Design, Synthesis, and Evaluation of X-ray Crystal Structure, Biological Activities, DFT Calculations, and Molecular Docking of Phenyl Imidazolidin-2-One Derivatives
Abstract
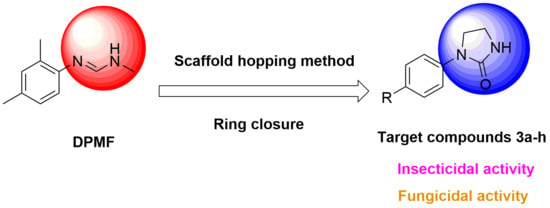
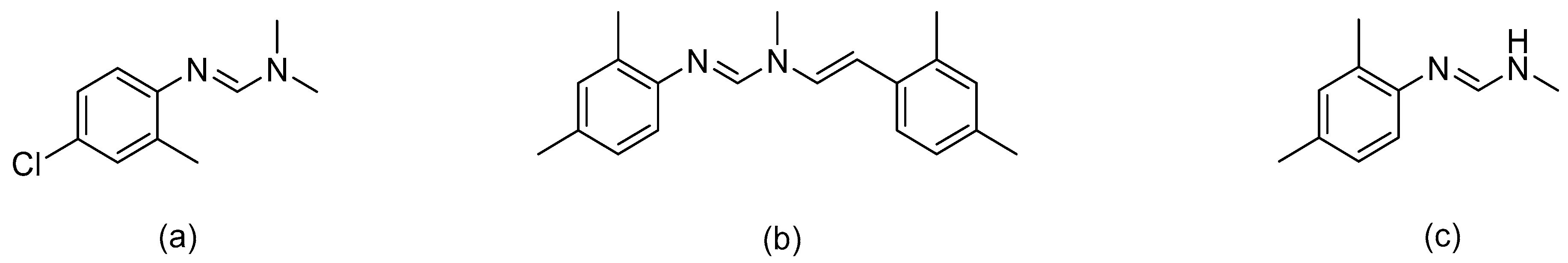
:1. Introduction
2. Materials and Methods
2.1. Materials and Physical Measurements
2.2. Synthetic Procedures
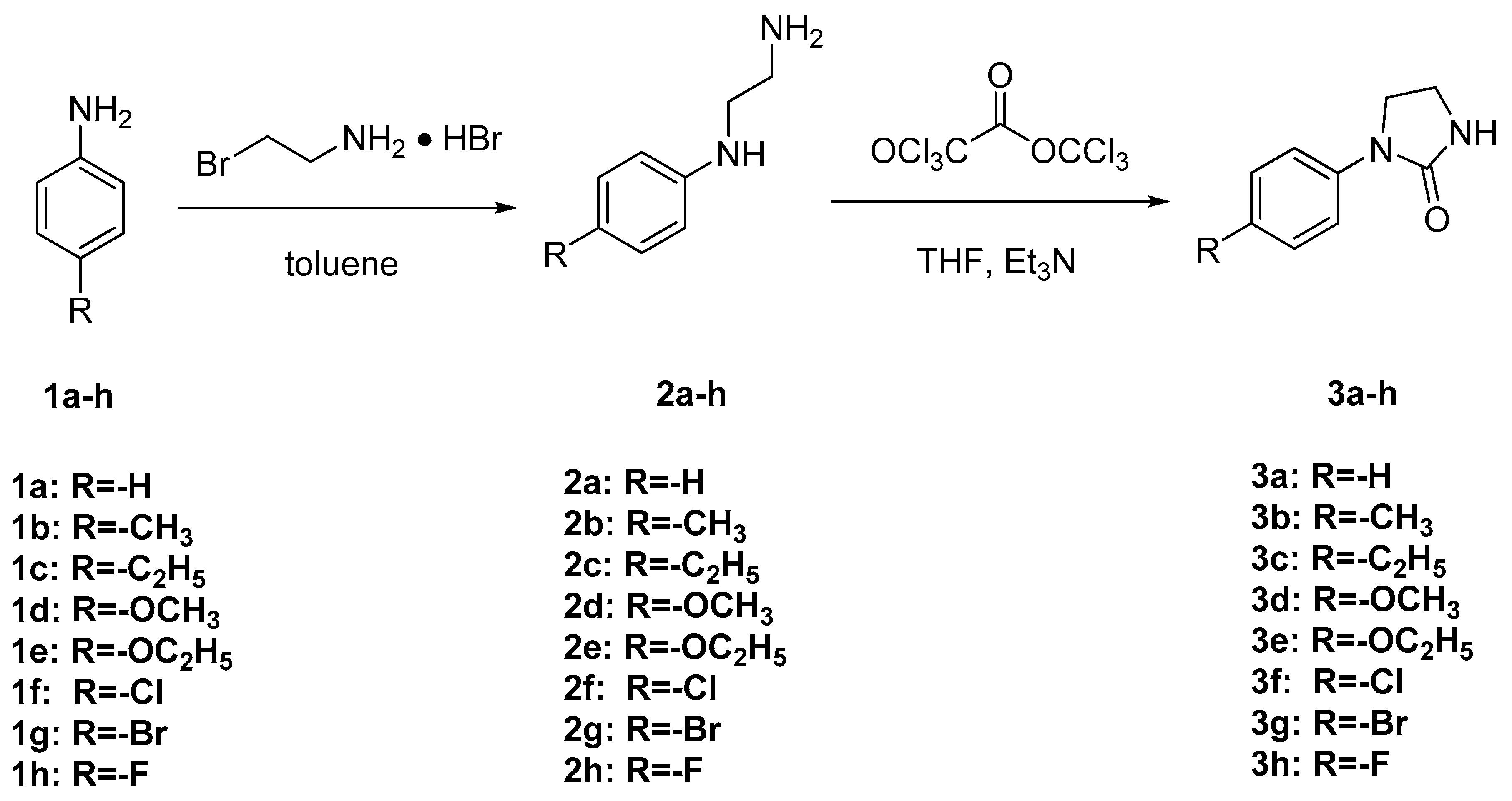
2.2.1. Synthesis of Intermediates 2a–h
2.2.2. Synthesis of Target Compounds 3a–h
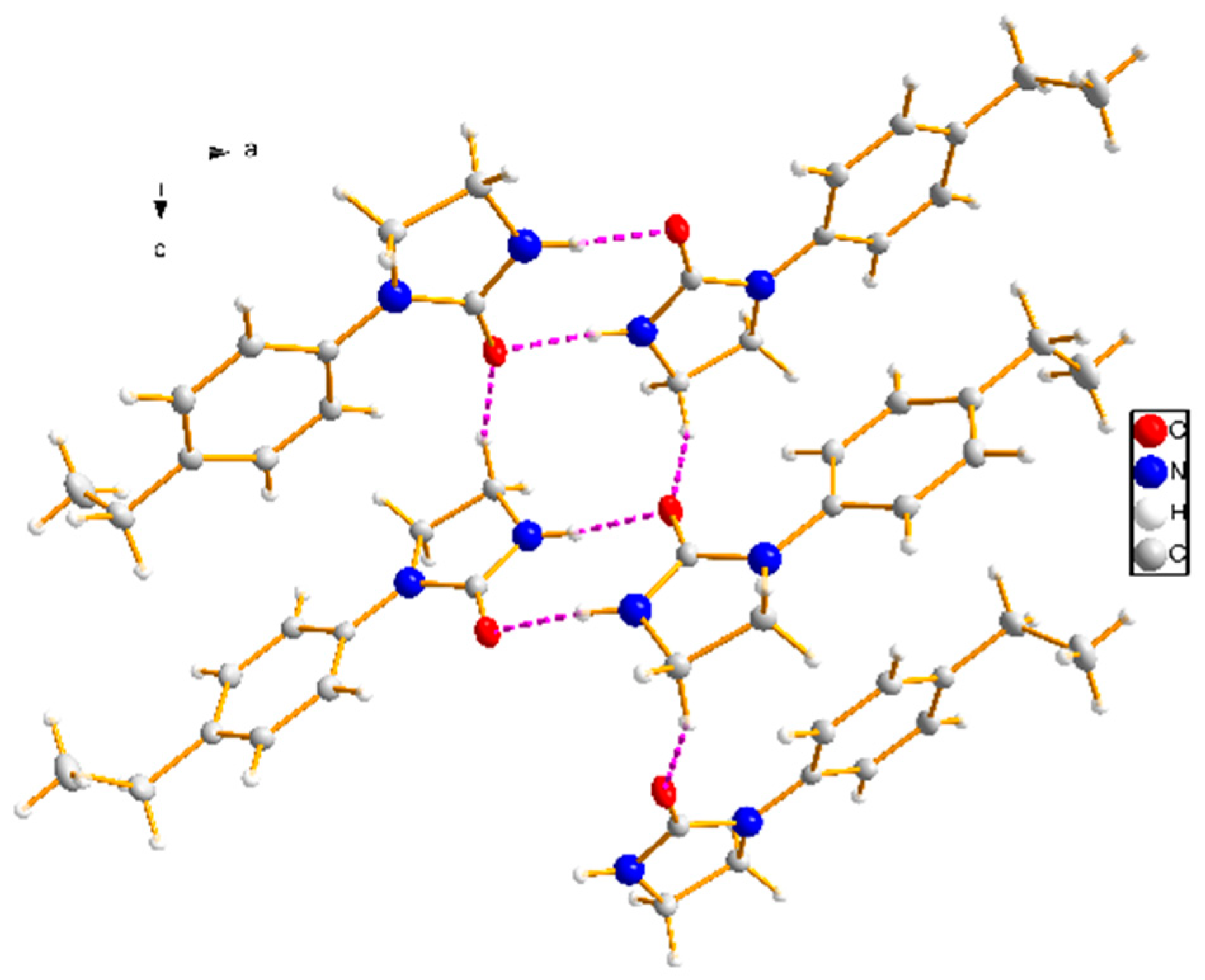
2.3. Crystal Structural Determination
2.4. Insecticidal Activities
2.5. Fungicidal Activities
2.6. Theoretical Calculations
2.7. Homology Modeling
2.8. Molecular Docking
3. Results and Discussion
3.1. Synthesis and Spectroscopic Properties
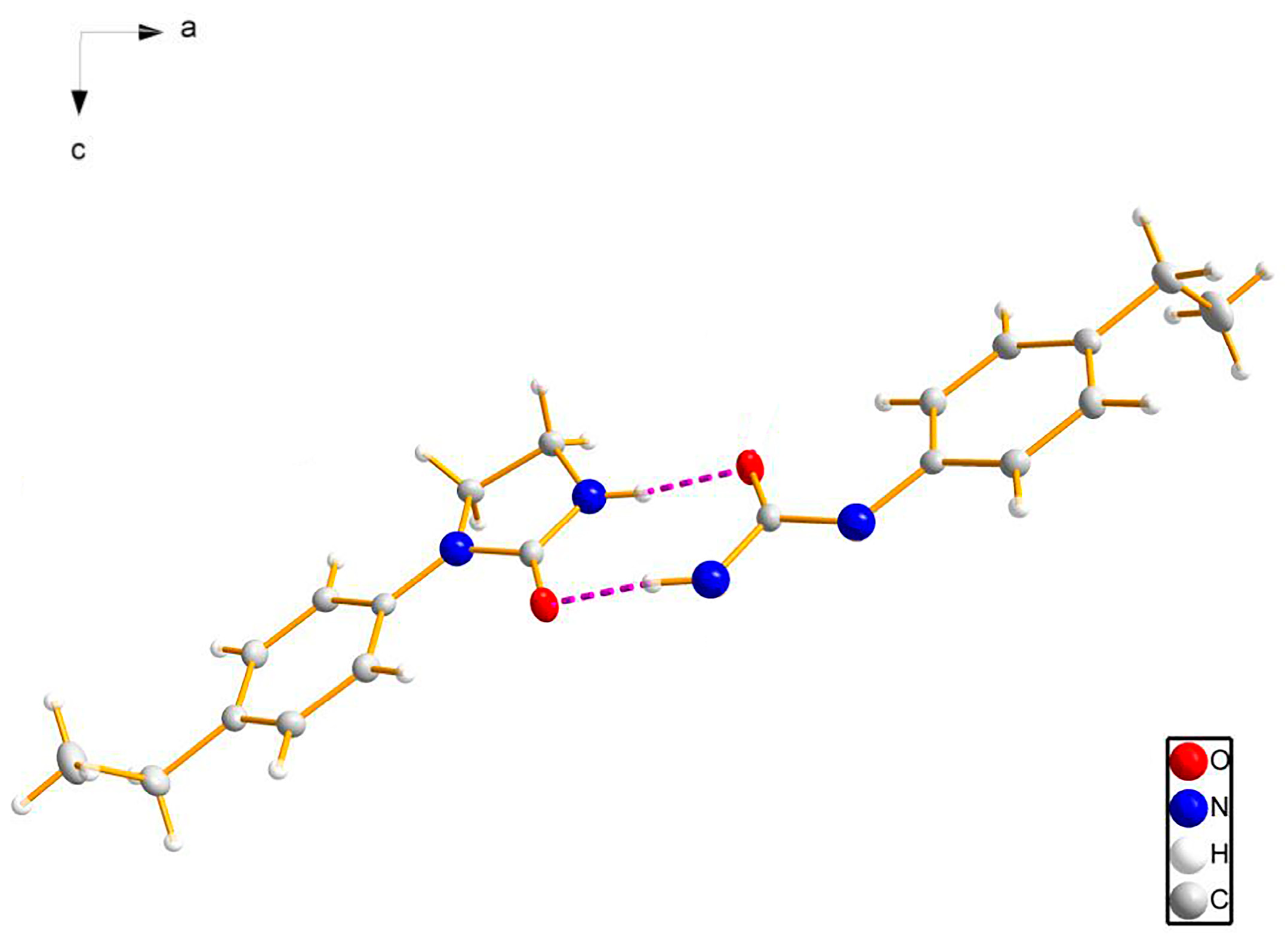
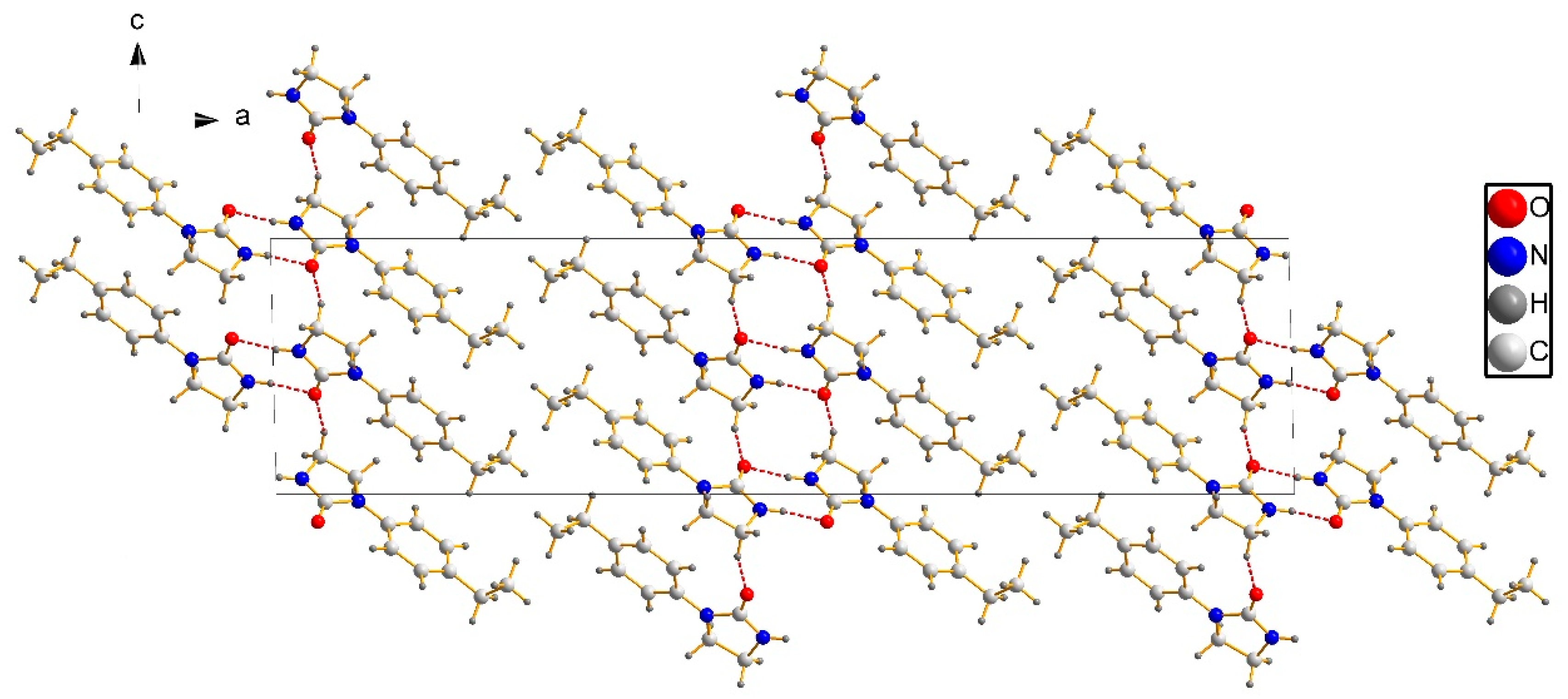
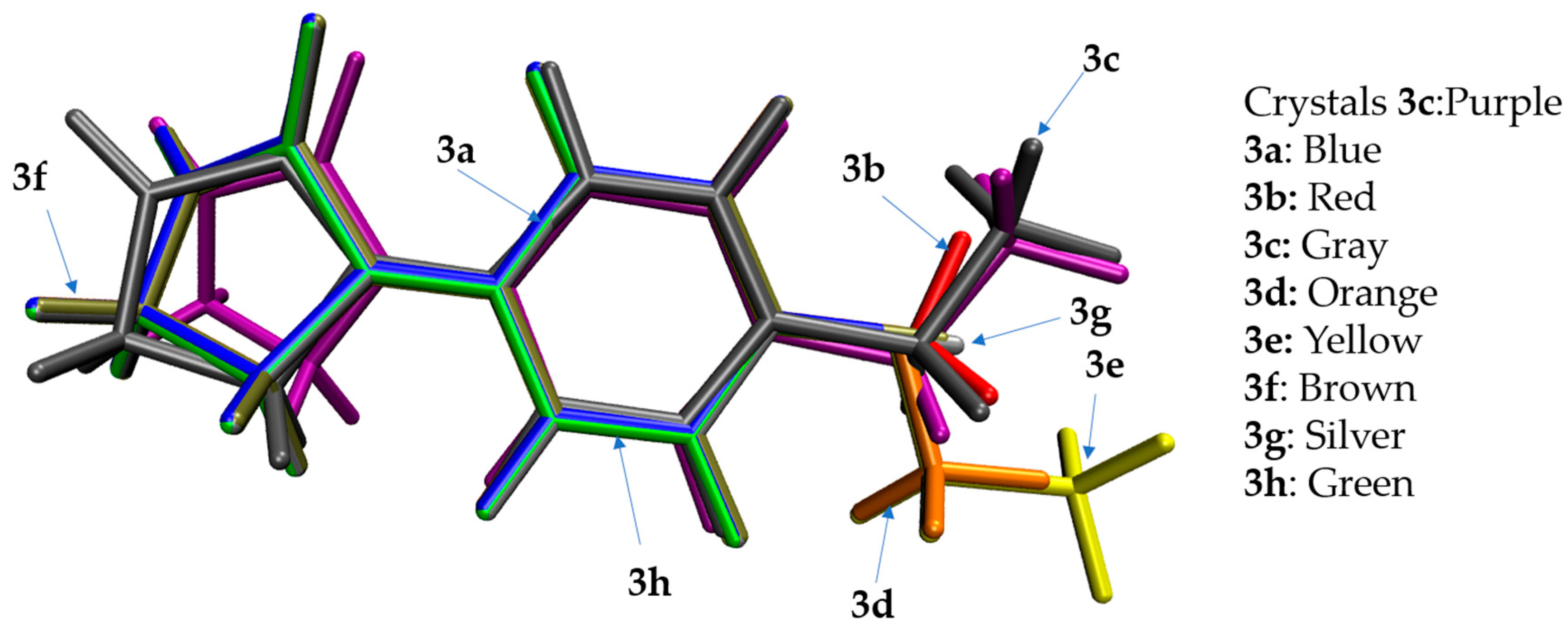
3.2. Crystal Structure
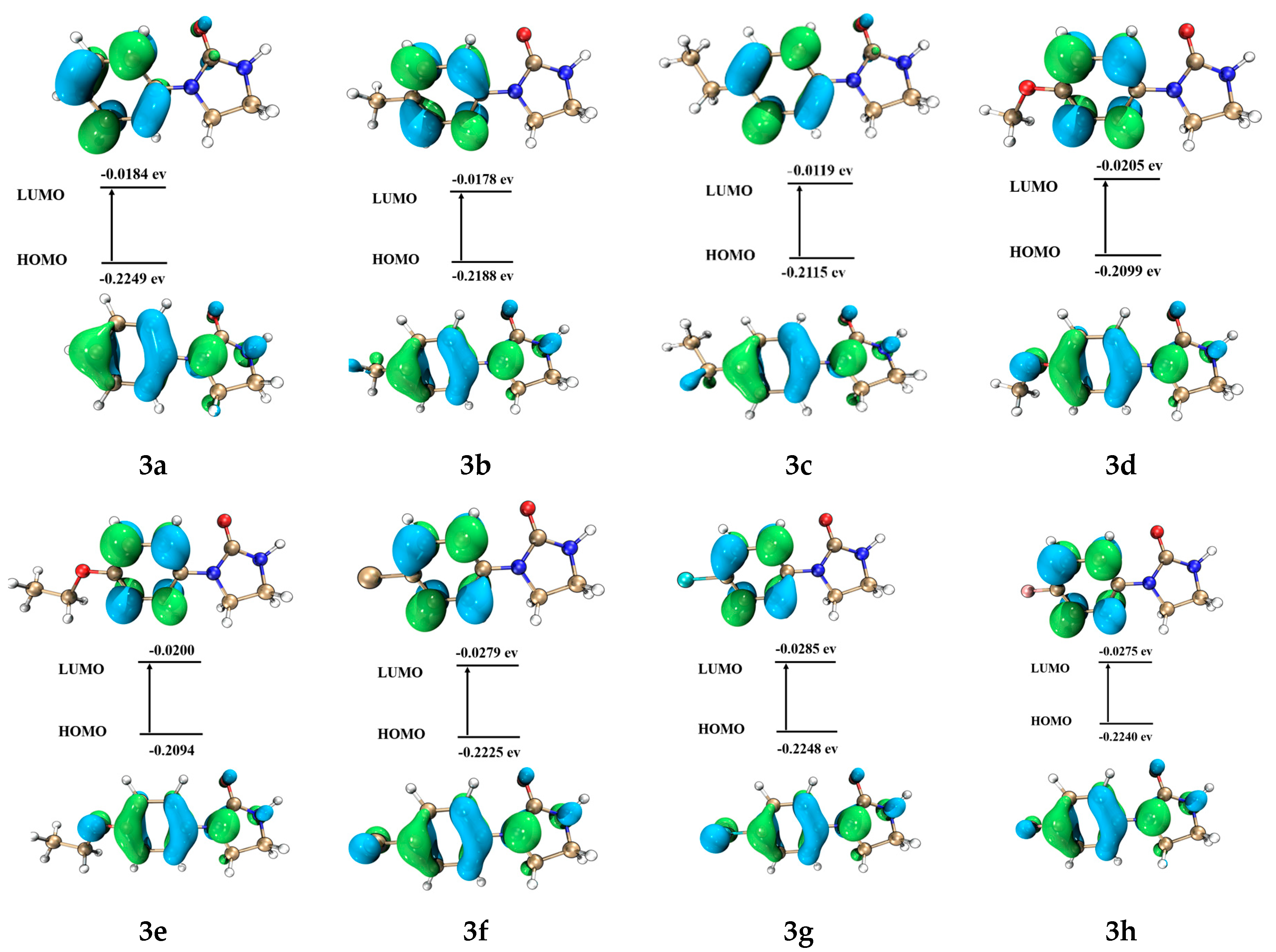
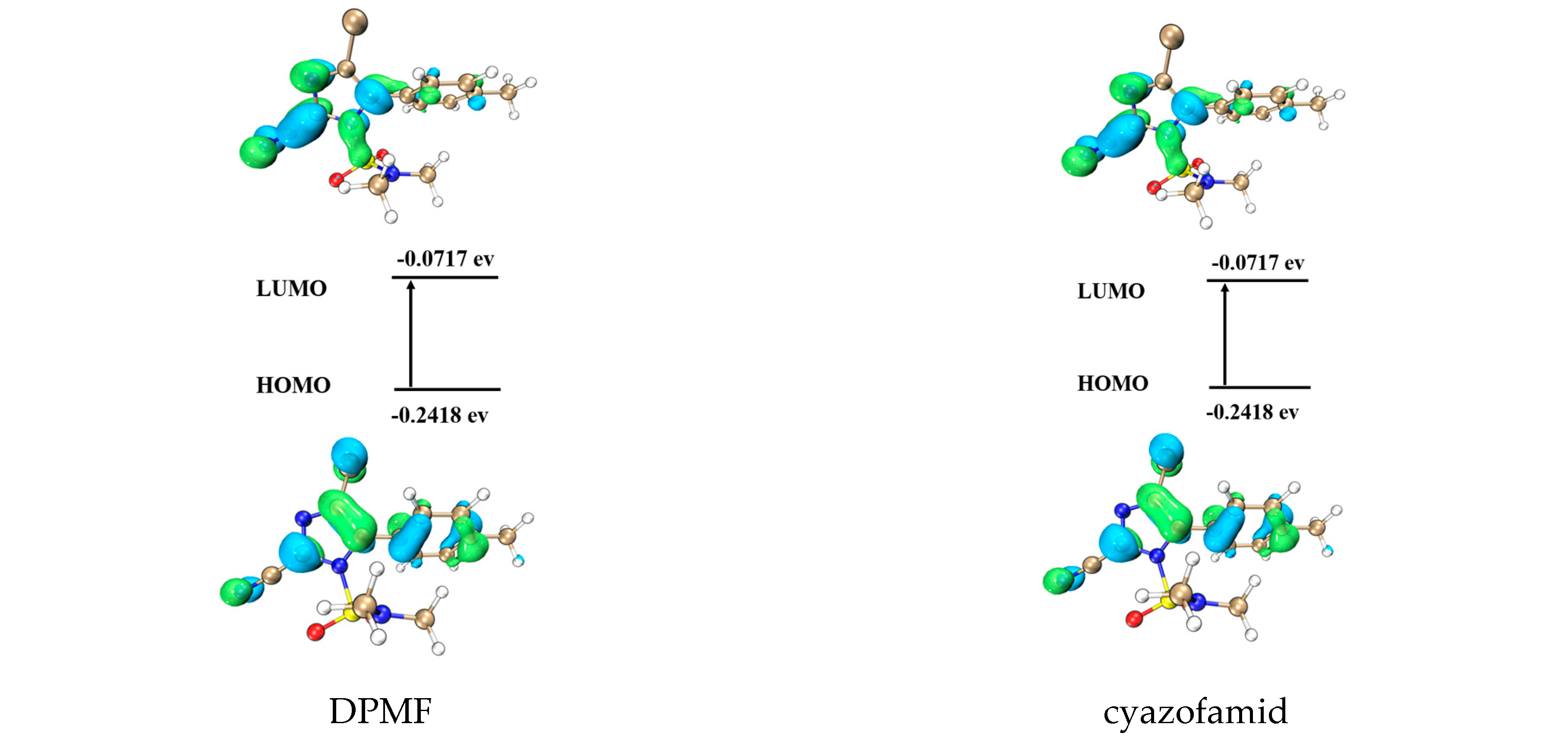
3.3. Quantum Chemistry Calculations of the Geometry and Electronic Structure
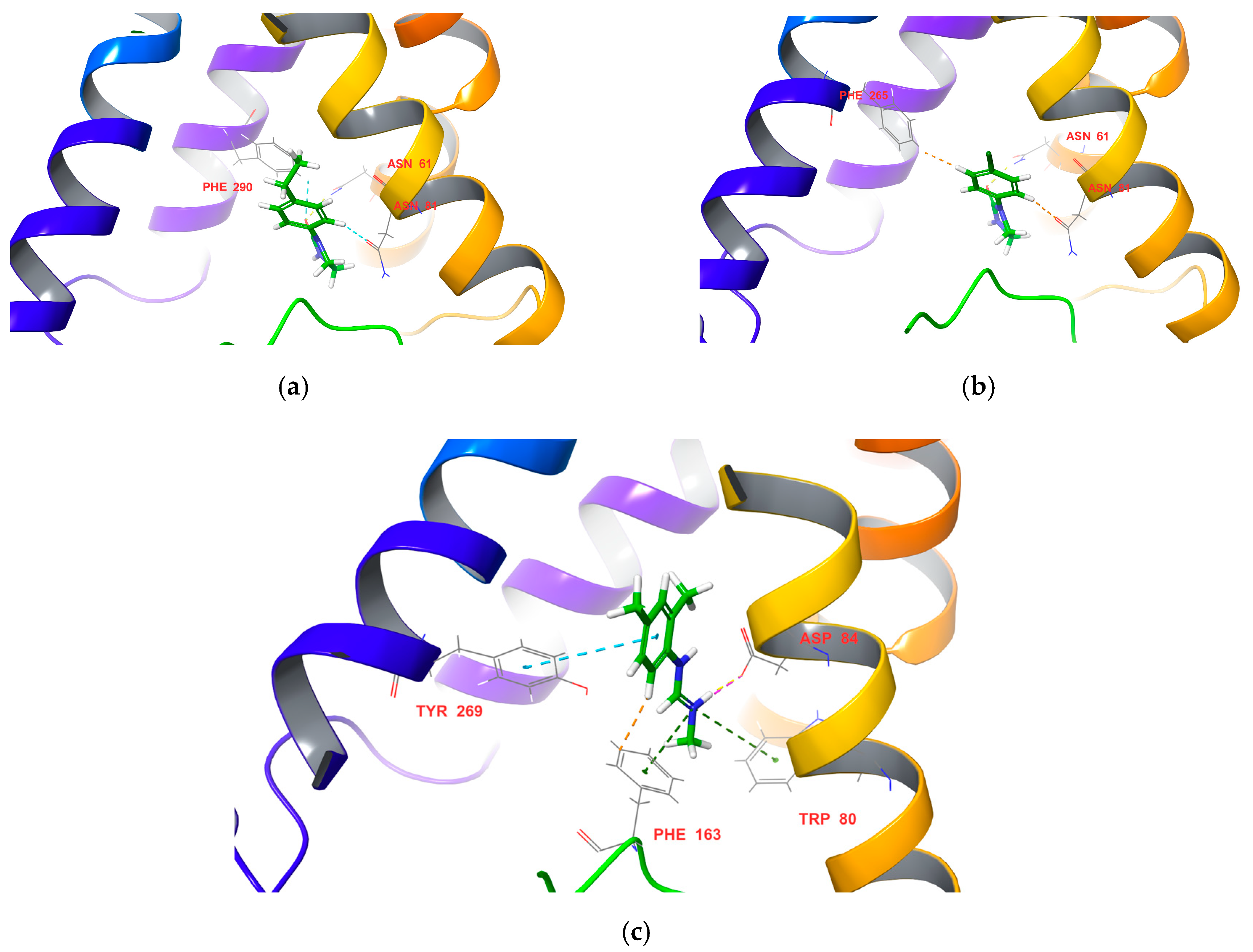
3.4. Molecular-Docking Stimulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Evans, P.D.; Maqueira, B. Insect octopamine receptors: A new classification scheme based on studies of cloned drosophila G-protein coupled receptors. Invertebr. Neurosci. 2005, 5, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Hamasaki, T.; Ozoe, F.; Ozoe, Y. Single amino acid of an octopamine receptor as a molecular switch for distinct G protein couplings. Biochem. Biophys. Res. Commun. 2008, 371, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Hamasaki, T.; Ozoe, F.; Ohta, H.; Enomoto, K.I.; Kataoka, H.; Sawa, Y.; Hirota, A.; Ozoe, Y. Identification of critical structural determinants responsible for octopamine binding to the α-adrenergic-like bombyx mori octopamine receptor. Biochemistry 2007, 46, 5896–5903. [Google Scholar] [PubMed]
- Chen, X.; Ohta, H.; Ozoe, F.; Miyazawa, K.; Huang, J.; Ozoe, Y. Functional and pharmacological characterization of a β-adrenergic-like octopamine receptor from the silkworm Bombyx mori. Insect Biochem. Mol. Biol. 2010, 40, 476–486. [Google Scholar] [PubMed]
- Ohta, H.; Ozoe, Y. Chapter two-molecular signalling, pharmacology, and physiology of octopamine and tyramine receptors as potential insect pest control targets. Adv. Insect Physiol. 2014, 46, 73–166. [Google Scholar]
- Hunter, J.S.; Baggott, D.; Everett, W.R.; Fourie, J.J.; Cramer, L.G.; Yoon, S.S.; Collidor, N.; Mallouk, Y.; Lee, L.; Blair, J.; et al. Efficacy of a novel topical combination of fipronil, amitraz and (S)-methoprene for treatment and control of induced infestations of brown dog ticks (Rhipicephalus sanguineus) on dogs. Vet. Parasitol. 2011, 179, 318–323. [Google Scholar]
- Martel, A.C.; Zeggane, S.; Aurieres, C.; Drajnudel, P.; Faucon, J.P.; Aubert, M. Acaricide residues in honey and wax after treatment of honey bee colonies with Apivar or Asuntol 50. Apidologie 2008, 38, 534–544. [Google Scholar] [CrossRef]
- Hellmann, K.; Adler, K.; Parker, L.; Pfister, K.; DeLay, R.L.; Rugg, D. Evaluation of the efficacy and safety of a novel formulation of metaflumizone plus amitraz in dogs naturally infested with fleas and ticks in Europe. Vet. Parasitol. 2007, 150, 239–245. [Google Scholar] [CrossRef]
- Thomas, W.D.; Craig, G.K.; Stacey, N.H. Effects of chlordimeform and its metabolite 4-chloro-o-toluidine on rat splenic T, B and tumoricidal effector cells. Immunopharmacology 1990, 19, 79–86. [Google Scholar] [CrossRef]
- Leslie, C.; Reidy, G.F.; Murray, M.; Stacey, N.H. Induction of xenobiotic biotransformation by the insecticide chlordimeform, a metabolite 4-chloro-o-toluidine and a structurally related chemical o-toluidine. Biochem. Pharmcol. 1988, 37, 2529–2535. [Google Scholar] [CrossRef]
- Mallott, M.; Hamm, S.; Troczka, B.J.; Randall, E.; Pym, A.; Grant, C.; Baxter, S.; Vogel, H.; Shelton, A.M.; Field, L.M.; et al. A flavin-dependent monooxgenase confers resistance to chlorantraniliprole in the diamondback moth, Plutella xylostella. Insect Biochem. Mol. Biol. 2019, 115, 103247. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Yusoff, N.; Aizat, W.M.; Othman, N.W.; Abd Ghani, I. Optimization method for proteomic analysis of the larva and adult tissues of Plutella xylostella (L.) (Lepidoptera: Plutellidae). Sains Malays. 2018, 47, 2975–2983. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, J. Optimization of artificial rearing methods for Plutella xylostella L. (Plutellidae) and its susceptibility to five types of insecticides. Agric. Sci. Technol. 2012, 13, 1713–1715, 1721. [Google Scholar]
- Wang, B.; Wang, H.; Liu, H.; Xiong, L.; Yang, N.; Zhang, Y.; Li, Z. Synthesis and structure-insecticidal activity relationship of novel phenylpyrazole carboxylic acid derivatives containing fluorine moiety. Chin. Chem. Lett. 2019, 19, 79–86. [Google Scholar] [CrossRef]
- Cao, G.; Han, Z. Tebufenozide resistance selected in Plutella xylostella and its cross-resistance and fitness cost. Pest Manag. Sci. 2006, 62, 746–751. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, X.H.; Ishaaya, I.; Cao, S.; Wu, J.J.; Yu, J.L.; Li, H.; Qian, X.H. Synthesis and insecticidal activity of heptafluoroisopropyl-containing benzoylphenylurea structures. J. Agric. Food Chem. 2010, 58, 2736–2740. [Google Scholar] [CrossRef]
- Huang, Q.T.; Ma, H.H.; Deng, X.L.; Zhu, H.; Liu, J.; Zhou, Y.; Zhou, X.M. Pharmacological characterization of a β-adrenergic-like octopamine receptor in Plutella xylostella. Arch. Insect Biochem. Physiol. 2018, 98, e21466. [Google Scholar] [CrossRef]
- Davenport, A.P.; Wright, D.J. Toxicity of chlordimeform and amitraz to the egyptian cotton leafworm (Spodoptera littoralis) and the tobacco budworm (Heliothis virescens). Pestic. Sci. 1985, 16, 81–87. [Google Scholar] [CrossRef]
- Knowles, C.O.; Roulston, W.J. Toxicity to boophilus microplus of formamidine acaricides and related compounds, and modification of toxicity by certain insecticide synergists. J. Econ. Entomol. 1973, 66, 1245–1251. [Google Scholar] [CrossRef]
- Kita, T.; Hayashi, T.; Ohtani, T.; Takao, H.; Takasu, H.; Liu, G.; Ohta, H.; Ozoe, F.; Ozoe, Y. Amitraz and its metabolite differentially activate α- and β-adrenergic-like octopamine receptors. Pest Manag. Sci. 2017, 73, 984–990. [Google Scholar] [CrossRef]
- Lamberth, C. Agrochemical lead optimization by scaffold hopping. Pest Manag. Sci. 2018, 74, 282–292. [Google Scholar] [CrossRef]
- Lv, K.; Li, L.H.; Wang, B.; Liu, M.L.; Wang, B.; Shen, W.Y.; Guo, H.Y.; Lu, Y. Design, synthesis and antimycobacterial activity of novel imidazo[1,2-a]pyridine-3-carboxamide derivatives. Eur. J. Med. Chem. 2017, 137, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Fortin, S.; Wei, L.H.; Moreau, E.; Lacroix, J.; Côté, M.; Petitclerc, É.; Kotra, L.P.; Gaudreault, R.C. Substituted phenyl 4-(2-oxoimidazolidin-1-yl)benzenesulfonamides as antimitotics. Antiproli ferative, antiangiogenic and antitumoral activity, and quantitative structure-activity relationships. Eur. J. Med. Chem. 2011, 46, 5327–5342. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, 64, 112–122. [Google Scholar]
- Deng, X.L.; Zhou, X.M.; Wang, Z.Y.; Rui, C.H.; Yang, X.L. Synthesis, crystal structure and insecticidal activity of N-(pyridin-2-ylmethyl)-1-phenyl-1,4,5,6,7,8-hexahydrocyclohepta[c]pyrazole-3-carbox amide. Chin. J. Struct. Chem. 2018, 37, 551–556. [Google Scholar]
- Zheng, W.N.; Zhu, Z.Y.; Deng, Y.N.; Wu, Z.C.; Zhou, Y.; Zhou, X.M.; Bai, L.Y.; Deng, X.L. Synthesis, crystal structure, herbicide safening, and antifungal activity of N-(4,6-dichloropyrimidine-2-yl)benzamide. Crystals 2018, 8, 75. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.B.; Lei, P.; Sun, T.; Jin, X.; Yang, X.L.; Ling, Y. Design, synthesis, and fungicidal activity of novel thiosemicarbazide derivatives containing piperidine fragments. Molecules 2017, 22, 2085. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Huang, C.Y.; Olieric, V.; Ma, P.; Howe, N.; Vogeley, L.; Liu, X.; Warshamanage, R.; Weinert, T.; Panepucci, E.; Kobilka, B.; et al. In meso in situ serial X-ray crystallography of soluble and membrane proteins at cryogenic temperatures. Acta Crystallogr. D Struct. Biol. 2016, 72, 93–112. [Google Scholar] [CrossRef] [Green Version]
- Webb, B.; Sali, A. Comparative protein structure modeling using modeler. Curr. Protoc. Bioinform. 2016, 54, 5.6.1–5.6.32. [Google Scholar] [CrossRef] [Green Version]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Olyaei, A.; Abbasi, A.; Ghandi, M.; Salimi, F.; Eriksson, L. 1-(1,3-benzothiazol-2-yl)-4,5-dihydroxy-3- phenylimidazolidin-2-one. Acta Crystallogr. Sect. E Struct. Rep. Online 2006, 62, O5326–O5327. [Google Scholar] [CrossRef] [Green Version]
- Treweeke, N.R.; Hitchcock, P.B.; Pardoe, D.A.; Caddick, S. Controlling diastereoselectivity in the reactions of enantiomerically pure α-bromoacyl-imidazolidinones with nitrogen nucleophiles: Substitution reactions with retention or inversion of configuration. Chem. Commun. 2005, 1868–1870. [Google Scholar] [CrossRef] [PubMed]
- Siegler, M.; Long, S. (4R,5R)-4,5-diphenylimidazolidin-2-one. Acta Crystallogr. Sect. E Struct Rep Online 2006, 62, O5310–O5311. [Google Scholar] [CrossRef]
- Shen, Z.H.; Shi, Y.X.; Yang, M.Y.; Sun, Z.H.; Weng, J.Q.; Tan, C.X.; Liu, X.H.; Li, B.J.; Zhao, W.G. Synthesis, crystal structure, DFT studies and biological activity of a novel schiff base containing triazolo 4,3-a pyridine moiety. Chin. J. Struct. Chem. 2016, 35, 457–464. [Google Scholar]
- Löser, R.; Pitzschler, R.; Köckerling, M. Synthesis and X-ray crystal structure of N’-cyano-N,N’–dimethyl -4-nitrobenzohydrazide. Crystals 2017, 7, 290. [Google Scholar] [CrossRef]
- Liu, X.H.; Wang, Q.; Sun, Z.H.; Wedge, D.E.; Becnel, J.J.; Estep, A.S.; Tan, C.X.; Weng, J.Q. Synthesis and insecticidal activity of novel pyrimidine derivatives containing urea pharmacophore against Aedes aegypti. Pest Manag. Sci. 2017, 73, 953–959. [Google Scholar] [CrossRef]
- Kuruvilla, T.K.; Muthu, S.; Prasana, J.C.; George, J.; Saji, R.S.; Geoffrey, B.; David, R.H.A. Molecular docking, spectroscopic studies on 4-[2-(dipropylamino)ethyl]-1,3-dihydro-2H-indol-2-one and QSAR study of a group of dopamine agonists by density functional method. Spectrochim. Acta A 2018, 222, 117185. [Google Scholar] [CrossRef]
- Deng, X.L.; Xie, J.; Li, Y.Q.; Yuan, D.K.; Zhang, X.P.; Wang, Q.M.; Chi, M.; Yang, X.L. Design, synthesis and biological activity of novel substituted pyrazole amide derivatives targeting EcR/USP receptor. Chin. Chem. Lett. 2016, 27, 566–570. [Google Scholar] [CrossRef]
- Deng, X.L.; Zhang, L.; Hu, X.P.; Yin, B.; Liang, P.; Yang, X.L. Target-based design, synthesis and biological activity of new pyrazole amide derivatives. Chin. Chem. Lett. 2016, 27, 251–255. [Google Scholar] [CrossRef]
- Hirashima, A.; Huang, H.W. Homology modeling, agonist binding site identification, and docking in octopamine receptor of Periplaneta americana. Comput. Biol. Chem. 2008, 32, 185–190. [Google Scholar] [CrossRef]
- Chen, X.; Ohta, H.; Sasaki, K.; Ozoe, F.; Ozoe, Y. Amino acid residues involved in the interaction with the intrinsic agonist (R)-octopamine in the β-adrenergic-like octopamine receptor from the silkworm Bombyx mori. J. Pestic. Sci. 2011, 36, 473. [Google Scholar] [CrossRef] [Green Version]



| Compound | 3c |
|---|---|
| CCDC No. | 1878063 |
| Empirical formula | C11H14N2O |
| Formula weight | 190.24 |
| Temperature/K | 100.00(10) |
| Crystal system | monoclinic |
| Space group | C2/c |
| a/[Å] | 37.331(3) |
| b/[Å] | 5.5684(3) |
| c/[Å] | 9.3753(7) |
| α/° | 90 |
| β/° | 91.350(6) |
| γ/° | 90 |
| Volume/Å3 | 1948.4(2) |
| Z | 8 |
| Dc g/cm3 | 1.297 |
| μ/mm−1 | 0.085 |
| F(000) | 816.0 |
| Crystal size/mm3 | 0.13 × 0.11 × 0.09 |
| Radiation | MoKα (λ = 0.71073) |
| 2Θ range for data collection/° | 4.366 to 58.916 |
| Index ranges | −41 ≤ h ≤ 49, −7 ≤ k ≤ 7, −9 ≤ l ≤ 12 |
| Reflections collected | 7305 |
| Independent reflections | 2377 [Rint = 0.0286, Rsigma = 0.0311] |
| Data/restraints/parameters | 2377/0/128 |
| Goodness-of-fit on F2 | 1.044 |
| Final R indexes [I > = 2σ (I)] | R1 = 0.0469, wR2 = 0.1192 |
| Final R indexes [all data] | R1 = 0.0542, wR2 = 0.1255 |
| Largest diff. peak/hole/e Å−3 | 0.26/−0.42 |
| Bond | d [Å] | Bond | d [Å] |
|---|---|---|---|
| O(1)–C(9) | 1.232(2) | N(1)–C(7) | 1.467(2) |
| N(1)–C(9) | 1.384(2) | N(2)–C(8) | 1.454(2) |
| N(1)–C(1) | 1.408(2) | C(1)–C(2) | 1.404(2) |
| C(1)–C(6) | 1.399(2) | C(4)–C(5) | 1.392(2) |
| C(2)–C(3) | 1.386(2) | C(3)–C(4) | 1.394(2) |
| Angle | (°) | Angle | (°) |
| C(9)–N(1)–C(1) | 126.9(1) | N(2)–C(9)–N(1) | 108.2(1) |
| C(9)–N(1)–C(7) | 110.6(1) | C(2)–C(1)–N(1) | 122.6(1) |
| C(1)–N(1)–C(7) | 121.3(1) | C(6)–C(1)–N(1) | 119.0(1) |
| C(9)–N(2)–C(8) | 112.9(1) | N(1)–C(7)–C(8) | 103.3(1) |
| O(1)–C(9)–N(1) | 126.0(1) | C(5)–C(4)–C(3) | 117.0(1) |
| O(1)–C(9)–N(2) | 125.8(1) | N(2)–C(8)–C(7) | 102.0(1) |
| D–H···A | d(D–H)/(Å) | d(H···A)/(Å) | d(D···A)/(Å) | <(DHA)/(°) |
|---|---|---|---|---|
| N(2)–H(2)···O(1) #1 | 0.86 | 2.08 | 2.885(2) | 155 |
| C(8)–H(8B)···O(1) #2 | 0.97 | 2.56 | 3.358(2) | 139 |
| C(2)–H(2A)···O(1) | 0.93 | 2.26 | 2.871(2) | 123 |
| Compd. | Mortality (%) 600 mg/L | Inhibitory Rate (%) 50 mg/L | ΔE Value (ev) | |||
|---|---|---|---|---|---|---|
| P. xylostella | P. capsici | P. sojae | P. litchi | P. infestans | ||
| 3a | 0 | 77.5 | 71.1 | 38.2 | 82.1 | 0.2065 |
| 3b | 16.7 | 71.3 | 53.7 | 27.7 | 55.1 | 0.2010 |
| 3c | 20.0 | 59.6 | 26.2 | 4.9 | 49.2 | 0.1996 |
| 3d | 40.0 | 93.7 | 84.5 | 46.5 | 85.9 | 0.1894 |
| 3e | 13.3 | 60.7 | 37.3 | 19.9 | 61.6 | 0.1894 |
| 3f | 86.7 | 59.6 | 30.9 | 8.8 | 39.7 | 0.1946 |
| 3g | 30.0 | 64.1 | 34.0 | 5.4 | 40.4 | 0.1993 |
| 3h | 60.0 | 90.9 | 83.2 | 49.2 | 89.6 | 0.1965 |
| DPMF | 23.0 | - | - | - | - | 0.2007 |
| cyazofamid | - | 100 | 100 | 95 | 100 | 0.1701 |
| Compound | Docking Score | Mortality (%) |
|---|---|---|
| 3a | −8.162 | 0 |
| 3b | −7.314 | 16.7 |
| 3c | −7.350 | 20.0 |
| 3d | −7.713 | 40.0 |
| 3e | −7.231 | 13.3 |
| 3f | −7.576 | 86.7 |
| 3g | −7.763 | 30.0 |
| 3h | −7.912 | 60.0 |
| DPMF | −6.214 | 23.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, X.; Jin, C.; Xie, Y.; Gao, J.; Zhou, X. Design, Synthesis, and Evaluation of X-ray Crystal Structure, Biological Activities, DFT Calculations, and Molecular Docking of Phenyl Imidazolidin-2-One Derivatives. Crystals 2020, 10, 713. https://doi.org/10.3390/cryst10080713
Deng X, Jin C, Xie Y, Gao J, Zhou X. Design, Synthesis, and Evaluation of X-ray Crystal Structure, Biological Activities, DFT Calculations, and Molecular Docking of Phenyl Imidazolidin-2-One Derivatives. Crystals. 2020; 10(8):713. https://doi.org/10.3390/cryst10080713
Chicago/Turabian StyleDeng, Xile, Can Jin, Yong Xie, Junbo Gao, and Xiaomao Zhou. 2020. "Design, Synthesis, and Evaluation of X-ray Crystal Structure, Biological Activities, DFT Calculations, and Molecular Docking of Phenyl Imidazolidin-2-One Derivatives" Crystals 10, no. 8: 713. https://doi.org/10.3390/cryst10080713