Synthesis of Two Novel Azilsartan Cocrystals: Preparation, Physicochemical Characterization and Solubility Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and General Methods
2.2. Synthesis of the AZ-BIP Cocrystal (2:1)
2.3. Synthesis of the AZ-BPE Cocrystal (2:1)
2.4. Solubility and Powder Dissolution Measurement
3. Results and Discussion
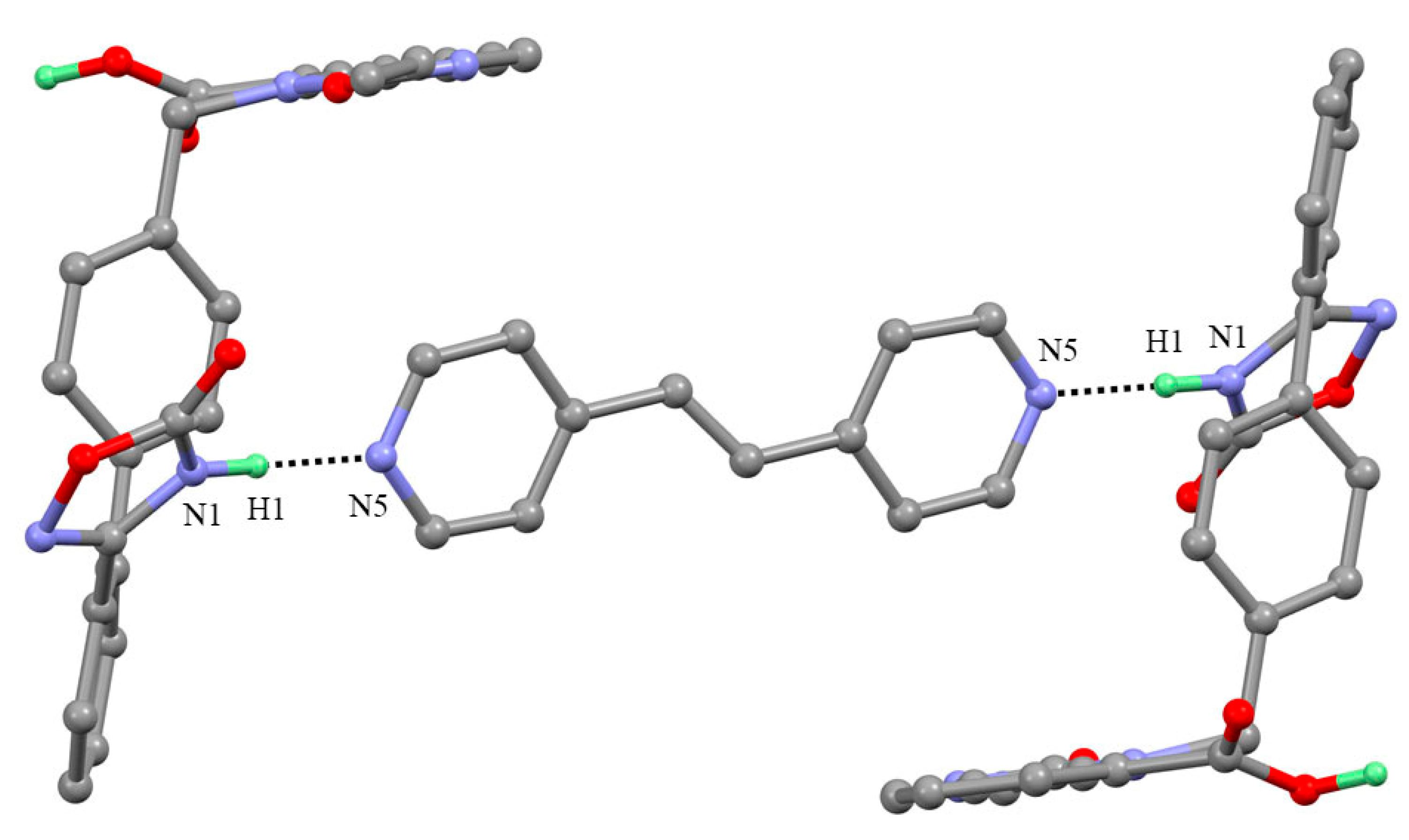
3.1. Crystal Structure Analysis
3.1.1. AZ-BIP Cocrystal (2:1)
3.1.2. AZ-BPE Cocrystal (2:1)
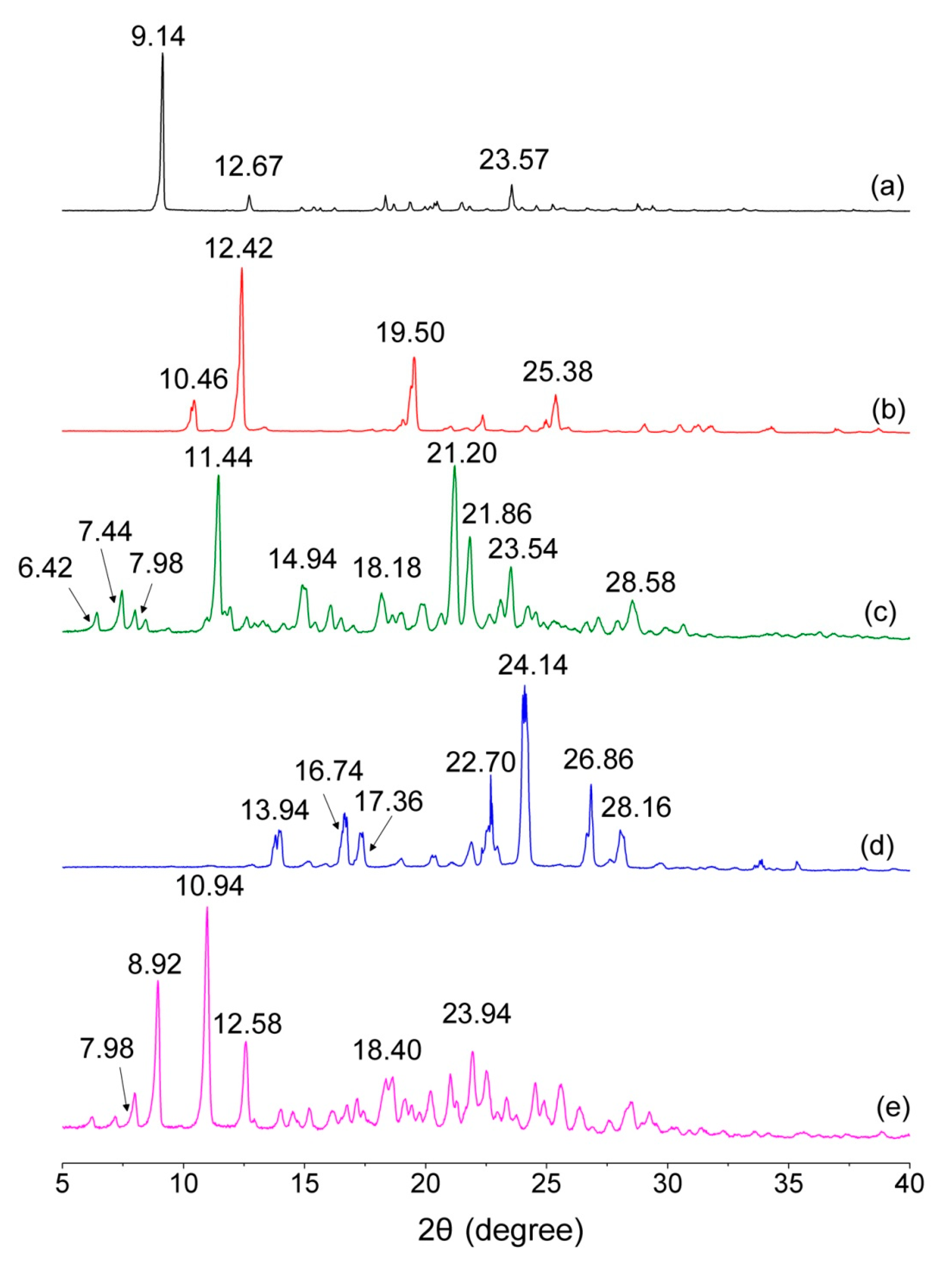
3.2. PXRD Analysis
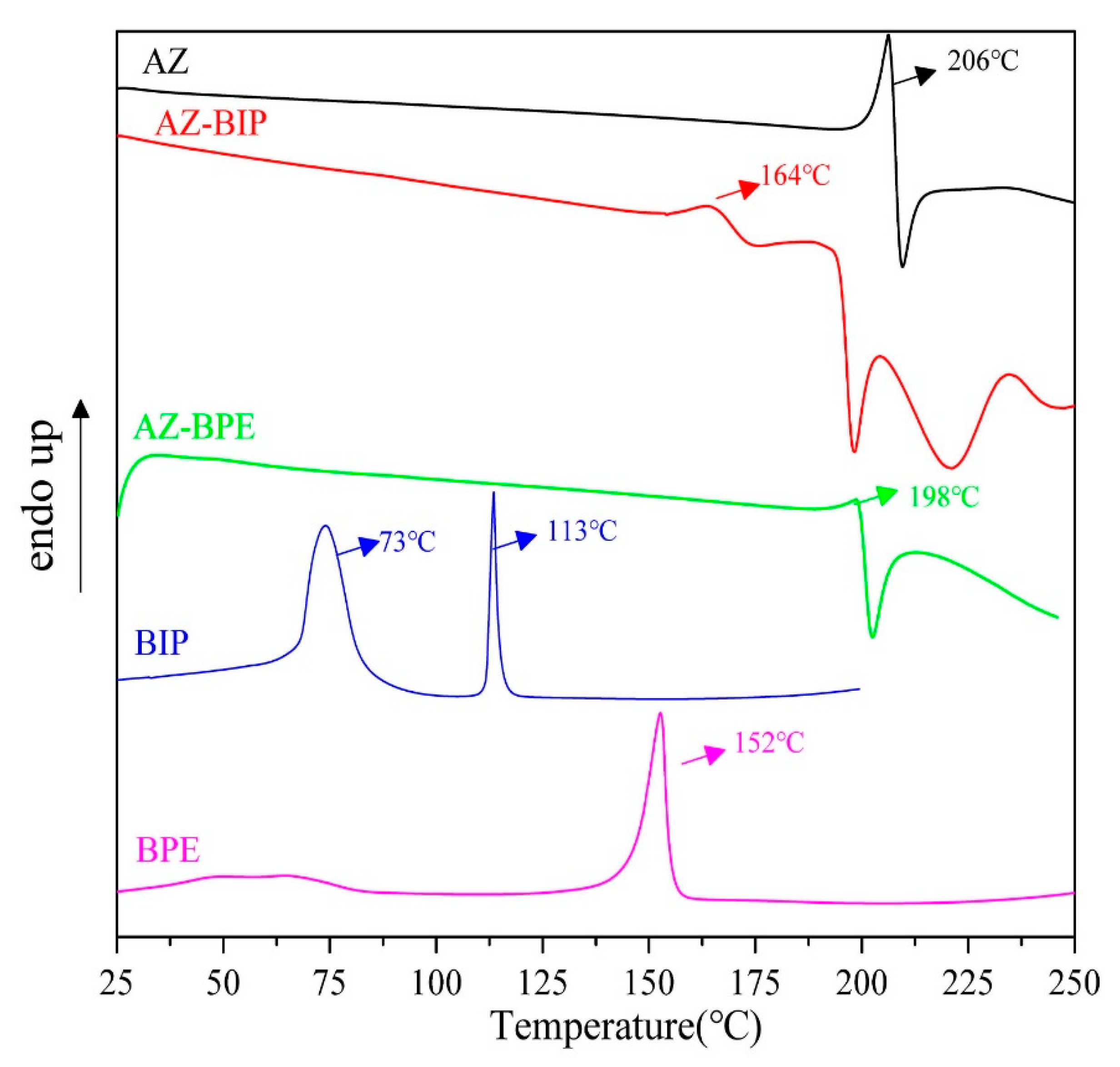
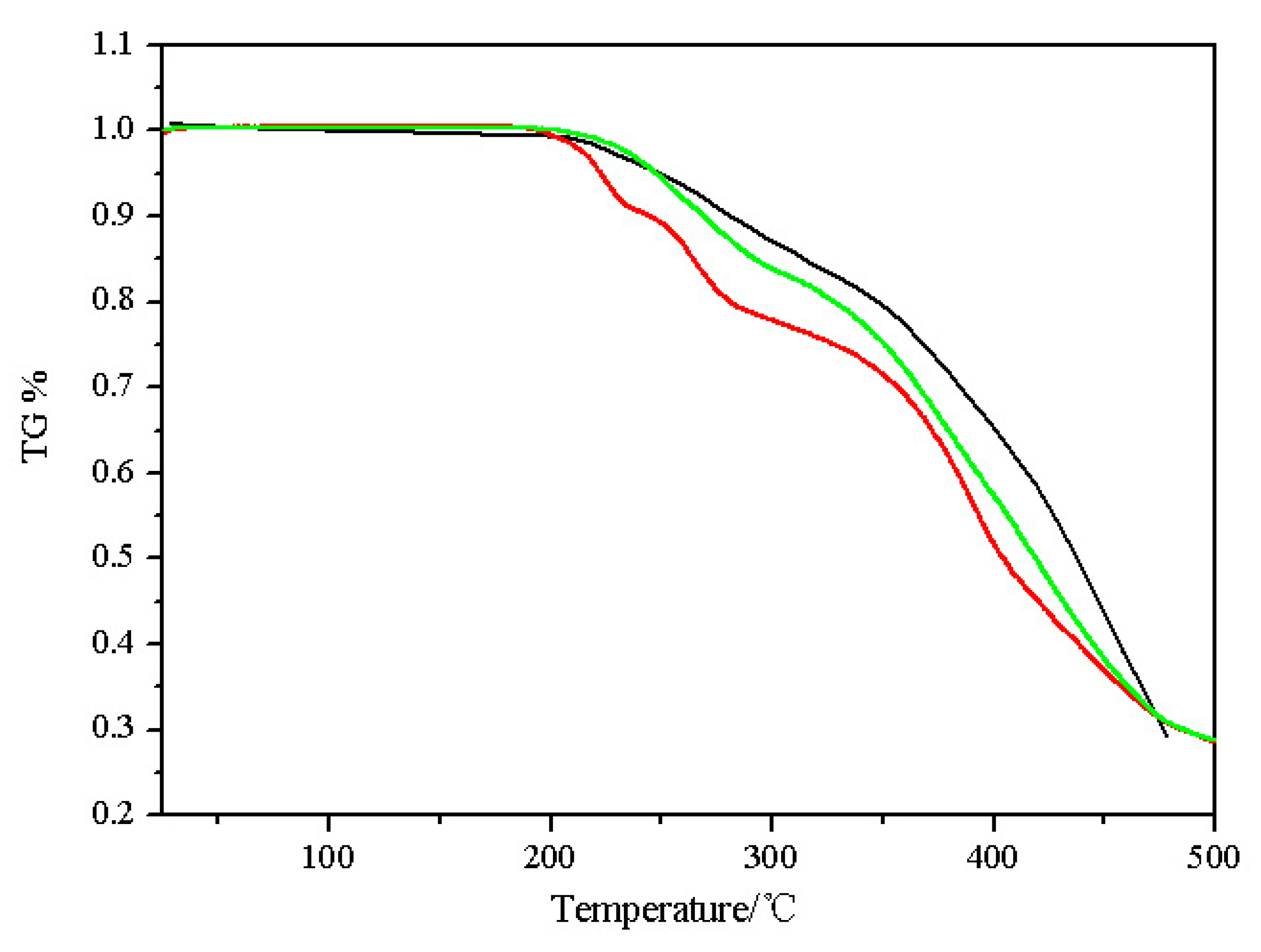
3.3. DSC and TGA Analysis
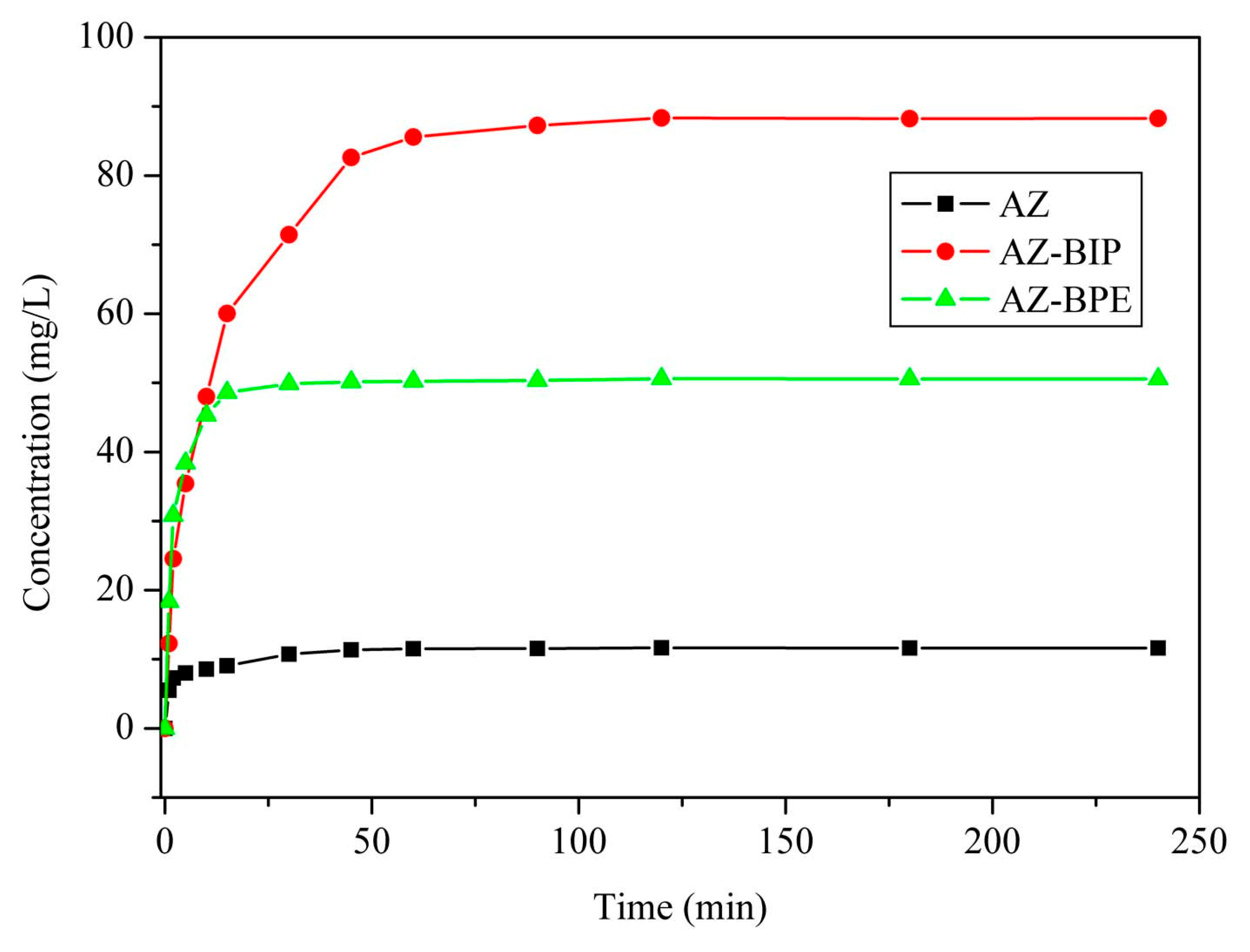
3.4. Solubility and Powder Dissolution Rate Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gao, L.; Zhang, X.R.; Yang, S.P.; Liu, J.J.; Chen, C.J. Improved solubility of vortioxetine using C2-C4 straight-chain dicarboxylic acid salt hydrates. Crystals 2018, 8, 352. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Zhang, X.R.; Chen, Y.F.; Liao, Z.L.; Wang, Y.Q.; Zou, X.Y. A new febuxostat imidazolium salt hydrate: Synthesis, crystal structure, solubility, and dissolution study. J. Mol. Struct. 2019, 1176, 633–640. [Google Scholar] [CrossRef]
- Mittapali, S.; Mannava, M.C.; Khandavilli, U.R.; Allu, S.; Nangia, A. Soluble salts and cocrystals of clotrimazole. Cryst. Growth Des. 2015, 15, 2493–2504. [Google Scholar] [CrossRef]
- Yadav, J.A.; Khomane, K.S.; Modi, S.R.; Ugale, B.; Yadav, R.N.; Nagaraja, C.M.; Kumar, N.; Bansal, A.K. Correlating single crystal structure, nanomechanical, and bulk compaction behavior of febuxostat polymorphs. J. Mol. Pharm. 2017, 14, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Suresh, K.; Nangia, A. Lornoxicam salts: Crystal structures, conformations, and solubility. Cryst. Growth Des. 2014, 14, 2945–2953. [Google Scholar] [CrossRef]
- Bolla, G.; Sanphui, P.; Nangia, A. Solubility advantage of tenoxicam phenolic cocrystals compared to salts. Cryst. Growth Des. 2013, 13, 1988–2003. [Google Scholar] [CrossRef]
- Sanphui, P.; Bolla, G.; Nangia, A.; Chernyshev, V. A cemetacin cocrystals and salts: Structure solution from powder X-ray data and form selection of the piperazine salt. IUCrJ 2014, 1, 136–150. [Google Scholar] [CrossRef] [Green Version]
- Maddileti, D.; Jayabun, S.K.; Nangia, A. Soluble cocrystals of the xanthine oxidase inhibitor febuxostat. Cryst. Growth Des. 2013, 13, 3188–3196. [Google Scholar] [CrossRef]
- Fala, L. Entresto (Sacubitril/Valsartan): First-in-class angiotensin receptor neprilysin inhibitor FDA approved for patients with heart failure. Am. Health Drug Benef. 2015, 8, 330. [Google Scholar]
- Scollo-Lavizzari, G.; Corbat, F.A. Clinical note on a new antiepileptic, ‘Depakine®’. Eur. Neurol. 1970, 4, 312–317. [Google Scholar] [CrossRef]
- Gascom, N.; Almansa, C.; Merlos, M.; Miguel Vela, J.; Encina, G.; Morte, A.; Smith, K.; Plata-Salaman, C. Co-crystals of tramadol-celecoxib: Preclinical and clinical evaluation of a novel analgesic. Expert Opin. Investig. Drugs 2019, 28, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Walsh, R.B.; Bradner, M.W.; Fleischman, S.; Morales, L.A.; Moulton, B.; Rodriguez-Hornedo, N.; Zaworotko, M.J. Crystal engineering of the composition of pharmaceutical phases. Chem. Commun. 2003, 2, 186–187. [Google Scholar] [CrossRef] [PubMed]
- Baker, W.L.; White, W.B. Azilsartan medoxomil: A new angiotensin II receptor antagonist for treatment of hypertension. Ann. Pharmacother. 2011, 45, 1506–1515. [Google Scholar] [CrossRef] [PubMed]
- French, C.J.; Zaman, A.T.; Sobel, B.E. The angiotensin receptor blocker, azilsartan medoxomil (TAK-491), suppresses vascular wall expression of plasminogen activator inhibitor typr-I protein protentially facilitating the stabilization of atherosclerotic plaques. J. Cardiovasc. Pharm. 2011, 58, 143–148. [Google Scholar] [CrossRef]
- Zhang, X.R.; Zhang, L. Solvent effect on the self-assembly of salt solvates of an antihypertensive drug azilsartan and 2-methylimidazole. J. Mol. Pharm. 2017, 1137, 320–327. [Google Scholar] [CrossRef]
- Su, X.; Zhang, Y.; Yin, H.; Liu, L.; Zhang, Y.; Wu, L.; Zhang, Q.; Wang, C.; Zhang, L.; Zhang, Y.; et al. Preparation of a 1:1.5 cocrystal of kaempferol with 4,4′-bipyridine based on analyzing intermolecular interaction of building units. J. Mol. Pharm. 2019, 1177, 107–116. [Google Scholar] [CrossRef]
- Du, S.; Wang, Y.; Wu, S.; Yu, B.; Shi, P.; Bian, L.; Zhang, D.; Hou, J.; Wang, J.; Gong, J. Two novel cocrystals of lamotrigine with isomeric bipyridines and in situ monitoring of the cocrystallization. Eur. J. Pharm. Sci. 2017, 110, 19–25. [Google Scholar] [CrossRef]
- Srivastava, K.; Khan, E.; Shimpi, M.R.; Tandon, P.; Sinha, K.; Velaga, S.P. Molecular structure and hydrogen bond interactions of a paracetamol-4,4′-bipyridine cocrystal studied using a vibrational spectroscopic and quantum chemical approach. CrystEngComm 2018, 20, 213–222. [Google Scholar] [CrossRef]
- Du, J.J.; Stanton, S.A.; Williams, P.A.; Ong, J.A.; Groundwater, P.W.; Overgaard, J.; Platts, J.A.; Hibbs, D.E. Using electron density to predict synthon formation in a 4-hydroxybenzoic acid: 4,4′-bipyridine cocrystal. Cryst. Growth Des. 2018, 18, 1786–1798. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; Lai, F.; Varadi, L.; Williams, P.; Groundwater, P.; Platts, J.A.; Hibbs, D.E.; Overgaard, J. Monoclinic paracetamol vs. paracetamol-4,4′-bipyridine co-crystal; What is the difference? A charge density study. Crystals 2018, 8, 46. [Google Scholar] [CrossRef] [Green Version]
- Nath, N.K.; Hazarika, M.; Gupta, P.; Ray, N.R.; Paul, A.K.; Nauha, E. Plastically bendable crystals of probenecid and its cocrystal with 4,4′-bipyridine. J. Mol. Struct. 2018, 1160, 20–25. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Yin, H.M.; Zhang, Y.; Zhang, D.J.; Su, X.; Kuang, H.X. Cocrystals of kaempferol, quercetin and myricetin with 4,4′-bipyridine: Crystal structures, analyses of intermolecular interactions and antibacterial properties. J. Mol. Struct. 2017, 1130, 199–207. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXL-97; PC version; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. SHELXTL; version 5.1; Bruker Analytical X-ray Instruments Inc.: Madison, WI, USA, 1998. [Google Scholar]
- Molecular Operating Environment (MOE); 2010.10; ChemicalComputing Group Inc.: Montreal, QC, Canada, 2012. Available online: https://www.chemcomp.com/ (accessed on 20 August 2020).
- Corbeil, C.R.; Williams, C.I.; Labute, P. Variability in docking success rates due to dataset preparation. J. Comput. Aided Mol. Des. 2012, 26, 775–786. [Google Scholar] [CrossRef] [Green Version]
- Guidelines for Industry: Regulatory Classification of Pharmaceutical Co-Crystals. U.S. Food and Drug Administration. Available online: http://www.fda.gov/downloads/Drugs/Guidances/UCM281764.pdf/ (accessed on 16 February 2014).
- Cruz-Cabeza, A. Acid–base crystalline complexes and the pKa rule. CrystEngComm 2012, 14, 6362–6365. [Google Scholar] [CrossRef]
- Aakeroy, C.B.; Fasulo, M.E.; Desper, J. Cocrystals or salts: Does it really matter? Mol. Pharm. 2007, 4, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Unal, H.; Gati, C.; Han, G.W.; Liu, W.; Zatsepin, N.A.; James, D.; Wang, D.; Xu, Q.; White, T.A.; et al. Structure of the Angiotensin receptor revealed by serial femtosecond crystallography. Cell 2015, 161, 833–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| AZ-BIP | AZ-BPE | |
|---|---|---|
| Empirical formula | C30H24N5O5 | C31H25N5O5 |
| Formula weight | 534.54 | 547.56 |
| Wavelength (A) | 0.71073 | 0.71073 |
| Crystal system | Monoclinic | Triclinic |
| Space group | C2/c | P-1 |
| a (Å) | 28.9266(17) | 7.8907(4) |
| b (Å) | 7.8175(5) | 12.6200(11) |
| c (Å) | 24.9867(16) | 14.3849(10) |
| α (°) | 90 | 72.206(7) |
| β (°) | 110.254(7) | 86.666(5) |
| γ (°) | 90 | 82.996(5) |
| V (Å3) | 5301.0(6) | 1353.42(17) |
| Z | 8 | 2 |
| T/K | 293(2) | 293(2) |
| Density (g/cm3) | 1.340 | 1.344 |
| μ (mm−1) | 0.094 | 0.093 |
| Parameters | 379 | 379 |
| F(000) | 2232 | 572 |
| Goodness-of-fit on F2 | 1.087 | 1.016 |
| reflns. Collected | 4672 | 4758 |
| unique reflns | 2950 | 3529 |
| Final R indices [I > 2σ(I)] | R1 = 0.0706 ωR2 = 0.1703 | R1 = 0.0451 ωR2 = 0.0979 |
| Δρmax/Δρmin (e Å−3) | 0.424/−0.377 | 0.346/−0.294 |
| CCDC | 1,899,062 | 1,899,063 |
| Interaction | d(D-H) | d(H⋯A) | d(D⋯A) | <(DHA) | Symmetry Code | |
|---|---|---|---|---|---|---|
| AZ-BIP | N1-H1⋯N5 | 0.90 | 1.88 | 2.774(4) | 172 | x, y − 1, z |
| O4-H2⋯N4 | 0.91 | 1.75 | 2.650(4) | 171 | x, y − 1, z | |
| AZ-BPE | N1-H1⋯N5 | 0.96 | 1.78 | 2.741(3) | 175 | x, y, z |
| O5-H2⋯N4 | 1.01 | 1.70 | 2.705(3) | 169 | X + 1, y, z |
| Compound | pKa | ΔpKa | Cocrystal/Salt |
|---|---|---|---|
| AZ | 3.51 | - | - |
| BIP | 5.25 | 1.74 | cocrystal |
| BPE | 4.99 | 1.48 | cocrystal |
| Bond | Bond | Distance | Angle | (°) |
|---|---|---|---|---|
| AZ-BIP | C25-O4 | 1.244(5) | C1-N1-C2 | 107.7(3) |
| C25-O5 (average value) | 1.384(5) | C26-N5-C30 | 114.2(4) | |
| AZ-BPE | C25-O4 | 1.204(2) | C1-N1-C2 | 108.03(19) |
| C25-O5 | 1.308(2) | C26-N5-C30 | 116.2(2) |
| A | B | D | E | τ1 | |
|---|---|---|---|---|---|
| optimal conformer | 78.8 | 79.7 | 61.7 | 122.9 | 0.9 |
| AZ | 114.1 | 111.6 | 60 | 103.3 | 167.0 |
| AZ-BIP | 75.3 | 76.3 | 51.8 | 56.7 | 60.4 |
| AZ-BPE | 85.9 | 86.9 | 48.0 | 47.2 | 23.3 |
| Sample | Solubility of AZ and Its Cocrystals (mg/L) |
|---|---|
| Azilsartan | 11.6 |
| AZ-BIP cocrystal | 88.3 |
| AZ-BPE cocrystal | 50.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, L.; Zhang, X.-R. Synthesis of Two Novel Azilsartan Cocrystals: Preparation, Physicochemical Characterization and Solubility Studies. Crystals 2020, 10, 739. https://doi.org/10.3390/cryst10090739
Gao L, Zhang X-R. Synthesis of Two Novel Azilsartan Cocrystals: Preparation, Physicochemical Characterization and Solubility Studies. Crystals. 2020; 10(9):739. https://doi.org/10.3390/cryst10090739
Chicago/Turabian StyleGao, Lei, and Xian-Rui Zhang. 2020. "Synthesis of Two Novel Azilsartan Cocrystals: Preparation, Physicochemical Characterization and Solubility Studies" Crystals 10, no. 9: 739. https://doi.org/10.3390/cryst10090739




