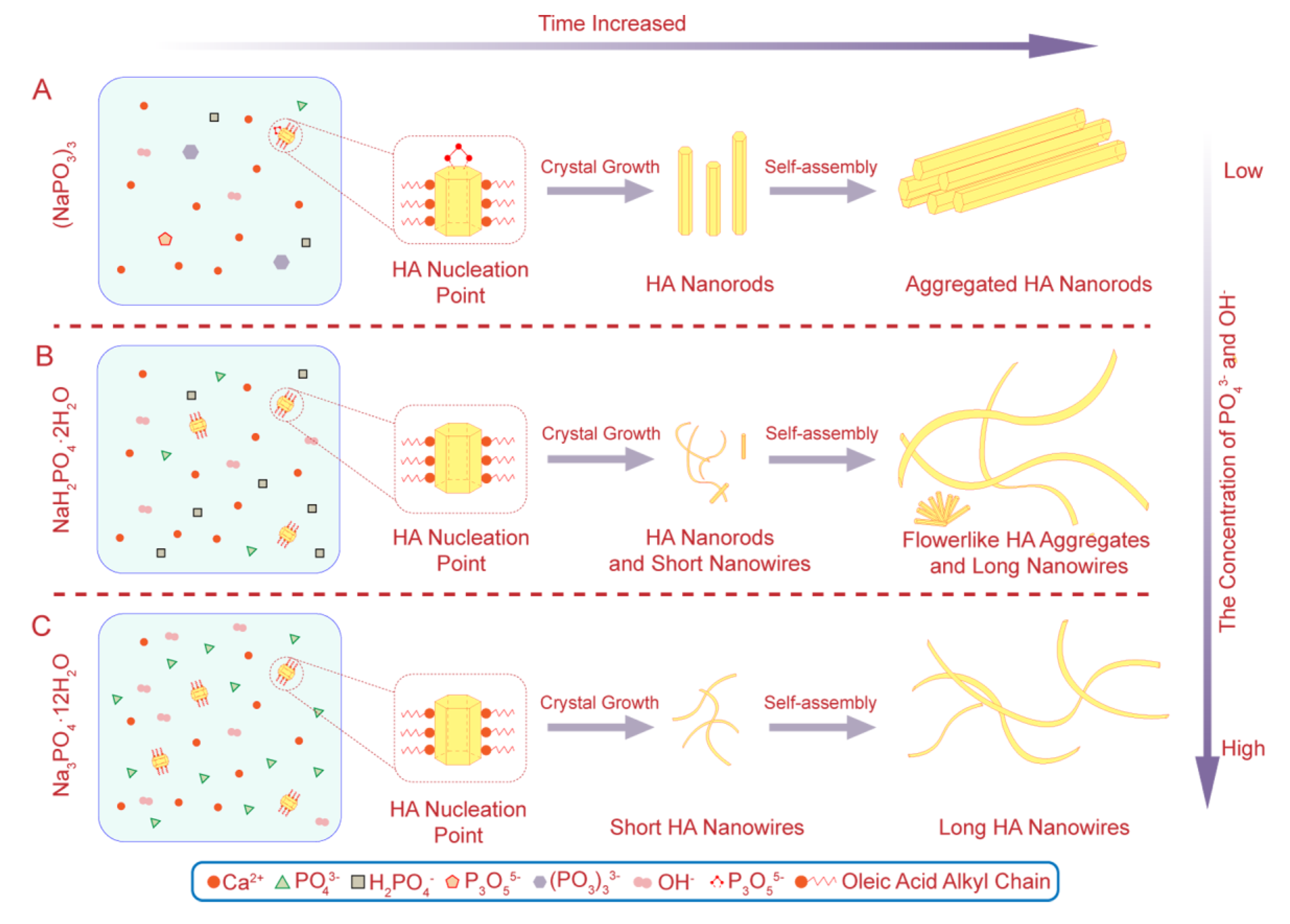
3.4. Formation Mechanism
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shojai, M.S.; Khorasani, M.T.; Khoshdargi, E.D.; Jamshidi, A. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 2013, 9, 7591–7621. [Google Scholar] [CrossRef]
- Kanasan, N.; Adzila, S.; Koh, C.T.; Panerselvan, G. Effects of magnesium doping on the properties of hydroxyapatite/sodium alginate biocomposite. Adv. Appl. Ceram. 2019, 118, 381–386. [Google Scholar] [CrossRef]
- Ratnayake, J.T.B.; Mucalo, R.; Dias, G.J. Substituted hydroxyapatites for bone regeneration: A review of current trends. J. Biomed. Mater. Res. B 2017, 105, 1285–1299. [Google Scholar] [CrossRef]
- Zakaria, S.M.; Sharif, Z.; Othman, S.H.; Yang, M.R.; Jansen, F.J.A. Nanophase hydroxyapatite as a biomaterial in advanced hard tissue engineering: A review. Tissue Eng. Part B Rev. 2013, 19, 431–441. [Google Scholar] [CrossRef]
- Sun, W.; Fan, J.; Wang, S.; Kang, Y.; Du, J.; Peng, X. Biodegradable drug-loaded hydroxyapatite nanotherapeutic agent for targeted drug release in tumors. ACS Appl. Mater. Inter. 2018, 10, 7832–7840. [Google Scholar] [CrossRef]
- Shen, J.W.; Wu, T.; Wang, Q.; Pan, H.H. Molecular simulation of protein adsorption and desorption on hydroxyapatite surfaces. Biomaterials 2008, 29, 513–532. [Google Scholar] [CrossRef]
- Phakatkar, A.H.; Shirdar, M.R.; Qi, M.; Taheri, M.M.; Narayanan, S.; Foroozan, T.; Sharifi-Asl, S.; Huang, Z.; Agrawal, M.; Lu, Y.; et al. Novel PMMA bone cement nanocomposites containing magnesium phosphate nanosheets and hydroxyapatite nanofibers. Mater. Sci. Eng. C 2020, 109, 110497. [Google Scholar] [CrossRef] [PubMed]
- Alves Cardoso, D.; Jansen, J.A.; Leeuwenburgh, S.C. Synthesis and application of nanostructured calcium phosphate ceramics for bone regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 2316–2326. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-C.; Xu, W.-L.; Lu, Y.-P.; Xu, W.-H.; Yin, H.; Xiao, G.-Y. Investigation of nature of starting materials on the construction of hydroxyapatite 1D/3D morphologies. Mater. Sci. Eng. C 2020, 108, 110408. [Google Scholar] [CrossRef] [PubMed]
- Cengiz, B.; Gokce, Y.; Yildiz, N.; Aktas, Z.; Calimli, A. Synthesis and characterization of hydroxyapatite nanoparticles. Colloids Surf. A 2008, 322, 29–33. [Google Scholar] [CrossRef]
- Sun, F.; Zhou, H.J.; Lee, J. Various preparation methods of highly porous hydroxyapatite/polymer nanoscale biocomposites for bone regeneration. Acta Biomater. 2011, 7, 3813–3828. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.Q.; Zhu, Y.J.; Chen, F. Highly flexible and nonflammable inorganic hydroxyapatite paper. Chem. Eur. J. 2014, 20, 1242–1246. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.Y.; Zhu, Y.J.; Chen, F.; Wu, J. Solvothermal synthesis of submillimeter ultralong hydroxyapatite nanowires using a calcium oleate precursor in a series of monohydroxy alcohols. Ceram Int. 2015, 41, 6098–6102. [Google Scholar] [CrossRef]
- Chen, F.F.; Zhu, Y.J.; Xiong, Z.C.; Dong, L.Y.; Chen, F.; Lu, B.Q.; Yang, R.L. Hydroxyapatite nanowire-based all-weather flexible electrically conductive paper with superhydrophobic and flame-retardant properties. ACS Appl. Mater. Inter. 2017, 9, 39534–39548. [Google Scholar] [CrossRef]
- Sun, T.W.; Yu, W.L.; Zhu, Y.J.; Chen, F.; Zhang, Y.G.; Jiang, Y.Y.; He, Y.H. Porous nanocomposite comprising ultralong hydroxyapatite nanowires decorated with zinc-containing nanoparticles and chitosan: Synthesis and application in bone defect repair. Chemistry 2018, 24, 8809–8821. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Zhu, Y.J.; Chen, F.; Lu, B.Q.; Qi, C.; Zhao, J.; Wu, J. Hydrothermal synthesis of hydroxyapatite nanorods and nanowires using riboflavin-5′-phosphate monosodium salt as a new phosphorus source and their application in proteinadsorption. Cryst. Eng. Comm. 2013, 15, 7926–7935. [Google Scholar] [CrossRef]
- Chen, F.; Zhu, Y.J.; Wang, K.W.; Zhao, K.L. Surfactant-free solvothermal synthesis of hydroxyapatite nanowire/nanotube ordered arrays with biomimetic structures. Cryst. Eng. Comm. 2011, 13, 1858–1863. [Google Scholar] [CrossRef]
- Cao, M.H.; Wang, Y.H.; Guo, C.X.; Qi, Y.J.; Hu, C.W. Preparation of ultrahigh-aspect-ratio hydroxyapatite nanofibers in reverse micelles under hydrothermal conditions. Langmuir 2004, 20, 4784–4786. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, Y.; Chen, S.T.; Zhao, Y.Q.; Li, H.L. Template synthesis of highly ordered hydroxyapatite nanowire arrays. J. Mater. Sci. 2005, 40, 1121–1125. [Google Scholar] [CrossRef]
- Costa, D.O.; Dixon, S.J.; Rizkalla, A.S. One- and Three-dimensional growth of hydroxyapatite nanowires during sol–gel–hydrothermal synthesis. ACS Appl. Mater. Inter. 2012, 4, 1490–1499. [Google Scholar] [CrossRef]
- Reardon, P.J.T.; Handoko, A.D.; Li, L.; Huang, J.; Tang, J.W. Dimensionally and compositionally controlled growth of calcium phosphate nanowires for bone tissue regeneration. J. Mater. Chem. B 2013, 1, 6170–6176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakagawa, K.; Arai, Y.; Umezaki, Y.A.; Yoshida, Y.; Kajiwara, S.A.; Matsuyama, H.; Sugiyama, S. Template effect of phosphate surfactant on formation of hydroxyapatite nanostructures with various shapes. Mater. Chem. Phys. 2018, 213, 183–190. [Google Scholar] [CrossRef]
- Zhang, Y.-G.; Zhu, Y.-J.; Chen, F.; Wu, J. Ultralong hydroxyapatite nanowires synthesized by solvothermal treatment using a series of phosphate sodium salts. Mater. Lett. 2015, 144, 135–137. [Google Scholar] [CrossRef]
- Sun, R.X.; Chen, K.Z.; Liao, Z.M.; Meng, N. Controlled synthesis and thermal stability of hydroxyapatite hierarchical microstructures. Mater. Res. Bull. 2013, 48, 1143–1147. [Google Scholar] [CrossRef]
- Qi, M.-L.; Qi, J.; Xiao, G.-Y.; Zhang, K.-Y.; Lu, C.-Y.; Lu, Y.-P. One-step hydrothermal synthesis of carbonated hydroxyapatite porous microspheres with a large and uniform size regulated by L-glutamic acid. Cryst. Eng. Comm. 2016, 18, 5876–5884. [Google Scholar] [CrossRef]
- Lin, K.; Chang, J.; Zhu, Y.; Wu, W.; Cheng, G.; Zeng, Y.; Ruan, M. A facile one-step surfactant-free and low-temperature hydrothermal method to prepare uniform 3D structured carbonated apatite flowers. Cryst. Growth Des. 2008, 9, 177–181. [Google Scholar] [CrossRef]
- Qi, M.-L.; Yao, S.; Liu, X.-C.; Wang, X.; Cui, F. Nanosheet-assembled carbonated hydroxyapatite microspheres prepared by an EDTA-assisted hydrothermal homogeneous precipitation route. Cryst. Eng. Comm. 2020, 22, 2884–2888. [Google Scholar] [CrossRef]
- Li, H.; Zhu, Y.J.; Jiang, Y.Y.; Yu, Y.D.; Chen, F.; Dong, L.Y.; Wu, J. Hierarchical assembly of monodisperse hydroxyapatite nanowires and construction of high-strength fire-resistant inorganic paper with high-temperature flexibility. Chem. Nano. Mat. 2017, 3, 259–268. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, M.-l.; Qin, S.; Wang, Y.-c.; Yao, S.; Qi, L.; Wu, Y.; Lu, Y.-p.; Cui, F. Controlled Synthesis of Hydroxyapatite Nanomaterials Regulated by Different Phosphorus Sources. Crystals 2020, 10, 678. https://doi.org/10.3390/cryst10080678
Qi M-l, Qin S, Wang Y-c, Yao S, Qi L, Wu Y, Lu Y-p, Cui F. Controlled Synthesis of Hydroxyapatite Nanomaterials Regulated by Different Phosphorus Sources. Crystals. 2020; 10(8):678. https://doi.org/10.3390/cryst10080678
Chicago/Turabian StyleQi, Mei-li, Sijia Qin, Yin-chuan Wang, Shengkun Yao, Liang Qi, Yanling Wu, Yu-peng Lu, and Fengkun Cui. 2020. "Controlled Synthesis of Hydroxyapatite Nanomaterials Regulated by Different Phosphorus Sources" Crystals 10, no. 8: 678. https://doi.org/10.3390/cryst10080678





