Residual Oxygen Effects on the Properties of MoS2 Thin Films Deposited at Different Temperatures by Magnetron Sputtering
Abstract
:1. Introduction
2. Materials and Methods
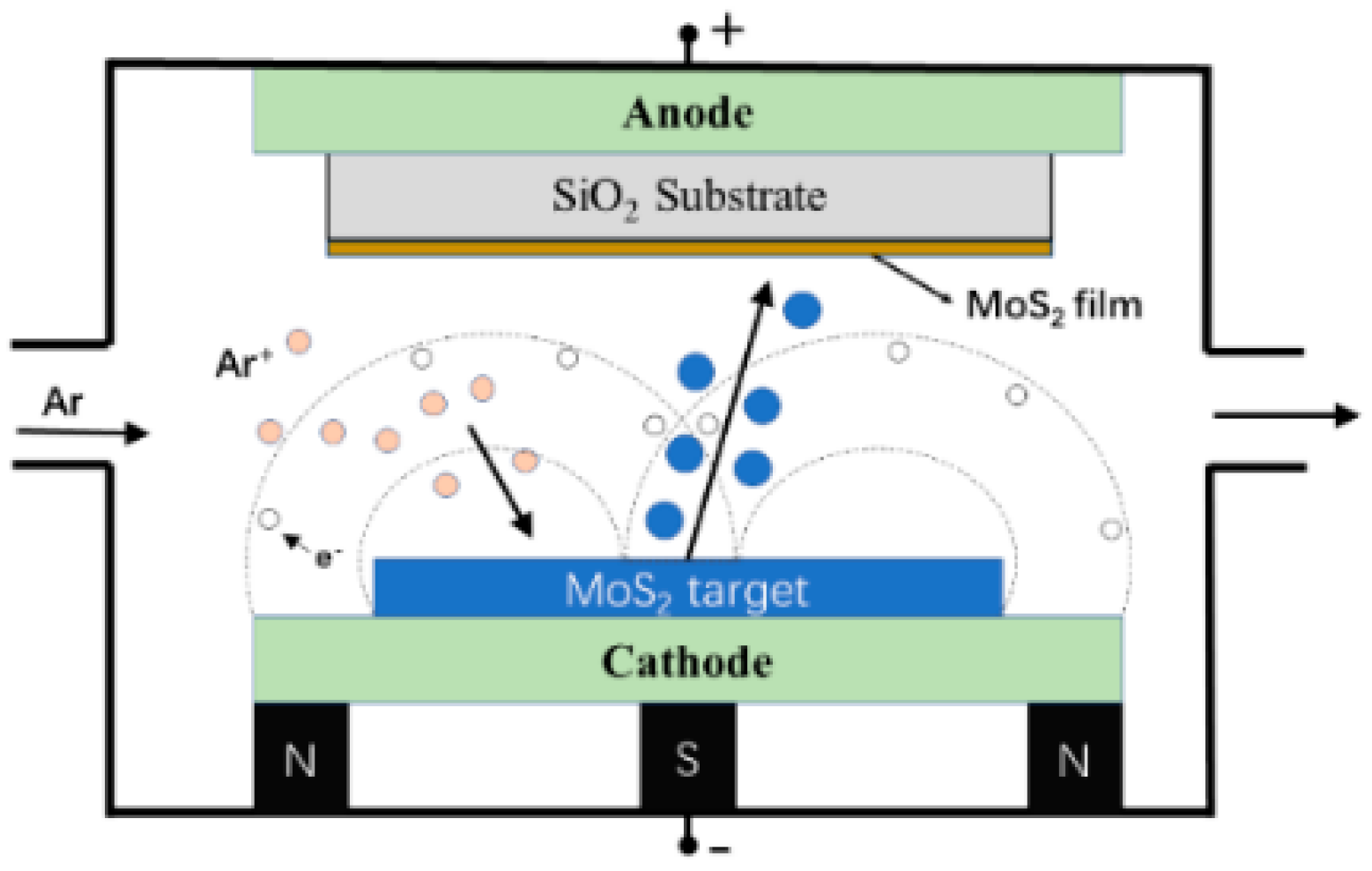
2.1. Preparation of Molybdenum Disulfide Film
2.2. Characterization of Properties
3. Results and Discussion
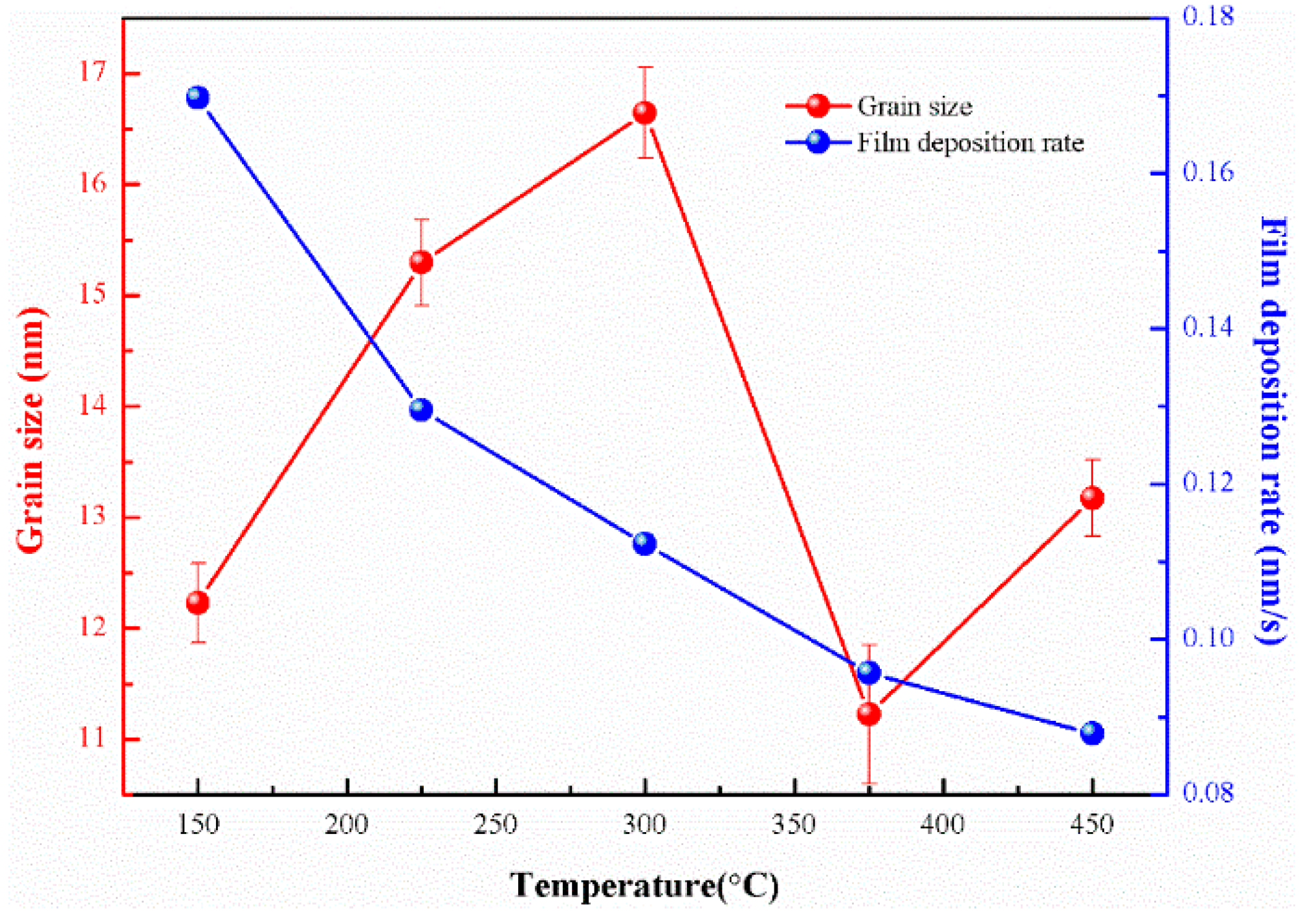
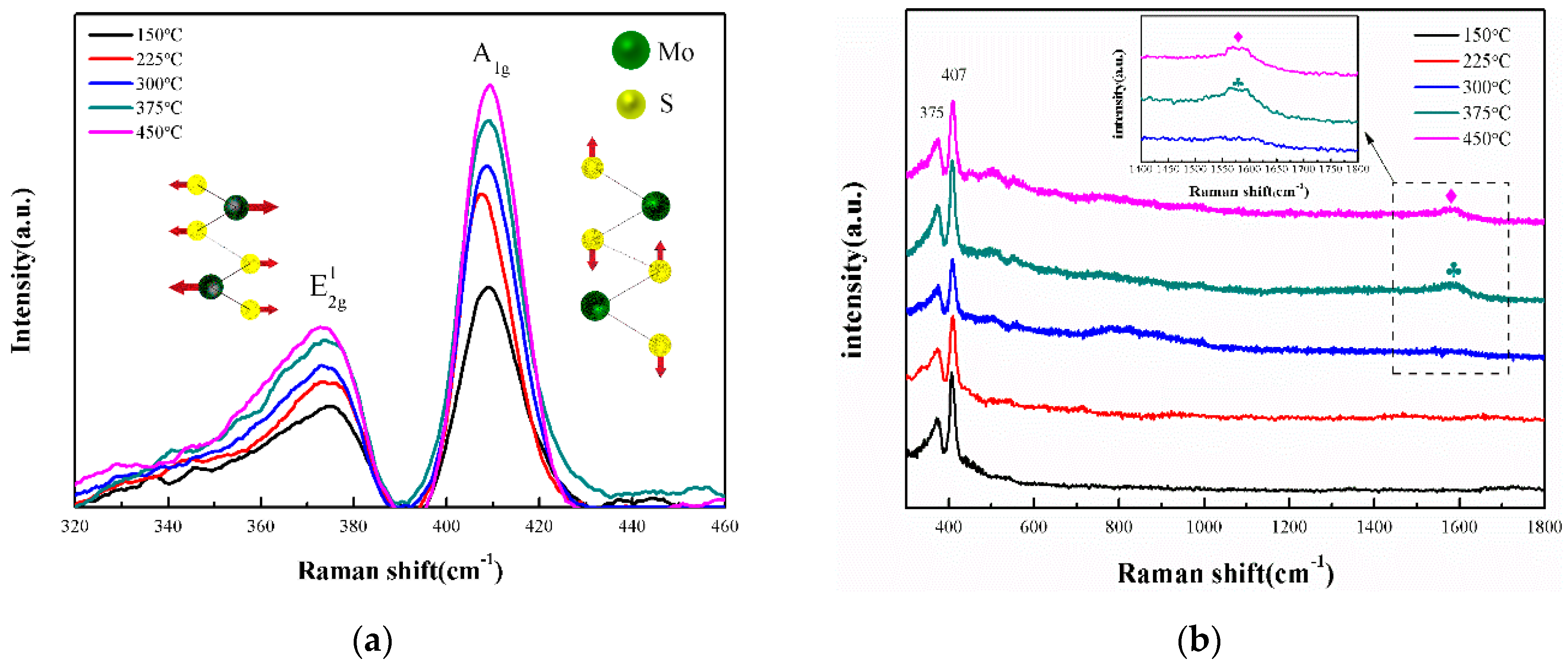
3.1. Structure of the Films
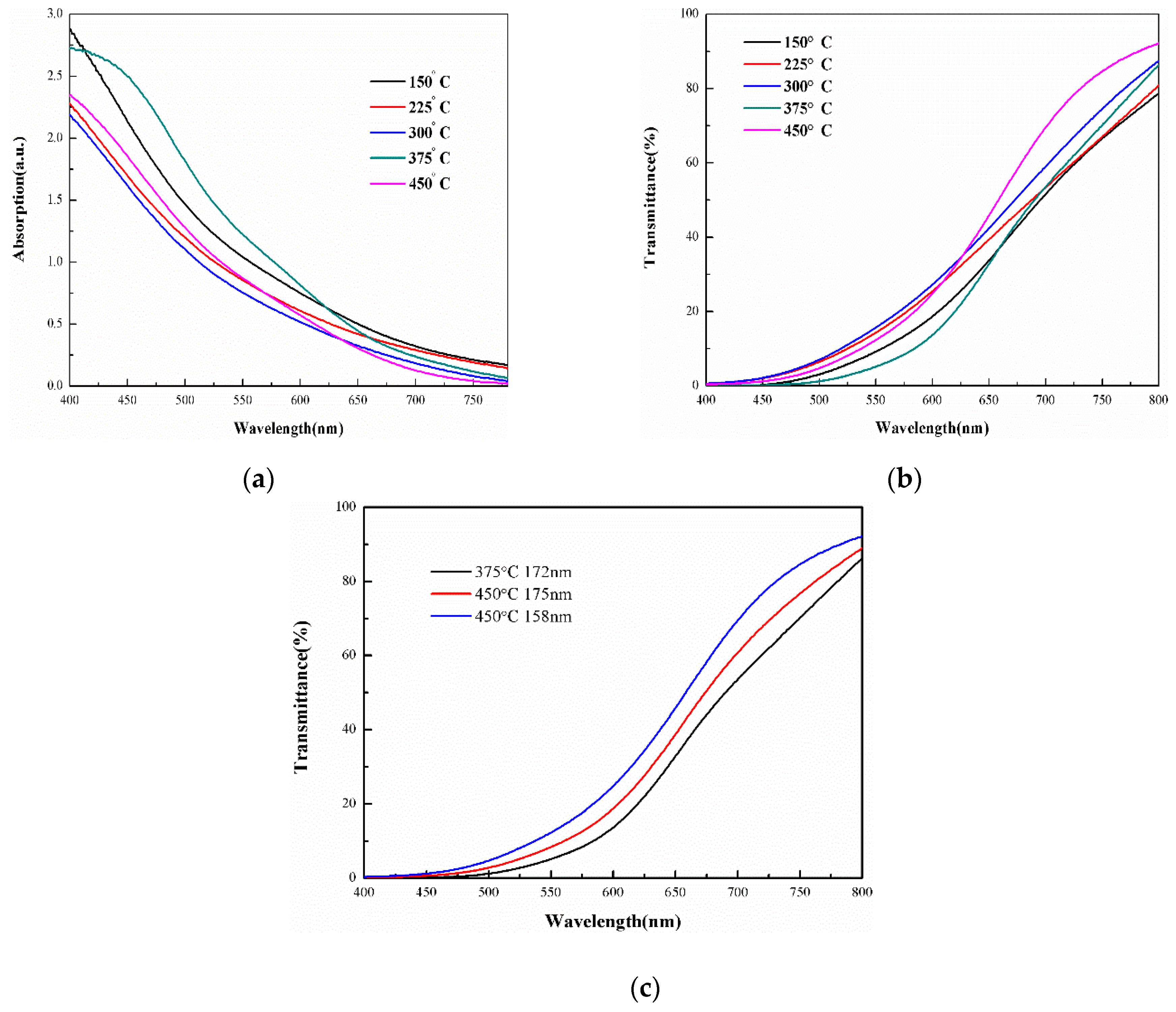
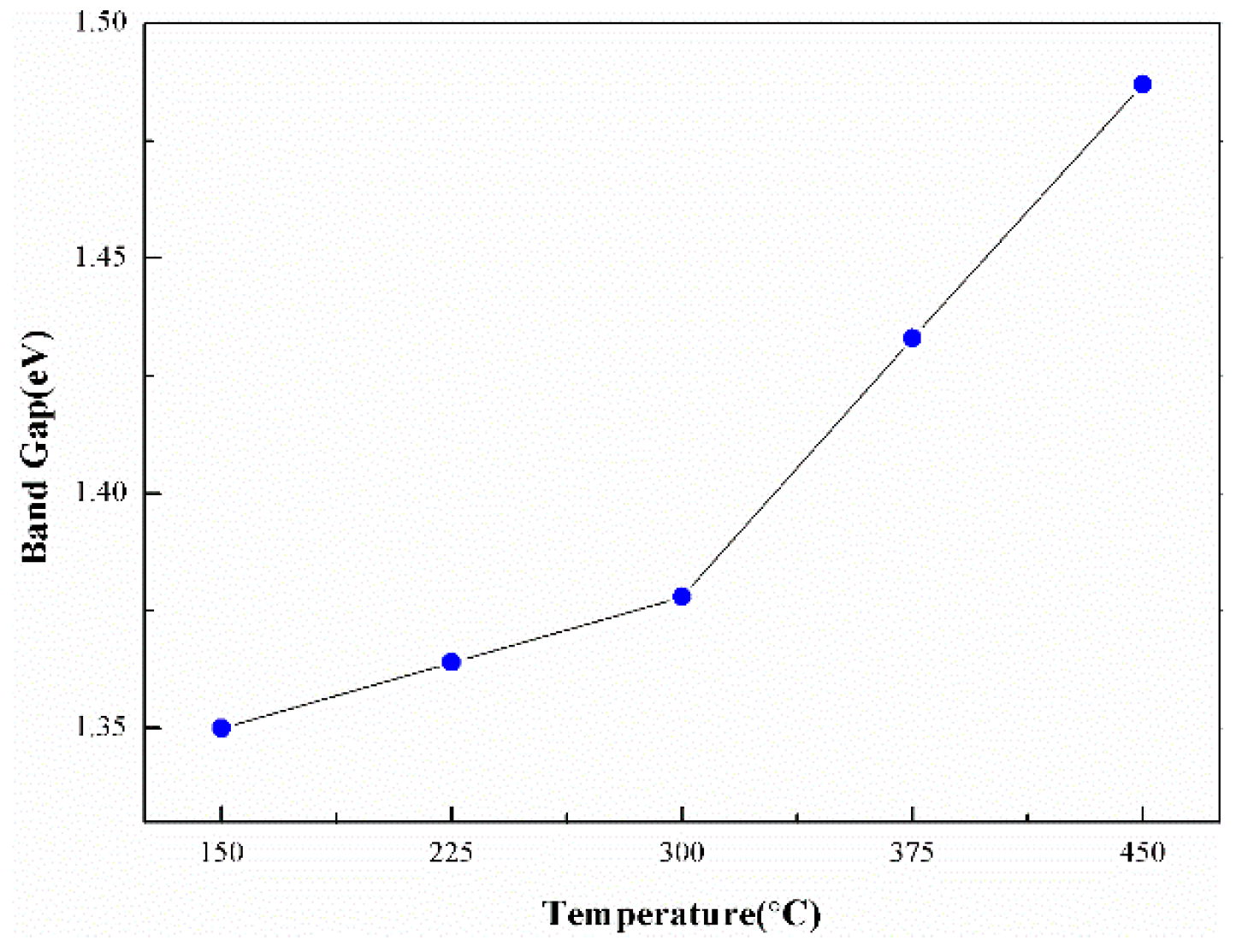
3.2. Optical Properties of the Films
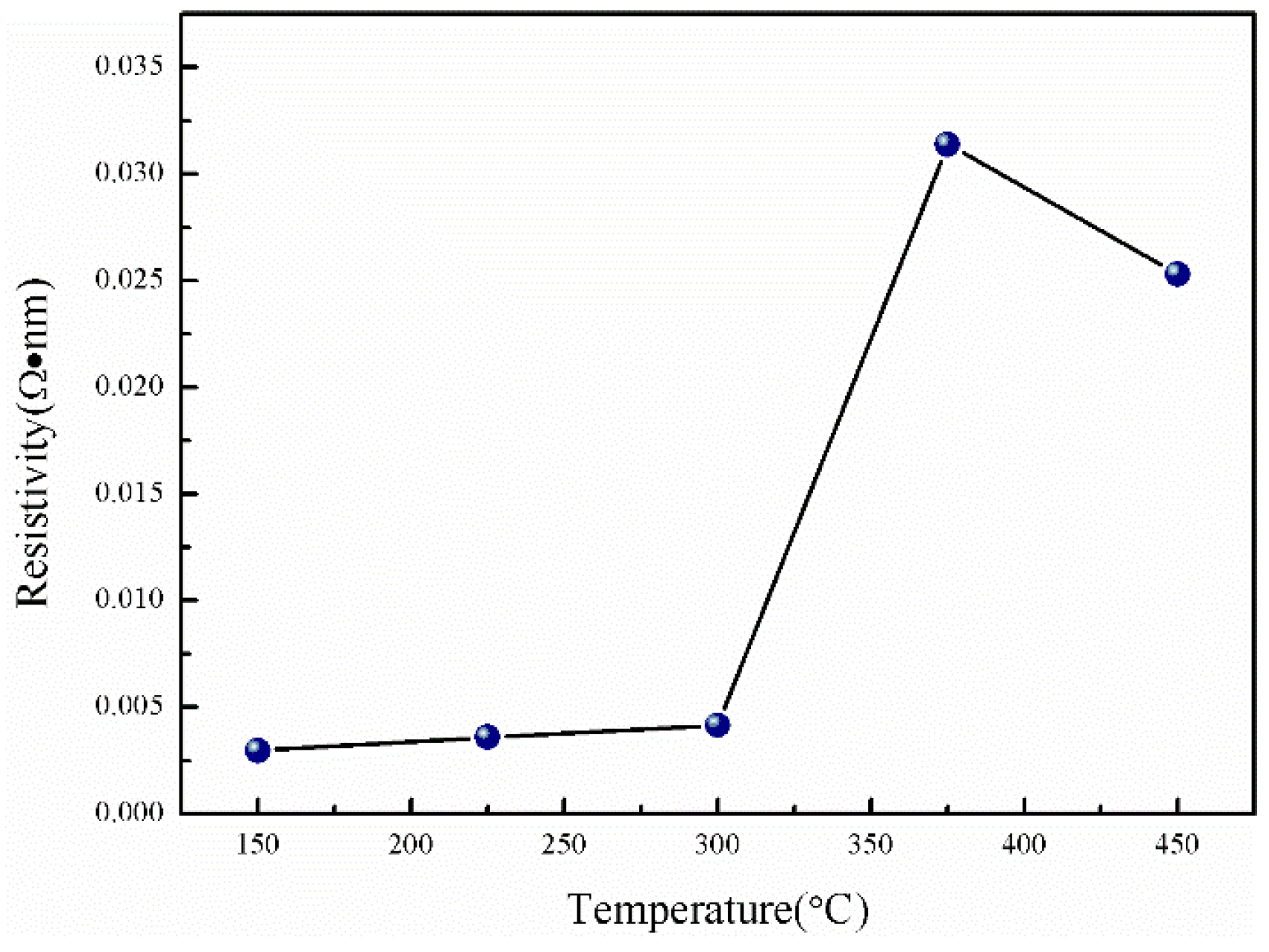
3.3. Electrical Properties of the Films
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mak, K.F.; Lee, C.; Hone, J.; Shan, J.; Heinz, T.F. Atomically thin MoS2: A new direct-gap semiconductor. Phys. Rev. Lett. 2010, 105, 136805. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Zhang, B.; Wang, S.; Wang, D.; Wang, A.; Wang, Z.; Yu, H.; Zhang, H.; Chen, Y.; Zhao, M.; et al. Ultrabroadband MoS2 photodetector with spectral response from 445 to 2717 nm. Adv. Mater. 2017, 29, 1605972. [Google Scholar] [CrossRef]
- Choudhary, N.; Patel, M.; Ho, Y.H.; Dahotre, N.B.; Lee, W.; Hwang, J.Y.; Choi, W. Directly deposited MoS2 thin-film electrodes for high-performance supercapacitors. J. Mater. Chem. A 2015, 3, 24049–24054. [Google Scholar] [CrossRef]
- Musil, J.; Čiperová, Z.; Čerstvý, R.; Haviar, S. Flexible hard (Zr, Si) alloy films prepared by magnetron sputtering. Thin Solid Film. 2019, 688, 137216. [Google Scholar] [CrossRef]
- Petrov, I.; Hall, A.; Mei, A.B.; Nedfors, N.; Zhirkov, I.; Rosen, J.; Reed, A.; Howe, B.; Greczynski, G.; Birch, J.; et al. Controlling the boron-to-titanium ratio in magnetron-sputter-deposited TiBx thin films. J. Vac. Sci. Technol. A Vac. Surf. Film. 2017, 35, 050601. [Google Scholar] [CrossRef]
- Sidelev, D.V.; Krivobokov, V.P. Angular thickness distribution and target utilization for hot Ni target magnetron sputtering. Vacuum 2019, 160, 418–420. [Google Scholar] [CrossRef]
- Tao, J.; Chai, J.; Lu, X.; Wong, L.M.; Wong, T.I.; Pan, J.; Xiong, Q.; Chi, D.; Wang, S. Growth of wafer-scale MoS2 monolayer by magnetron sputtering. Nanoscale 2015, 7, 2497–2503. [Google Scholar] [CrossRef]
- Samassekou, H.; Alkabsh, A.; Wasala, M.; Eaton, M.; Walber, A.; Walker, A.; Pitkänen, O.; Kordas, K.; Talapatra, S.; Jayasekera, T.; et al. Viable route towards large-area 2D MoS2 using magnetron sputtering. 2D Mater. 2017, 4, 021002. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Kang, Y.C. Effect of Radio Frequency Power on the Physicochemical Properties of MoS2 Films Obtained by rf Magnetron Sputtering. Bull. Korean Chem. Soc. 2016, 37, 1326–1330. [Google Scholar] [CrossRef]
- Zhong, W.; Deng, S.; Wang, K.; Li, G.; Li, G.; Chen, R.; Kwok, H.S. Feasible route for a large area few-layer MoS2 with magnetron sputtering. Nanomaterials 2018, 8, 590. [Google Scholar] [CrossRef] [Green Version]
- Gong, C.; Xiao, J.; Zhu, L.; Wang, Z.; Ma, S. Effect of deposition pressure on the microstructure and optical bandgap of molybdenum disulfide films prepared by magnetron sputtering. Coatings 2019, 9, 570. [Google Scholar] [CrossRef] [Green Version]
- Qiao, L.; Wang, P.; Chai, L.; Zhang, X.; Liu, W. Influence of the incident flux angles on the structures and properties of magnetron sputtered MoS2 films. J. Phys. D Appl. Phys. 2015, 48, 175304. [Google Scholar] [CrossRef]
- Hamilton, M.A.; Alvarez, L.A.; Mauntler, N.A.; Argibay, N.; Colbert, R.; Burris, D.L.; Muratore, C.; Voevodin, A.A.; Perry, S.S.; Sawyer, W.G. A possible link between macroscopic wear and temperature-dependent friction behaviors of MoS2 coatings. Tribol. Lett. 2008, 32, 91–98. [Google Scholar] [CrossRef]
- Kubart, T.; Polcar, T.; Kopecký, L.; Novak, R.; Novakova, D. Temperature dependence of tribological properties of MoS2 and MoSe2 coatings. Surf. Coat. Technol. 2005, 193, 230–233. [Google Scholar] [CrossRef]
- Spalvins, T. Structure of sputtered molybdenum disulfide films at various substrate temperatures. ASLE Trans. 1974, 17, 1–7. [Google Scholar] [CrossRef]
- Chen, G.; Lu, B.; Cui, X.; Xiao, J. Effects of Deposition and Annealing Temperature on the Structure and Optical Band Gap of MoS2 Films. Materials 2020, 13, 5515. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Qiao, L.; Xu, J.; Li, W.; Liu, W. Erosion mechanism of MoS2-based films exposed to atomic oxygen environments. ACS Appl. Mater. Interfaces 2015, 7, 12943–12950. [Google Scholar] [CrossRef]
- Buck, V. Preparation and properties of different types of sputtered MoS2 films. Wear 1987, 114, 263–274. [Google Scholar] [CrossRef]
- Zhou, Z.; Xu, T.; Zhang, C.; Li, S.; Xu, J.; Sun, L.; Gao, L. Enhancing stability by tuning element ratio in 2D transition metal chalcogenides. Nano Res. 2021, 14, 1704–1710. [Google Scholar] [CrossRef]
- Gao, J.; Li, B.; Tan, J.; Chow, P.; Lu, T.M.; Koratkar, N. Aging of transition metal dichalcogenide monolayers. ACS Nano 2016, 10, 2628–2635. [Google Scholar] [CrossRef]
- Serpini, E.; Rota, A.; Ballestrazzi, A.; Marchetto, D.; Gualtieri, E.; Valeri, S. The role of humidity and oxygen on MoS2 thin films deposited by RF PVD magnetron sputtering. Surf. Coat. Technol. 2017, 319, 345–352. [Google Scholar] [CrossRef]
- Nikpay, M.A.; Mortazavi, S.Z.; Reyhani, A.; Elahi, S.M. Sputtered MoS2 layer as a promoter in the growth of MoS2 nanoflakes by TCVD. Mater. Res. Express 2018, 5, 015032. [Google Scholar] [CrossRef]
- Duan, Z.; Qiao, L.; Chai, L.; Xu, J.; Wang, P.; Liu, W. Structure, properties and growth mechanism of a self-assembled nanocylindrical MoS2/Mo-SC composite film. Appl. Surf. Sci. 2019, 465, 564–574. [Google Scholar] [CrossRef]
- Miranda MA, R.; Sasaki, J.M. The limit of application of the Scherrer equation. Acta Crystallogr. Sect. A Found. Adv. 2018, 74, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Sun, Y. From tremella-like MoS2 to α-Mo3 nanoplates: Sintering synthesis and adsorption properties. Micro Nano Lett. 2017, 12, 652–655. [Google Scholar] [CrossRef]
- Zhong, M.; Wei, Z.; Meng, X.; Wu, F.; Li, J. From MoS2 Microspheres to α-MoO3 Nanoplates: Growth Mechanism and Photocatalytic Activities. Eur. J. Inorg. Chem. 2014, 2014, 3245–3251. [Google Scholar] [CrossRef]
- Liu, J.; Sun, K.; Zheng, X.; Wang, S.; Lian, S.; Deng, C.; Xie, H.; Zhang, X.; Gao, Y.; Song, F.; et al. Evolutions of morphology and electronic properties of few-layered MoS2 exposed to UVO. Results Phys. 2020, 19, 103634. [Google Scholar] [CrossRef]
- Choudhary, N.; Park, J.; Hwang, J.Y.; Choi, W. Growth of large-scale and thickness-modulated MoS2 nanosheets. ACS Appl. Mater. Interfaces 2014, 6, 21215–21222. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.P.; Yang, R.; Chai, J.W.; Wang, S.J.; Leong, W.S.; Tong, Y.; Lei, D.; Zhou, Q.; Gong, X.; Chi, D.Z.; et al. Large-scale two-dimensional MoS2 photodetectors by magnetron sputtering. Opt. Express 2015, 23, 13580–13586. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yan, H.; Brus, L.E.; Heinz, T.F.; Hone, J.; Ryu, S. Anomalous lattice vibrations of single-and few-layer MoS2. ACS Nano 2010, 4, 2695–2700. [Google Scholar] [CrossRef] [Green Version]
- Li, S.L.; Miyazaki, H.; Song, H.; Kuramochi, H.; Nakaharai, S.; Tsukagoshi, K. Quantitative Raman spectrum and reliable thickness identification for atomic layers on insulating substrates. ACS Nano 2012, 6, 7381–7388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sirota, B.; Glavin, N.; Voevodin, A.A. Room temperature magnetron sputtering and laser annealing of ultrathin MoS2 for flexible transistors. Vacuum 2019, 160, 133–138. [Google Scholar] [CrossRef]
- Guo, Y.; Zhuang, Z.; Liu, Z.; Fan, W.; Zhong, H.; Zhang, W.; Ni, Y.; Guo, Z. Facile hot spots assembly on molybdenum oxide nanosheets via in situ decoration with gold nanoparticles. Appl. Surf. Sci. 2019, 480, 1162–1170. [Google Scholar] [CrossRef]
- Fodjo, E.K.; Li, D.W.; Marius, N.P.; Albert, T.; Long, Y.T. Low-temperature synthesis and SERS application of silver molybdenum oxides. J. Mater. Chem. A 2013, 1, 2558–2566. [Google Scholar] [CrossRef]
- Niu, Z.; Zhou, C.; Wang, J.; Xu, Y.; Gu, C.; Jiang, T.; Zeng, S.; Zhang, Y.; Ang, D.S.; Zhou, J. UV-light-assisted preparation of MoO3− x/Ag NPs film and investigation on the SERS performance. J. Mater. Sci. 2020, 55, 8868–8880. [Google Scholar] [CrossRef]
- Nath, A.; Raman, R.; Yadav, V.K.; Sannibabu, P.; Sarkar, M.B. Bandgap modulation of glancing angle deposition aided Ag nanoparticles covered TiO2 thin film by high-temperature annealing. J. Nanosci. Nanotechnol. 2020, 20, 7636–7643. [Google Scholar] [CrossRef]
- Coulter, J.B.; Birnie, D.P., III. Assessing Tauc plot slope quantification: ZnO thin films as a model system. Phys. Status Solidi 2018, 255, 1700393. [Google Scholar] [CrossRef]
- Dhakal, K.P.; Duong, D.L.; Lee, J.; Nam, H.; Kim, M.; Kan, M.; Lee, Y.H.; Kim, J. Confocal absorption spectral imaging of MoS2: Optical transitions depending on the atomic thickness of intrinsic and chemically doped MoS2. Nanoscale 2014, 6, 13028–13035. [Google Scholar] [CrossRef] [Green Version]
- Li, X.L.; Han, W.P.; Wu, J.B.; Qiao, X.F.; Zhang, J.; Tan, P.H. Layer-number dependent optical properties of 2D materials and their application for thickness determination. Adv. Funct. Mater. 2017, 27, 1604468. [Google Scholar] [CrossRef]
- Koike, K.; Wada, R.; Yagi, S.; Harada, Y.; Sasa, S.; Yano, M. Characteristics of MoO3 films grown by molecular beam epitaxy. Jpn. J. Appl. Phys. 2014, 53, 05FJ02. [Google Scholar] [CrossRef]
- Qi, M.; Xiao, J.; Gong, C. Thermal annealing effects on the electrophysical characteristics of sputtered MoS2 thin films by Hall effect measurements. Semicond. Sci. Technol. 2019, 34, 045017. [Google Scholar] [CrossRef]

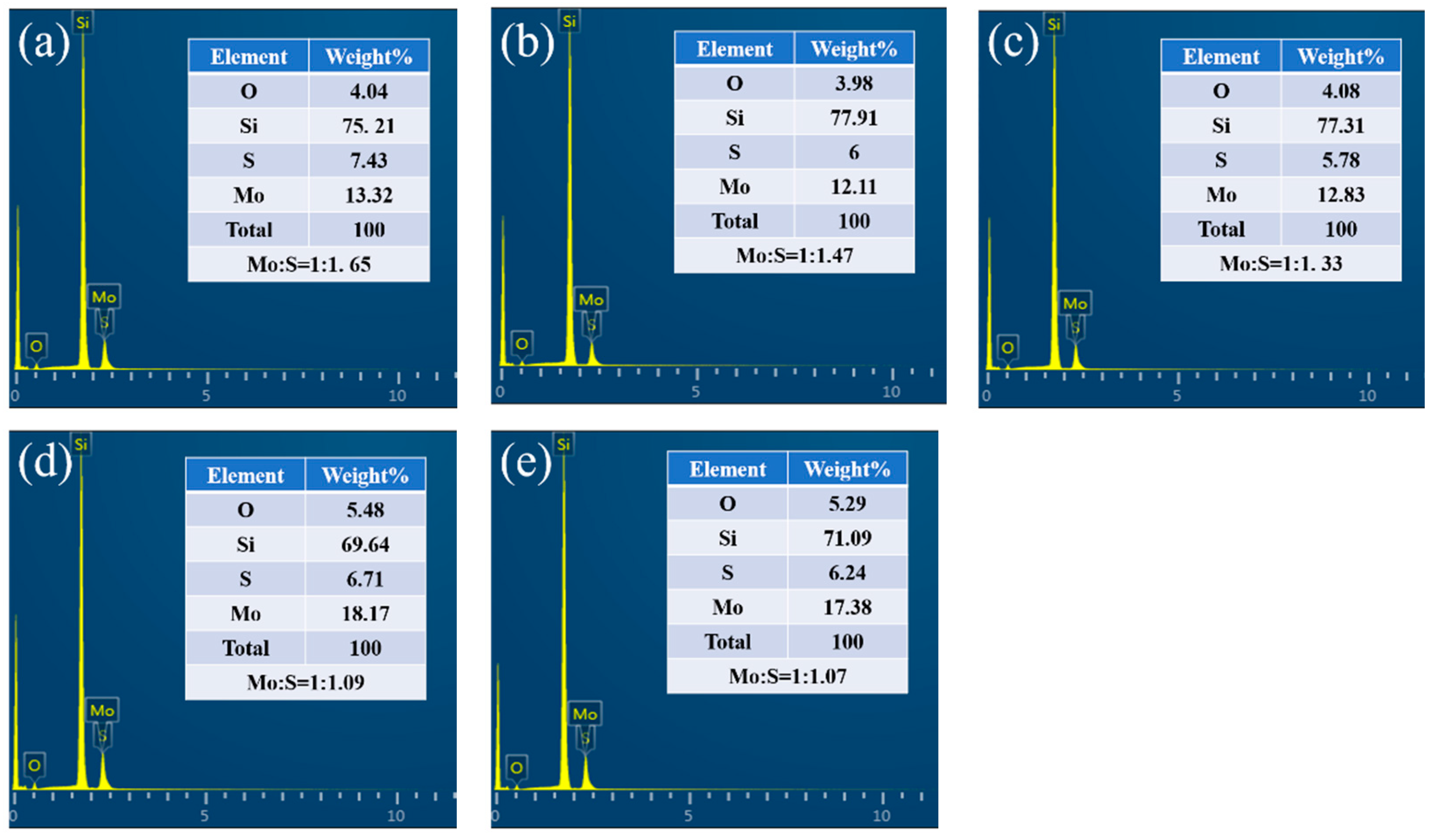

| Temperature (°C) | 150 | 225 | 300 | 375 | 450 |
| Thickness (nm) | 305 | 233 | 202 | 172 | 158 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Wang, X.; Tan, F.; Zhang, R. Residual Oxygen Effects on the Properties of MoS2 Thin Films Deposited at Different Temperatures by Magnetron Sputtering. Crystals 2021, 11, 1183. https://doi.org/10.3390/cryst11101183
Wang P, Wang X, Tan F, Zhang R. Residual Oxygen Effects on the Properties of MoS2 Thin Films Deposited at Different Temperatures by Magnetron Sputtering. Crystals. 2021; 11(10):1183. https://doi.org/10.3390/cryst11101183
Chicago/Turabian StyleWang, Peiyu, Xin Wang, Fengyin Tan, and Ronghua Zhang. 2021. "Residual Oxygen Effects on the Properties of MoS2 Thin Films Deposited at Different Temperatures by Magnetron Sputtering" Crystals 11, no. 10: 1183. https://doi.org/10.3390/cryst11101183
APA StyleWang, P., Wang, X., Tan, F., & Zhang, R. (2021). Residual Oxygen Effects on the Properties of MoS2 Thin Films Deposited at Different Temperatures by Magnetron Sputtering. Crystals, 11(10), 1183. https://doi.org/10.3390/cryst11101183





