Efficient Synthesis and X-ray Structure of [1,2,4]Triazolo[4,3-a]pyridines via Oxidative Cyclization Using N-Chlorosuccinimide (NCS)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Physical Measurements
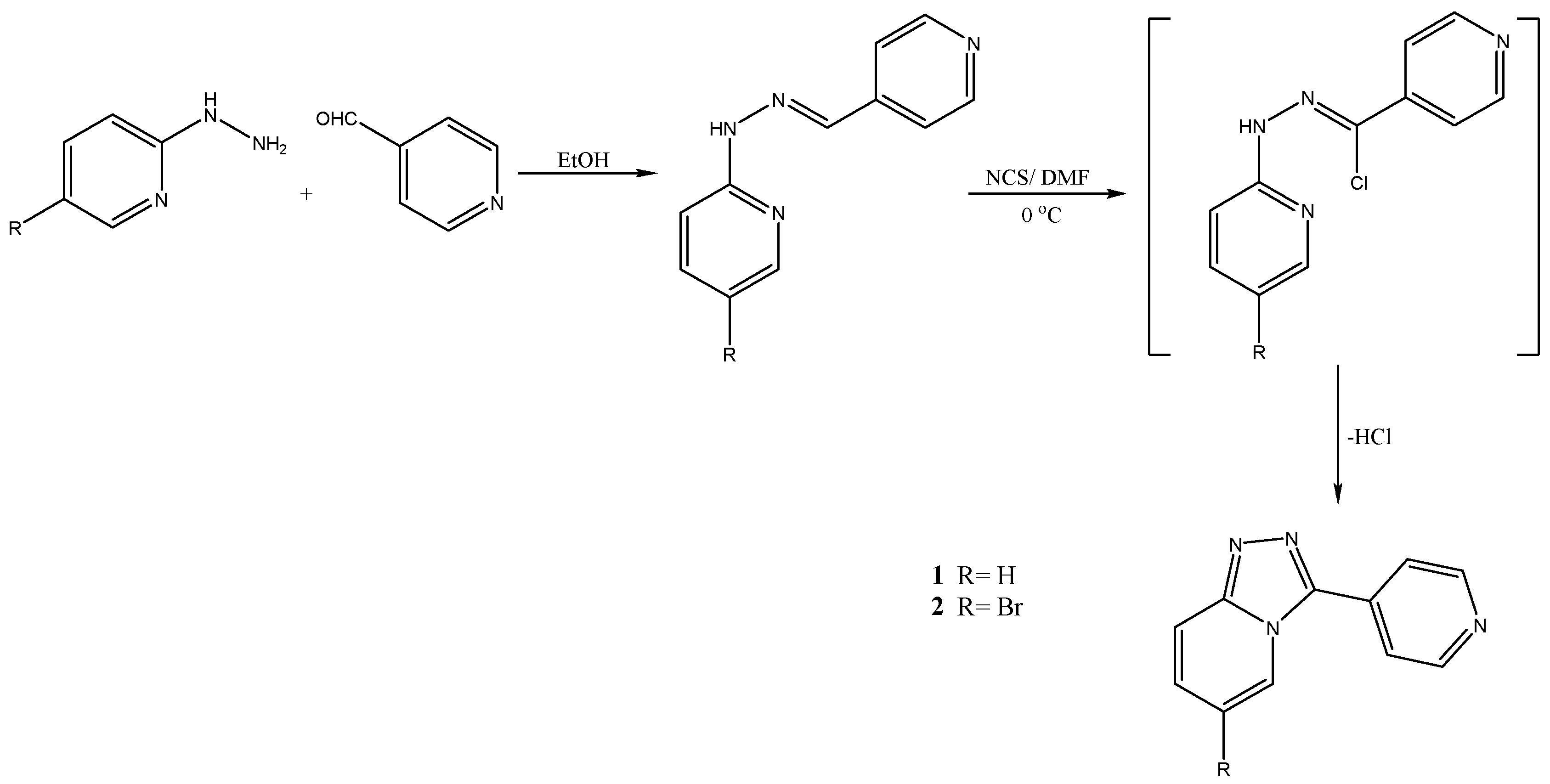
2.2. Chemistry
2.2.1. General Procedure for Synthesis of Hydrazones
2.2.2. General Procedure for Synthesis of [1,2,4]Triazolo[4,3-a]pyridines Derivatives
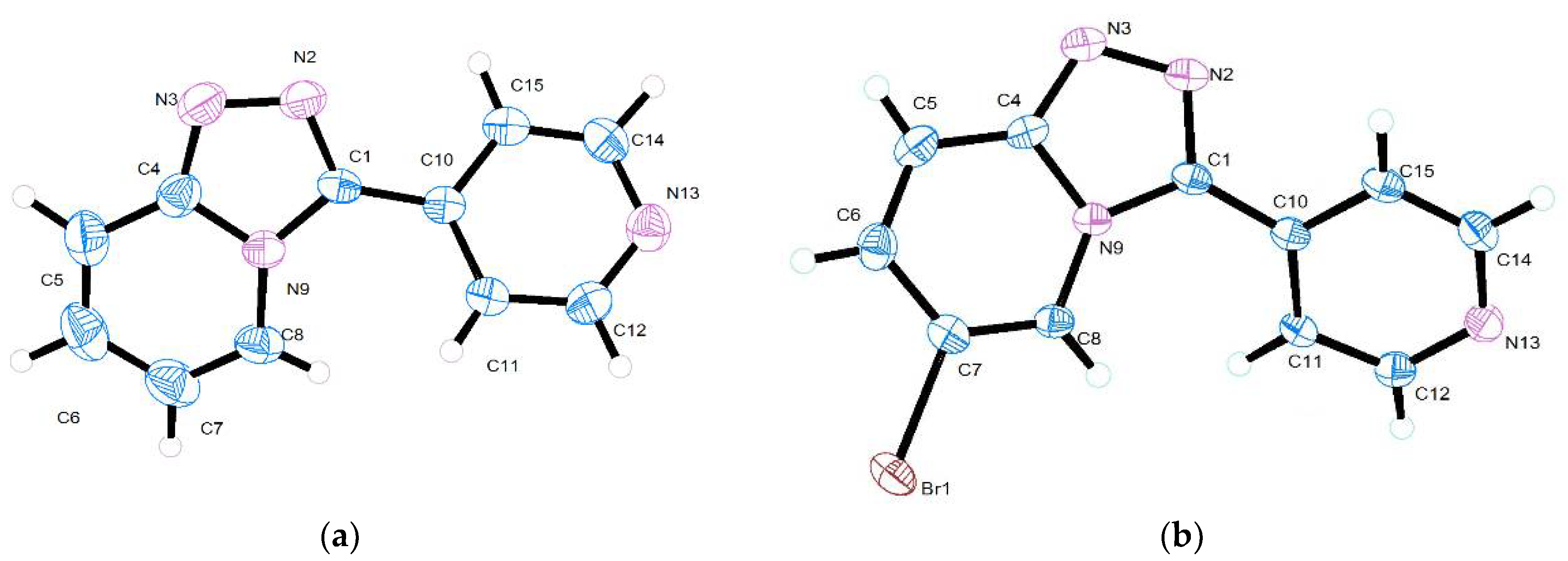
2.3. Crystal Structural Determination
3. Results and Discussion
3.1. Chemistry
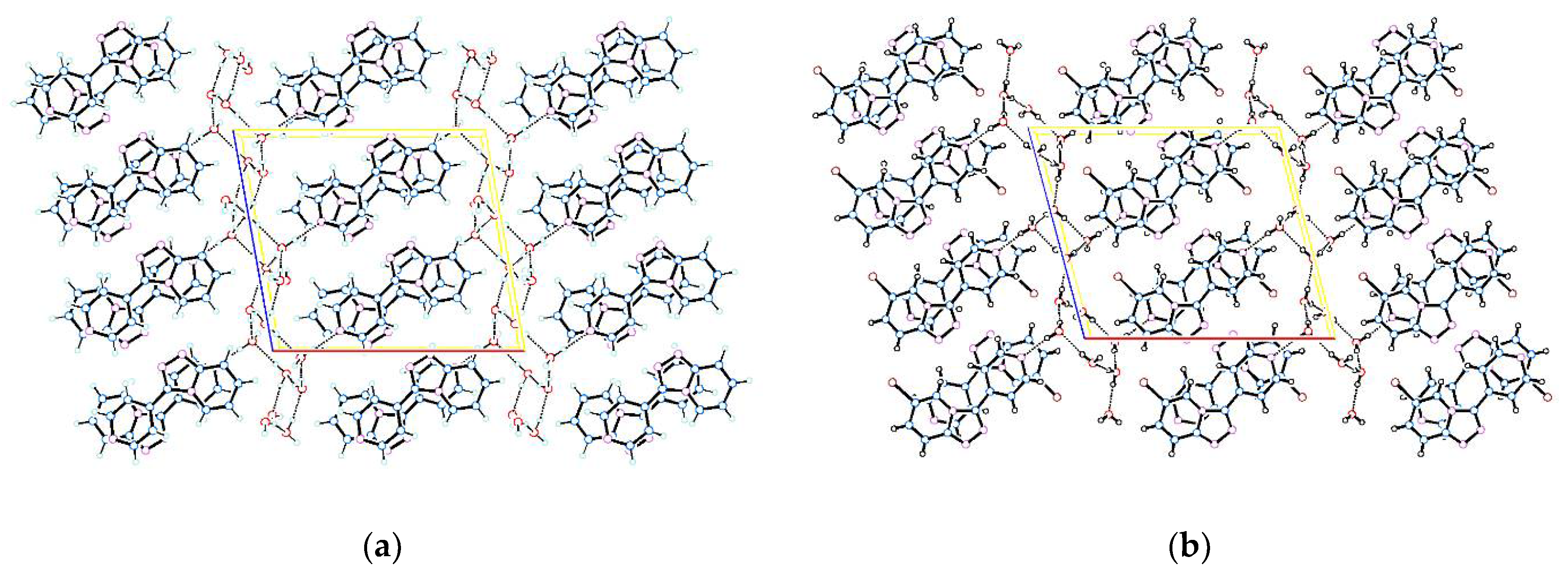
3.2. Crystal Structure and Formation of Hydrogen Bond
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sadana, A.K.; Mirza, Y.; Aneja, K.R.; Prakash, O. Hypervalent iodine mediated synthesis of 1-aryl/hetryl-1,2,4-triazolo[4,3-a] pyridines and 1-aryl/hetryl 5-methyl-1,2,4-triazolo[4,3-a]quinolines as antibacterial agents. Eur. J. Med. Chem. 2003, 38, 533–536. [Google Scholar] [CrossRef]
- Prakash, O.; Hussain, K.; Aneja, D.K.; Sharma, C.; Aneja, K.R. A facile iodine(III)-mediated synthesis of 3-(3-aryl-1-phenyl-1H-pyrazol-4-yl)-[1,2,4]triazolo[4,3-a]pyridines via oxidation of 2-((3-aryl-1-phenyl-1H-pyrazol-4-yl)methylene)-1-(pyridin-2-yl)hydrazines and their antimicrobial evaluations. Org. Med. Chem. Lett. 2011, 1, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cid-Núñez, J.M.; Trabanco-Suárez, A.A.; Lavreysen, H.; Ceusters, M. 1,2,4-triazolo[4,3-a]pyridine Compounds and Their Use as Positive Allosteric Modulators of mGluR2 Receptors. WO 2015032790 A1, 12 March 2015. [Google Scholar]
- Liu, X.H.; Xu, X.Y.; Tan, C.X.; Weng, J.Q.; Xin, J.H.; Chen, J. Synthesis, crystal structure, herbicidal activities and 3D-QSAR study of some novel 1,2,4-triazolo[4,3-a]pyridine derivatives. Pest Manag. Sci. 2015, 71, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Maehata, R.; Shimomura, M. Preparation of Fused Heterocyclic Compounds as Pesticides against Harmful Arthropods. WO 2018139436 A1, 2 August 2018. [Google Scholar]
- Kalgutkar, A.S.; Hatch, H.L.; Kosea, F.; Nguyen, H.T.; Choo, E.F.; McClure, K.F.; Taylor, T.J.; Henne, K.R.; Kuperman, A.V.; Dombroski, M.A.; et al. Preclinical pharmacokinetics and metabolism of 6-(4-(2,5-difluorophenyl)oxazol-5-yl)-3-isopropyl-[1,2,4]-triazolo[4,3-a]pyridine, a novel and selective p38α inhibitor: Identification of an active metabolite in preclinical species and human liver microsomes. Biopharm. Drug Dispos. 2006, 27, 371–386. [Google Scholar] [CrossRef] [PubMed]
- McClure, K.F.; Abramov, Y.A.; Laird, E.R.; Barberia, J.T.; Cai, W.; Carty, T.J.; Cotina, S.R.; Danley, D.E.; Dipesa, A.J.; Donahue, K.M.; et al. Theoretical and Experimental Design of Atypical Kinase Inhibitors: Application to p38 MAP Kinase. J. Med. Chem. 2005, 48, 5728–5737. [Google Scholar] [CrossRef]
- Bektas, H.; Karaali, N.; Sahin, D.; Demirbas, A.; Karaoglu, S.A.; Demirbas, N. Synthesis and Antimicrobial Activities of Some New 1,2,4-Triazole Derivatives. Molecules 2010, 15, 2427–2438. [Google Scholar] [CrossRef] [Green Version]
- Lawson, E.C.; Hoekstra, W.J.; Addo, M.F.; Andrade-Gordon, P.; Damiano, B.P.; Kauffman, J.A.; Mitchell, J.A.; Maryanoff, B.E. 1,2,4-triazolo[3,4-a]pyridine as a novel, constrained template for fibrinogen receptor (GPIIb/IIIa) antagonists. Bioorg. Med. Chem. Lett. 2001, 11, 2619–2622. [Google Scholar] [CrossRef]
- Karpina, V.R.; Kovalenko, S.S.; Kovalenko, S.M.; Drushlyak, O.G.; Bunyatyan, N.D.; Georgiyants, V.A.; Ivanov, V.V.; Langer, T.; Maes, L. A Novel Series of [1,2,4]Triazolo[4,3-a]Pyridine Sulfonamides as Potential Antimalarial Agents: In Silico Studies, Synthesis and In Vitro Evaluation. Molecules 2020, 25, 4485. [Google Scholar] [CrossRef]
- Ryu, H.; Nam, K.-Y.; Kim, H.J.; Song, J.-Y.; Hwang, S.-G.; Kim, J.S.; Kim, J.; Ahn, J. Discovery of a Novel Triazolopyridine Derivative as a Tankyrase Inhibitor. Int. J. Mol. Sci. 2021, 22, 7330. [Google Scholar] [CrossRef]
- Moreau, S.; Coudert, P.; Rubat, C.; Vallee-Goyet, D.; Gardette, D.; Jean-Claude Gramain, J.-C.; Couquelet, J. Synthesis and anticonvulsant properties of triazolo- and imidazopyridazinyl carboxamides and carboxylic acids. Bioorg. Med. Chem. 1998, 6, 983–991. [Google Scholar] [CrossRef]
- Nitlikar, L.H.; Darandale, S.N.; Shinde, D.B. Exploring the Unexplored Practical and Alternative Synthesis of 3-(Trifluoromethyl)-triazolopiperazine the Key Intermediate for Sitagliptin. Lett. Org. Chem. 2013, 10, 348–352. [Google Scholar] [CrossRef]
- Al-Issa, S.A.R. Synthesis of a New Series of Pyridine and Fused Pyridine Derivatives. Molecules 2012, 17, 10902–10915. [Google Scholar] [CrossRef] [Green Version]
- Lankau, H.-J.; Langen, B.; Grunwald, C.; Hoefgen, N.; Stange, H.; Dost, R.; Egerland, U. (1,2,4)Triazolo[4,3-a]quinoxaline Derivatives as Inhibitors of Phosphodiesterases. WO 2012/104293, 9 August 2012. (A1) English. [Google Scholar]
- Nelson, P.J.; Potts, K.T. A New One-Step Synthesis of Substituted Coumarins. J. Org. Chem. 1962, 27, 3243–3247. [Google Scholar] [CrossRef]
- El Khadem, H.S.; Kawai, J.; Swartz, D.L. Synthesis and Rearrangements of Imidazolo- and Triazolo-Diazines. Heterocycles 1989, 28, 239–248. [Google Scholar] [CrossRef]
- Zavodskaya, A.V.; Bakharev, V.V.; Parfenov, V.E.; Gidaspov, A.A.; Slepukhin, P.A.; Isenov, M.L.; Eltsov, O.S. Synthesis of new 5-aza-isosteres of guanine containing aryl and hetaryl substituents on the 1,2,4-triazole ring. Tetrahedron Lett. 2015, 56, 1103–1106. [Google Scholar] [CrossRef]
- Butler, R.N.; O’Sullivan, P.; Scott, F.L. The reactions of lead tetra-acetate with substituted benzothiazolylhydrazones. J. Chem. Soc. C 1971, 2265–2268. [Google Scholar] [CrossRef]
- Aggarwal, R.; Sumran, G.; Kumar, V.; Mittal, A. Copper(II) chloride mediated synthesis and DNA photocleavage activity of 1-aryl/heteroaryl-4-substituted-1,2,4-triazolo[4,3-a]quinoxalines. Eur. J. Med. Chem. 2011, 46, 6083–6088. [Google Scholar] [CrossRef] [PubMed]
- Oliver, R.; Thiel, O.R.; Achmatowicz, M.M.; Reichelt, A.; Larsen, R.D. Palladium-Catalyzed Coupling of Aldehyde-Derived Hydrazones: Practical Synthesis of Triazolopyridines and Related Heterocycles. Angew. Chem. Int. Ed. 2010, 49, 8395–8398. [Google Scholar] [CrossRef]
- Shawali, A.S. Tandem in situ generation and 1,5-electrocyclization of N-hetaryl nitrilimines. A facile methodology for synthesis of annulated 1,2,4-triazoles and their acyclo C-nucleosides. Arkivoc 2010, 33–97. [Google Scholar] [CrossRef]
- Chen, H.; Shang, Z.; Chang, J. Novel Synthesis of 7-β-D-Ribofuranosyl-7H-1,2,4-triazolo[3,4-i]purines with Use of NBS. Synthetic Commun. 2006, 36, 445–450. [Google Scholar] [CrossRef]
- Sun, X.; Yu, M.; Mu, X.; Zhou, Z.; Wang, L.; Liu, J.; Liu, X. A Facile Approach to [1,2,3]Triazolo[3,4-i]Purine via PIDA Oxidation Ring-closing Reaction. J. Heterocyclic Chem. 2021. [Google Scholar] [CrossRef]
- Andreas, R.; James, R.F.; Robert, M.R.; Oliver, R.T.; Michal, M.A.; Robert, D.L.; Dawei, Z. Palladium-Catalyzed Chemoselective Monoarylation of Hydrazides for the Synthesis of [1,2,4]Triazolo[4,3-a]pyridines. Org. Lett. 2010, 12, 792–795. [Google Scholar] [CrossRef]
- Park, Y.; Kim, Y.; Chang, S. Transition Metal-Catalyzed C–H Amination: Scope, Mechanism, and Applications. Chem. Rev. 2017, 117, 9247–9301. [Google Scholar] [CrossRef] [PubMed]
- Tsang, W.C.P.; Zheng, N.; Buchwald, S.L. Combined C−H Functionalization/C−N Bond Formation Route to Carbazoles. J. Am. Chem. Soc. 2005, 127, 14560–14561. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Shimizu, S.; Imamura, Y.; Ueki, T. Incompatibilities between N-Bromosuccinimide and Solvents. Org. Process Res. Dev. 2014, 18, 354–358. [Google Scholar] [CrossRef]
- Process Wednesday: 10% NBS in DMF will Exotherm--Who Knew? Available online: http://chemjobber.blogspot.com/2014/02/process-wednesday-10-nbs-in-dmf-will.html (accessed on 26 August 2021).
- Butler, R.N.; Johnston, S.M. Stereoisomerization in heterocyclic hydrazones derived from 2-acylpyridines and their oxidative cyclization with mercury(II) acetate and lead tetra-acetate to fused 1,2,4-triazoles and 1,2,3-triazolium systems. J. Chem. Soc. Perkin Trans. 1984, 1, 2109–2116. [Google Scholar] [CrossRef]
- Mu, J.-X.; Yang, M.-Y.; Sun, Z.-H.; Tan, C.-X.; Weng, J.-Q.; Wu, H.-K.; Liu, X.-H. Synthesis, Crystal Structure and DFT Studies of 8-chloro-3-((3-chlorobenzyl)thio)-[1,2,4]triazolo[4,3-a]pyridine. Crystals 2015, 5, 491–500. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.H.; Pan, L.; Tan, C.X.; Weng, J.Q.; Wang, B.L.; Li, Z.M. Synthesis, crystal structure, bioactivity and DFT calculation of new oxime ester derivatives containing cyclopropane moiety. Pestic. Biochem. Phys. 2011, 101, 143–147. [Google Scholar] [CrossRef]
- Yang, M.Y.; Zhao, W.; Liu, X.H.; Tan, C.X.; Weng, J.Q. Synthesis, crystal structure and antifungal activity of 4-(5-((2,4-Dichlorobenzyl)thio)-4-phenyl-4H-1,2,4-triazol-3-yl)pyridine. Chin. J. Struct. Chem. 2015, 34, 203–207. [Google Scholar] [CrossRef]
- Weng, J.Q.; Wang, L.; Liu, X.H. Synthesis, Crystal structure and herbicidal activity of a 1,2,4-triazol-5(4H)-one derivative. J. Chem. Soc. Pak. 2012, 34, 1248–1252. [Google Scholar]
- Wang, Z.X.; Jian, F.F.; Duan, C.Y.; Bai, Z.P.; You, X.Z. 2-(2-Hydroxybenzylidene)-1-(2-picoloyl)hydrazine Hemihydrate. Acta Cryst. 1998, C54, 1927–1929. [Google Scholar] [CrossRef]
- Tong, J.Y.; Wu, H.K.; Sun, N.B.; Liu, X.H. Synthesis, crystal structure and biological activity of a new 1,2,4-triazole derivative. Chin. J. Struct. Chem. 2013, 32, 607–611. [Google Scholar]
- Shao, X.; Xu, Z.; Zhao, X.; Xu, X.; Tao, L.; Zhong, L.; Xuhong, Q. Synthesis, crystal structure, and insecticidal activities of highly congested hexahydroimidazo[1,2-a] pyridine derivatives: Effect of conformation on activities. J. Agric. Food Chem. 2010, 58, 2690–2695. [Google Scholar] [CrossRef] [PubMed]
| Parameter | 1 | 2 |
|---|---|---|
| Chemical formula | C11H8N4 + 3H2O | C11H7N4Br + 3H2O |
| Mr | 250.26 | 329.16 |
| Crystal system, space group | Monoclinic, P 21/c | Monoclinic, P 21/c |
| Temperature (K) | 193 | 193 |
| a, b, c (Å) | 14.3213(11), 6.9452(4), 12.6860(8) | 15.1413(12), 6.9179(4), 13.0938(8) |
| β (°) | 100.265(6)° | 105.102(6) |
| V (Å3) | 1241.62(14) | 1324.16(16) |
| Z | 4 | 4 |
| Radiation type | Mo-Kα Graphite monochromator | Mo-Kα Graphite monochromator |
| µ (mm−1) | 0.1 | 3.115 |
| Crystal size (mm) | 0.06 × 0.1 × 0.45 | 0.1 × 0.32 × 0.34 |
| Dc (g/cm3) | 1.339 | 1.651 |
| Diffractometer | STOE IPDS 2T | STOE IPDS 2T |
| F(000) | 528 | 664 |
| Index ranges | −19 ≤ h ≤ 19 −9 ≤ k ≤ 8 −16 ≤ l ≤ 16 | −20 ≤ h ≤ 16 −9 ≤ k ≤ 9 −17 ≤ l ≤ 17 |
| 6903, 3058, 1545 | 7323, 3252, 2727 | |
| Rint | 0.0451 | 0.0167 |
| GOF | 0.953 | 1.126 |
| H-atom treatment | H-atoms localized and refined with isotropic displacement parameters | H-atoms localized and refined with isotropic displacement parameters |
| (Δ)max, (Δ)min (e Å−3) | 0.24, −0.22 | 0.41, −0.47 |
| 3-(pyridin-4-yl)-[1,2,4]triazolo[4,3-a]pyridine 1 | |||
| C(1)-N(2) | 1.318(3) | C(8)-N(9) | 1.385(3) |
| N(2)-N(3) | 1.360(3) | C(12)-N(13) | 1.336(3) |
| N(3)-C(4) | 1.324(3) | N(13)-C(14) | 1.346(3) |
| C(1)-N(9) | 1.383(3) | C(10)-C(11) | 1.398(3) |
| C(4)-N(9) | 1.391(3) | C(11)-C(12) | 1.385(3) |
| N(2)-C(1)-N(9) | 108.95(18) | N(9)-C(1)-C(10) | 128.43(17) |
| N(2)-C(1)-C(10) | 122.61(19) | C(4)-N(3)-N(2) | 107.48(17) |
| C(1)-N(2)-N(3) | 109.36(19) | N(3)-C(4)-C(5) | 130.7(2) |
| N(3)-C(4)-N(9) | 109.6(2) | C(6)-C(5)-C(4) | 118.6(3) |
| N(9)-C(4)-C(5) | 119.7(2) | C(4)-C(5)-H(5) | 119.2(15) |
| C(6)-C(5)-H(5) | 122.1(15) | C(5)-C(6)-H(6) | 118.3(17) |
| C(5)-C(6)-C(7) | 120.2(3) | C(8)-C(7)-C(6) | 121.8(3) |
| C(7)-C(6)-H(6) | 121.5(17) | C(6)-C(7)-H(7) | 119.3(17) |
| 6-bromo-3-(pyridin-4-yl)-[1,2,4]triazolo-[4,3-a]pyridine 2 | |||
| Br(1)-C(7) | 1.886(2) | C(1)-N(2) | 1.321(3) |
| C(1)-N(9) | 1.377(3) | C(1)-C(10) | 1.461(3) |
| N(2)-N(3) | 1.370(3) | N(3)-C(4) | 1.328(3) |
| C(4)-N(9) | 1.386(3) | C(4)-C(5) | 1.408(4) |
| C(8)-N(9) | 1.384(3) | C(12)-N(13) | 1.341(3) |
| N(13)-C(14) | 1.347(3) | C(11)-C(12) | 1.386(3) |
| N(2)-C(1)-N(9) | 109.3(2) | C(8)-C(7)-Br(1) | 118.63(18) |
| N(2)-C(1)-C(10) | 122.3(2) | N(9)-C(1)-C(10) | 128.30(19) |
| C(1)-N(2)-N(3) | 108.95(19) | C(4)-N(3)-N(2) | 107.04(18) |
| N(3)-C(4)-N(9) | 109.9(2) | N(3)-C(4)-C(5) | 130.6(2) |
| N(9)-C(4)-C(5) | 119.5(2) | C(6)-C(5)-C(4) | 119.4(2) |
| C(6)-C(5)-H(5) | 123.5(19) | C(4)-C(5)-H(5) | 116.9(19) |
| D−H...A | d(D−H) | d(H...A) | d(D...A) | <(DHA) |
|---|---|---|---|---|
| 3-(pyridin-4-yl)-[1,2,4]triazolo[4,3-a]pyridine 1 | ||||
| O1W—H1W⋅⋅⋅O3W | 0.862(17) | 1.954(18) | 2.810(3) | 172.(3) |
| O1W—H2W⋅⋅⋅N13 | 0.906(17) | 1.857(18) | 2.759(3) | 174.(3) |
| O2W—H3W⋅⋅⋅O3W#1 | 0.856(18) | 1.98(2) | 2.810(3) | 164.(3) |
| O2W—H4W⋅⋅⋅O1W | 0.880(18) | 1.906(18) | 2.786(3) | 179.(3) |
| O3W—H5W⋅⋅⋅O2W#2 | 0.812(18) | 1.991(19) | 2.791(3) | 169.(3) |
| O3W—H6W⋅⋅⋅O1W#3 | 0.866(17) | 1.942(18) | 2.797(3) | 169.(3) |
| 6-bromo-3-(pyridin-4-yl)-[1,2,4]triazolo-[4,3-a]pyridine 2 | ||||
| O1W—H1W⋅⋅⋅N13 | 0.826(19) | 1.96(2) | 2.783(3) | 173.(3) |
| O1W—H2W⋅⋅⋅O2W | 0.814(19) | 1.96(2) | 2.771(3) | 174.(4) |
| O2W—H3W⋅⋅⋅O3W#1 | 0.82(2) | 2.06(2) | 2.873(3) | 175.(4) |
| O2W—H4W⋅⋅⋅O3W#2 | 0.81(2) | 2.07(2) | 2.873(3) | 171.(4) |
| O3W—H5W⋅⋅⋅O1W | 0.817(19) | 2.00(2) | 2.804(3) | 170.(4) |
| O3W—H6W⋅⋅⋅O1W#3 | 0.825(19) | 2.00(2) | 2.805(3) | 167.(4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Kurdi, S.; Abu Thaher, B.; Wahedy, K.; Schollmeyer, D.; Nopper, L.; Riester, O.; Deigner, H.-P. Efficient Synthesis and X-ray Structure of [1,2,4]Triazolo[4,3-a]pyridines via Oxidative Cyclization Using N-Chlorosuccinimide (NCS). Crystals 2021, 11, 1156. https://doi.org/10.3390/cryst11101156
El-Kurdi S, Abu Thaher B, Wahedy K, Schollmeyer D, Nopper L, Riester O, Deigner H-P. Efficient Synthesis and X-ray Structure of [1,2,4]Triazolo[4,3-a]pyridines via Oxidative Cyclization Using N-Chlorosuccinimide (NCS). Crystals. 2021; 11(10):1156. https://doi.org/10.3390/cryst11101156
Chicago/Turabian StyleEl-Kurdi, Said, Bassam Abu Thaher, Kanan Wahedy, Dieter Schollmeyer, Levin Nopper, Oliver Riester, and Hans-Peter Deigner. 2021. "Efficient Synthesis and X-ray Structure of [1,2,4]Triazolo[4,3-a]pyridines via Oxidative Cyclization Using N-Chlorosuccinimide (NCS)" Crystals 11, no. 10: 1156. https://doi.org/10.3390/cryst11101156
APA StyleEl-Kurdi, S., Abu Thaher, B., Wahedy, K., Schollmeyer, D., Nopper, L., Riester, O., & Deigner, H.-P. (2021). Efficient Synthesis and X-ray Structure of [1,2,4]Triazolo[4,3-a]pyridines via Oxidative Cyclization Using N-Chlorosuccinimide (NCS). Crystals, 11(10), 1156. https://doi.org/10.3390/cryst11101156






