Crystallization Behaviors of Composites Comprising Biodegradable Polyester and Functional Nucleation Agent
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Preparation
2.2. Apparatus and Procedures
2.2.1. Polarized Optical Microscopy (POM)
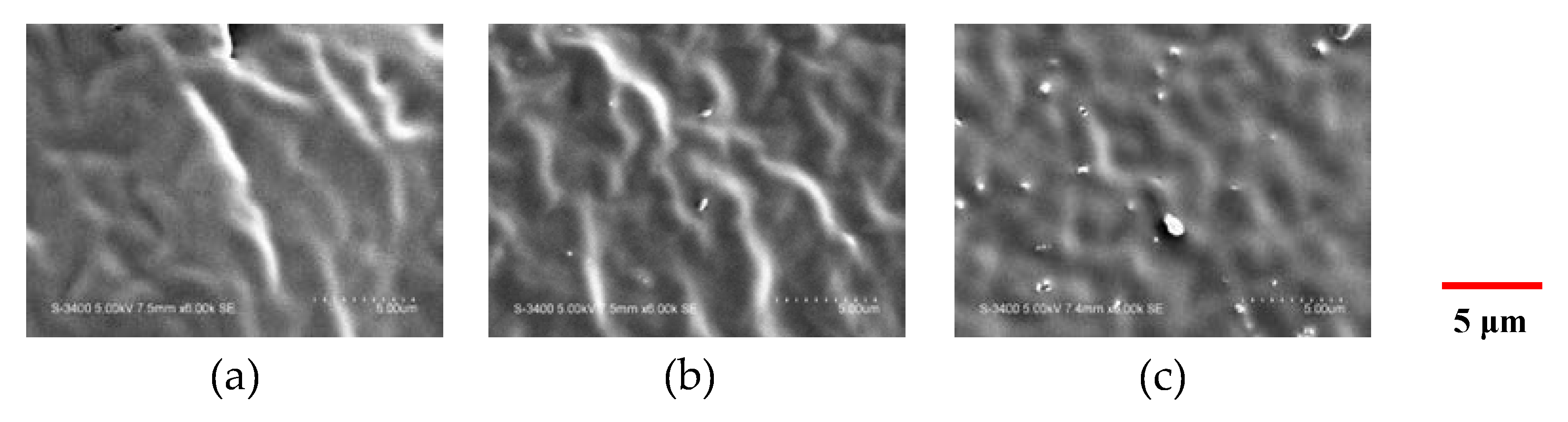
2.2.2. Scanning Electron Microscopy (SEM)
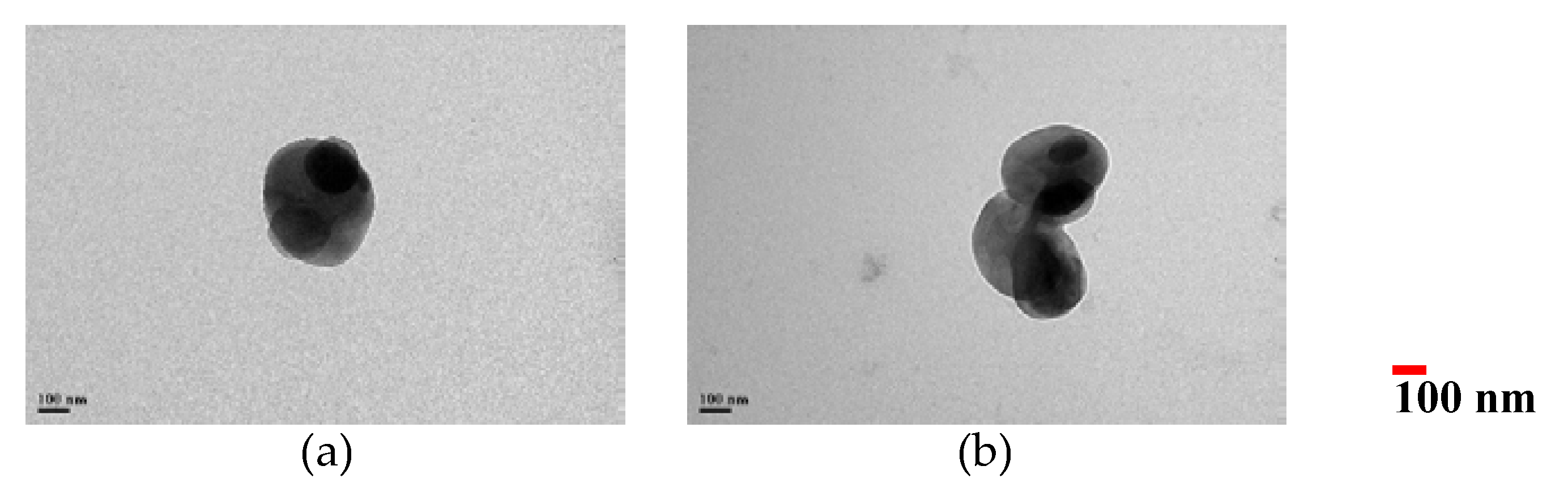
2.2.3. Transmission Electron Microscopy (TEM)
2.2.4. Differential Scanning Calorimetry (DSC)
2.2.5. Wide-Angle X-ray Diffraction (WAXD)
3. Results and Discussion
3.1. Morphological Observation
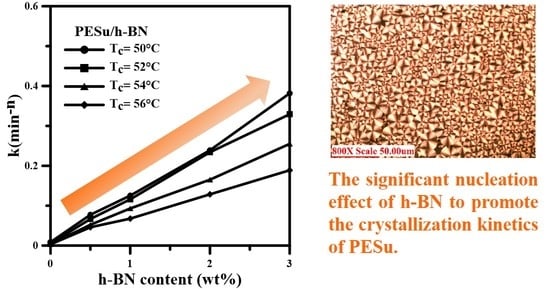
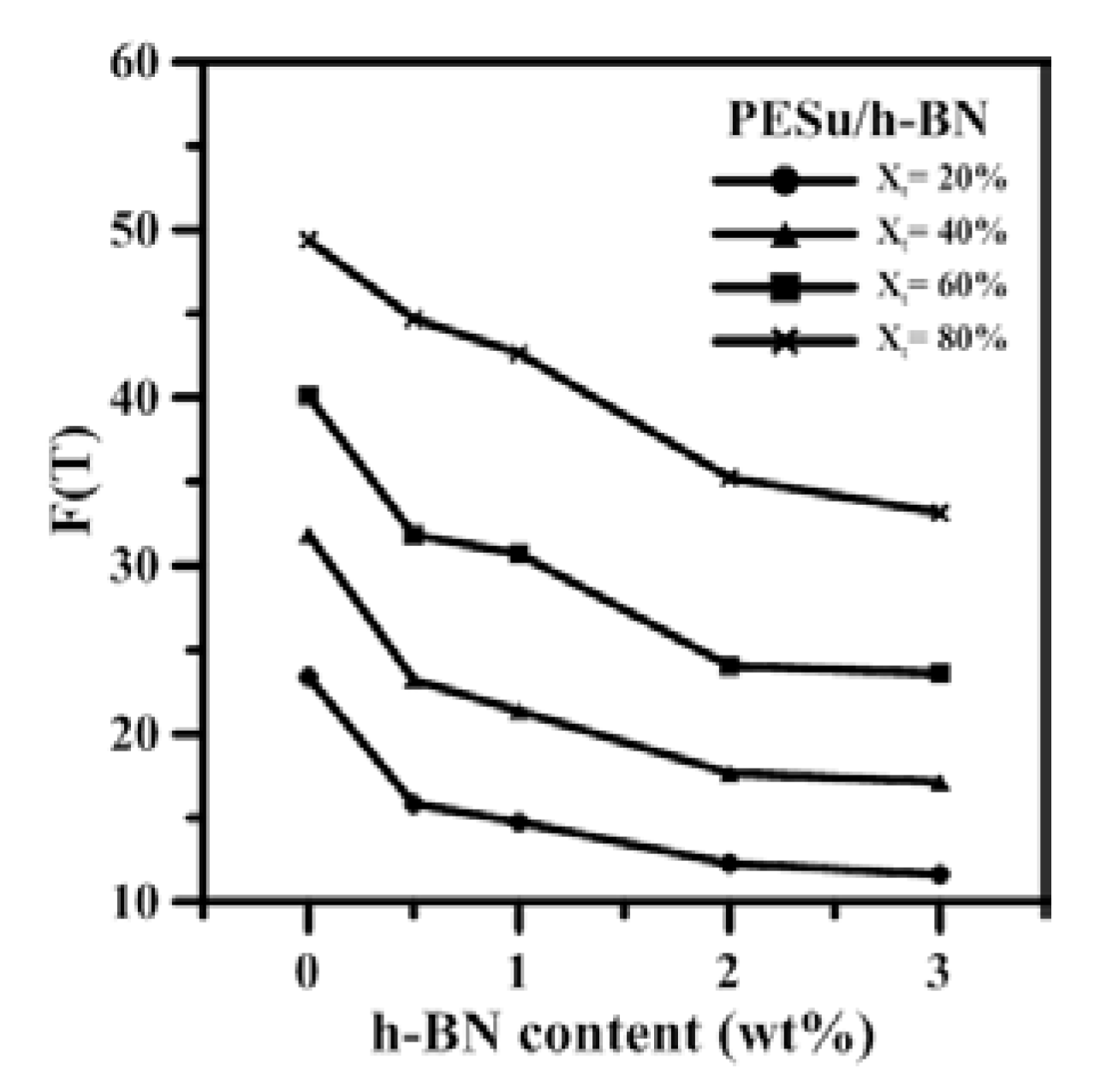
3.2. Isothermal Crystallization Investigation of PESu/h-BN Composites
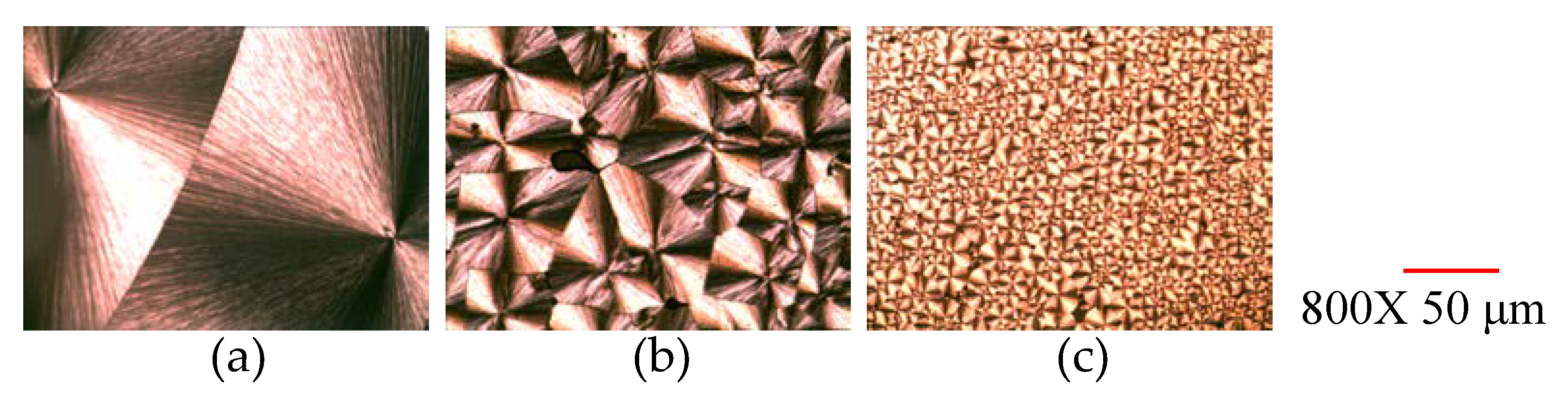
3.3. Spherulite Morphology Revealed by POM
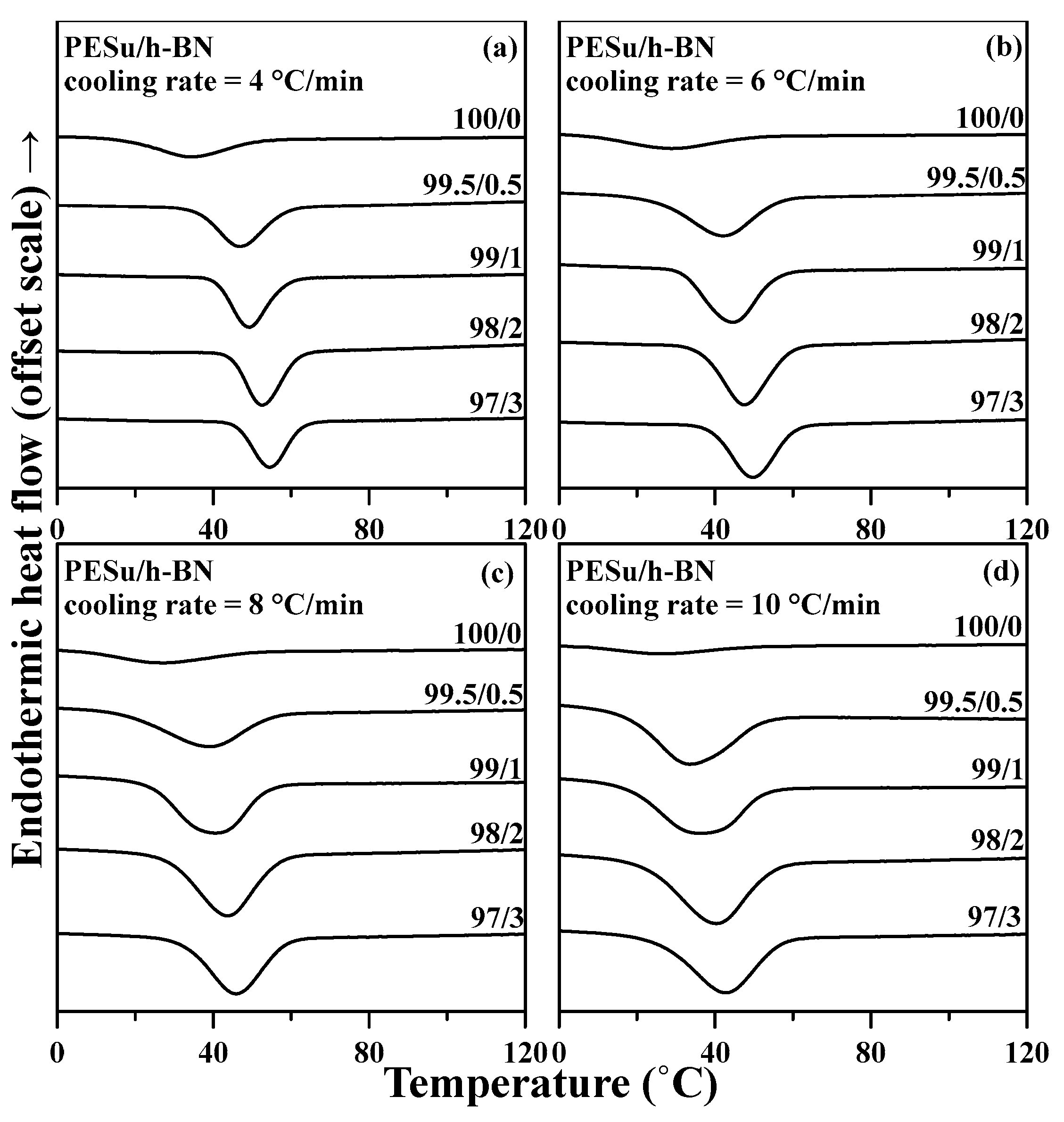
3.4. Non-Isothermal Crystallization Behaviors of PESu/h-BN Composites
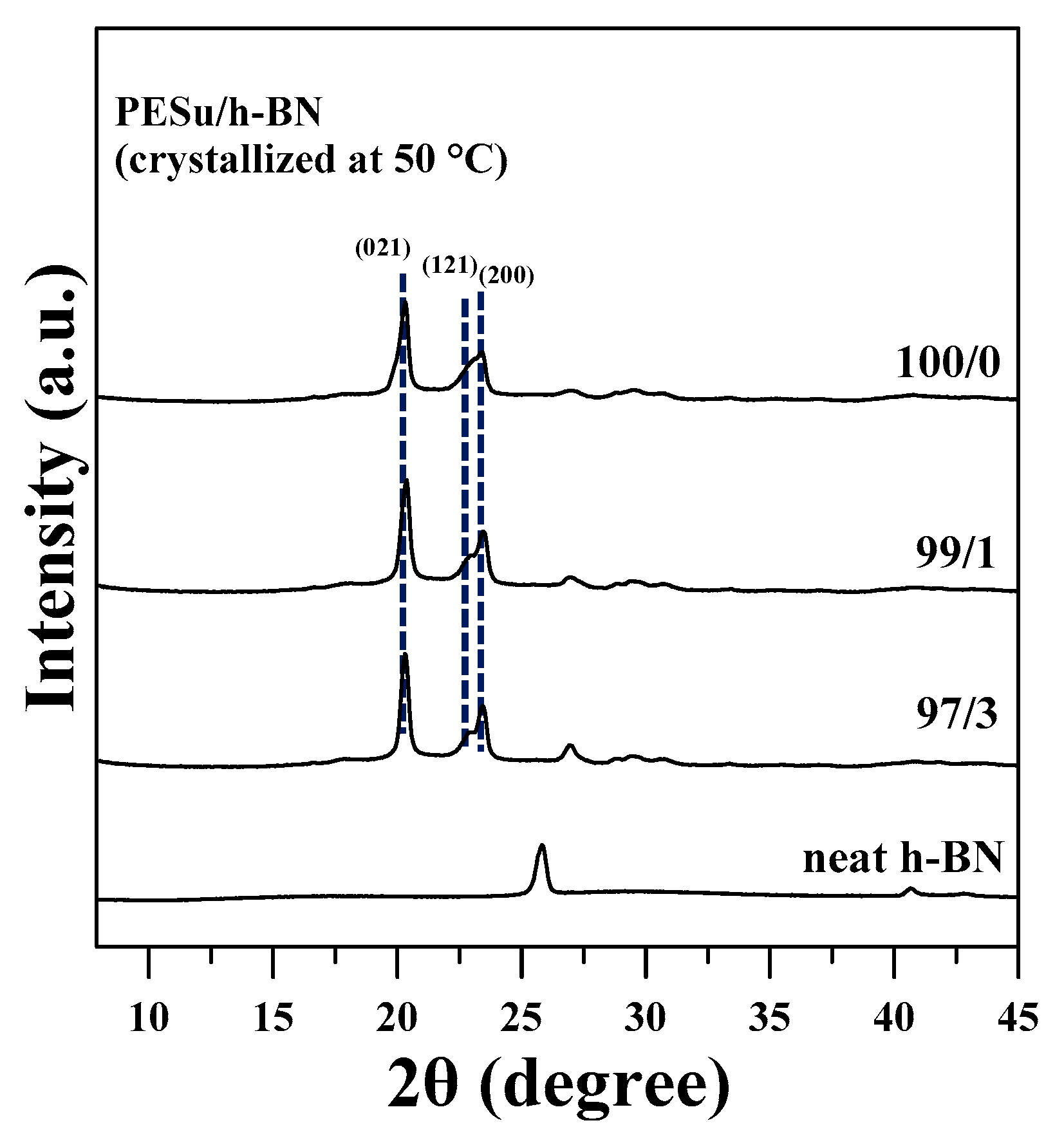
3.5. WAXD Studies of PESu/h-BN Composites
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Woo, E.M.; Yen, K.-C.; Yeh, Y.-T.; Wang, L.-Y. Biomimetically Structured Lamellae Assembly in Periodic Banding of Poly(ethylene adipate) Crystals. Macromolecules 2018, 51, 3845–3854. [Google Scholar] [CrossRef]
- Ray, S.S.; Bousmina, M. Biodegradable polymers and their layered silicate nanocomposites: In greening the 21st century materials world. Prog. Mater. Sci. 2005, 50, 962–1079. [Google Scholar] [CrossRef]
- Santagata, G.; Valerio, F.; Cimmino, A.; Poggetto, G.D.; Masi, M.; Di Biase, M.; Malinconico, M.; Lavermicocca, P.; Evidente, A. Chemico-physical and antifungal properties of poly(butylene succinate)/cavoxin blend: Study of a novel bioactive polymeric based system. Eur. Polym. J. 2017, 94, 230–247. [Google Scholar] [CrossRef]
- Khan, A.K.; Ho, J.C.S.; Roy, S.; Liedberg, B.; Nallani, M. Facile Mixing of Phospholipids Promotes Self-Assembly of Low-Molecular-Weight Biodegradable Block Co-Polymers into Functional Vesicular Architectures. Polymers 2020, 12, 979. [Google Scholar] [CrossRef] [Green Version]
- Nanaki, S.; Viziridou, A.; Zamboulis, A.; Kostoglou, M.; Papageorgiou, G.Z.; Bikiaris, D.N. New Biodegradable Poly(l-lactide)-Block-Poly(propylene adipate) Copolymer Microparticles for Long-Acting Injectables of Naltrexone Drug. Polymers 2020, 12, 852. [Google Scholar] [CrossRef] [Green Version]
- Corres, M.Á.; Mayor, Á.; Sangroniz, A.; del Río, J.; Iriarte, M.; Etxeberria, A. Blends based on biodegradable poly(caprolactone) with outstanding barrier properties for packaging applications: The role of free volume and interactions. Eur. Polym. J. 2020, 135, 109869. [Google Scholar] [CrossRef]
- Gu, J.-D. Microbiological deterioration and degradation of synthetic polymeric materials: Recent research advances. Int. Biodeterior. Biodegrad. 2003, 52, 69–91. [Google Scholar] [CrossRef]
- Woo, E.M.; Lugito, G. Origins of periodic bands in polymer spherulites. Eur. Polym. J. 2015, 71, 27–60. [Google Scholar] [CrossRef]
- Courgneau, C.; Ducruet, V.; Avérous, L.; Grenet, J.; Domenek, S. Nonisothermal crystallization kinetics of poly(lactide)-effect of plasticizers and nucleating agent. Polym. Eng. Sci. 2013, 53, 1085–1098. [Google Scholar] [CrossRef]
- Gumede, T.P.; Luyt, A.S.; Hassan, M.K.; Pérez-Camargo, R.A.; Tercjak, A.; Müller, A.J. Morphology, Nucleation, and Isothermal Crystallization Kinetics of Poly(ε-caprolactone) Mixed with a Polycarbonate/MWCNTs Masterbatch. Polymers 2017, 9, 709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, Z.; Li, Z. Effect of Orotic Acid on the Crystallization Kinetics and Morphology of Biodegradable Poly(l-lactide) as an Efficient Nucleating Agent. Ind. Eng. Chem. Res. 2011, 50, 12299–12303. [Google Scholar] [CrossRef]
- Tang, L.; Qiu, Z. Enhanced nonisothermal and isothermal cold crystallization kinetics of biodegradable poly(l-lactide) by trisilanolisobutyl-polyhedral oligomeric silsesquioxanes in their nanocomposites. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Hua, L.; Kai, W.H.; Inoue, Y. Crystallization behavior of poly(ε-caprolactone)/graphite oxide composites. J. Appl. Polym. Sci. 2007, 106, 4225–4232. [Google Scholar] [CrossRef]
- Chen, Y.-A.; Wu, T.-M. Crystallization Kinetics of Poly(1,4-butylene adipate) with Stereocomplexed Poly(lactic acid) Serving as a Nucleation Agent. Ind. Eng. Chem. Res. 2014, 53, 16689–16695. [Google Scholar] [CrossRef]
- Prasannan, A.; Bich-Tram, T.-L.; Hsu, D.-Y.; Hong, P.-D.; Pan, G.-R. Nucleation effects of α-cyclodextrin inclusion complexes on the crystallization behavior of biodegradable poly(1,4-butylene adipate). CrystEngComm 2013, 15, 5119–5126. [Google Scholar] [CrossRef]
- Bosq, N.; Aht-Ong, D. Isothermal and non-isothermal crystallization kinetics of poly(butylene succinate) with nanoprecipitated calcium carbonate as nucleating agent. J. Therm. Anal. Calorim. 2018, 132, 233–249. [Google Scholar] [CrossRef]
- Lee, L.-T.; Hsu, C.-Y.; Hung, S.-P. Promoted crystallization kinetics of biodegradable poly(butylene succinate) by a nucleation agent of green chemical. J. Polym. Res. 2019, 26, 1–9. [Google Scholar] [CrossRef]
- Tang, J.; Li, L.; Wang, X.; Yang, J.; Liang, X.; Li, Y.; Ma, H.; Zhou, S.; Wang, J. Tailored crystallization behavior, thermal stability, and biodegradability of poly(ethylene adipate): Effects of a biocompatible diamide nucleating agent. Polym. Test. 2020, 81, 106116. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Terzopoulou, Z.; Achilias, D.S.; Bikiaris, D.N.; Kapnisti, M.; Gournis, D. Biodegradable poly(ethylene succinate) nanocomposites. Effect of filler type on thermal behaviour and crystallization kinetics. Polymers 2013, 54, 4604–4616. [Google Scholar] [CrossRef]
- Morales-Huerta, J.; De Ilarduya, A.; Muñoz-Guerra, S. A green strategy for the synthesis of poly(ethylene succinate) and its copolyesters via enzymatic ring opening polymerization. Eur. Polym. J. 2017, 95, 514–519. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Terzopoulou, Z.; Tsanaktsis, V.; Achilias, D.; Triantafyllidis, K.; Diamanti, E.K.; Gournis, D.; Bikiaris, D.N. Effect of graphene oxide and its modification on the microstructure, thermal properties and enzymatic hydrolysis of poly(ethylene succinate) nanocomposites. Thermochim. Acta 2015, 614, 116–128. [Google Scholar] [CrossRef]
- Tang, L.; Qiu, Z. Crystallization Kinetics and Morphology of Biodegradable Poly(ethylene succinate)/Octavinyl-Polyhedral Oligomeric Silsesquioxanes Nanocomposites. Ind. Eng. Chem. Res. 2014, 53, 11365–11370. [Google Scholar] [CrossRef]
- Jing, X.; Qiu, Z. Influence of Thermally Reduced Graphene Low-Loadings on the Crystallization Behavior and Morphology of Biodegradable poly(ethylene succinate). Ind. Eng. Chem. Res. 2014, 53, 498–504. [Google Scholar] [CrossRef]
- Vasileiou, A.A.; Papageorgiou, G.Z.; Kontopoulou, M.; Docoslis, A.; Bikiaris, D. Covalently bonded poly(ethylene succin-ate)/SiO2 nanocomposites prepared by in situ polymerisation. Polymer 2013, 54, 1018–1032. [Google Scholar] [CrossRef]
- Ray, S.S.; Makhatha, M.E. Thermal properties of poly(ethylene succinate) nanocomposite. Polymers 2009, 50, 4635–4643. [Google Scholar] [CrossRef]
- Teng, S.; Jiang, Z.; Qiu, Z. Effect of different POSS structures on the crystallization behavior and dynamic mechanical properties of biodegradable Poly(ethylene succinate). Polymers 2019, 163, 68–73. [Google Scholar] [CrossRef]
- Li, Z.; Kong, J.; Han, L.; Zhang, H.; Dong, L. Effect of crystallinity on the thermal conductivity of poly(3-hydroxybutyrate)/BN composites. Polym. Bull. 2017, 75, 1651–1666. [Google Scholar] [CrossRef]
- Refaa, Z.; Boutaous, M.; Xin, S.; Fulchiron, R. Synergistic effects of shear flow and nucleating agents on the crystallization mechanisms of Poly (Lactic Acid). J. Polym. Res. 2017, 24, 18. [Google Scholar] [CrossRef]
- Gumede, T.P.; Luyt, A.S.; Camargo, R.A.P.; Tercjak, A.; Müller, A.J. Morphology, Nucleation, and Isothermal Crystallization Kinetics of Poly(Butylene Succinate) Mixed with a Polycarbonate/MWCNT Masterbatch. Polymers 2018, 10, 424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arjmandi, R.; Hassan, A.; Eichhorn, S.J.; Haafiz, M.K.M.; Zakaria, Z.; Tanjung, F. Enhanced ductility and tensile properties of hybrid montmorillonite/cellulose nanowhiskers reinforced polylactic acid nanocomposites. J. Mater. Sci. 2015, 50, 3118–3130. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Weng, Q.; Wang, X.-B.; Li, X.; Zhang, J.; Golberg, D.; Bando, Y. Recent Progress on Fabrications and Applications of Boron Nitride Nanomaterials: A Review. J. Mater. Sci. Technol. 2015, 31, 589–598. [Google Scholar] [CrossRef]
- Düzcükoğlu, H.; Ekinci, Ş.; Sahin, Ö.S.; Avci, A.; Ekrem, M.; Ünaldi, M. Enhancement of Wear and Friction Characteristics of Epoxy Resin by Multiwalled Carbon Nanotube and Boron Nitride Nanoparticles. Tribol. Trans. 2015, 58, 635–642. [Google Scholar] [CrossRef]
- Farshid, B.; Lalwani, G.; Mohammadi, M.S.; Simonsen, J.; Sitharaman, B. Boron nitride nanotubes and nanoplatelets as rein-forcing agents of polymeric matrices for bone tissue engineering. J. Biomed. Mater. Res. Part B 2017, 105, 406–419. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhang, X.; Yang, M.; Yang, S.; Wu, H.; Guo, S.; Wang, Y. Ordered multilayer film of (graphene oxide/polymer and boron nitride/polymer) nanocomposites: An ideal EMI shielding material with excellent electrical insulation and high thermal conductivity. Compos. Sci. Technol. 2016, 136, 104–110. [Google Scholar] [CrossRef]
- Wang, H.; Su, X.; Song, T.; Li, Z.; Zhao, Y.; Lou, H.; Wang, J. Scalable exfoliation and dispersion of few-layer hexagonal boron nitride nanosheets in NMP-salt solutions. Appl. Surf. Sci. 2019, 488, 656–661. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Zhu, W.; Chen, Z.; Pan, J.; Xu, K. Effect of nucleation agents on the crystallization of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) (P3/4HB). J. Appl. Polym. Sci. 2010, 116, 1116–1123. [Google Scholar] [CrossRef]
- Hsu, T.-C.; Lee, L.-T.; Wu, X.-Y. Investigations on Novel Ternary Green Polymer Composite. Processes 2019, 8, 31. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, J.; Luciano, G.; Maggiore, S. Nonisothermal crystallization behavior of polylactide/polyethylene glycol/graphene oxide nanosheets composite films. Polym. Compos. 2020, 41, 2108–2119. [Google Scholar] [CrossRef]
- Lee, L.-T.; Ke, Y.-L. Superior Crystallization Kinetics Caused by the Remarkable Nucleation Effect of Graphene Oxide in Novel Ternary Biodegradable Polymer Composites. ACS Omega 2020, 5, 30643–30656. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Zhang, K.; Su, Z.; Qiu, Z. Thermal behavior, mechanical and rheological properties, and hydrolytic degradation of novel branched biodegradable poly(ethylene succinate) copolymers. Polym. Test. 2018, 66, 64–69. [Google Scholar] [CrossRef]
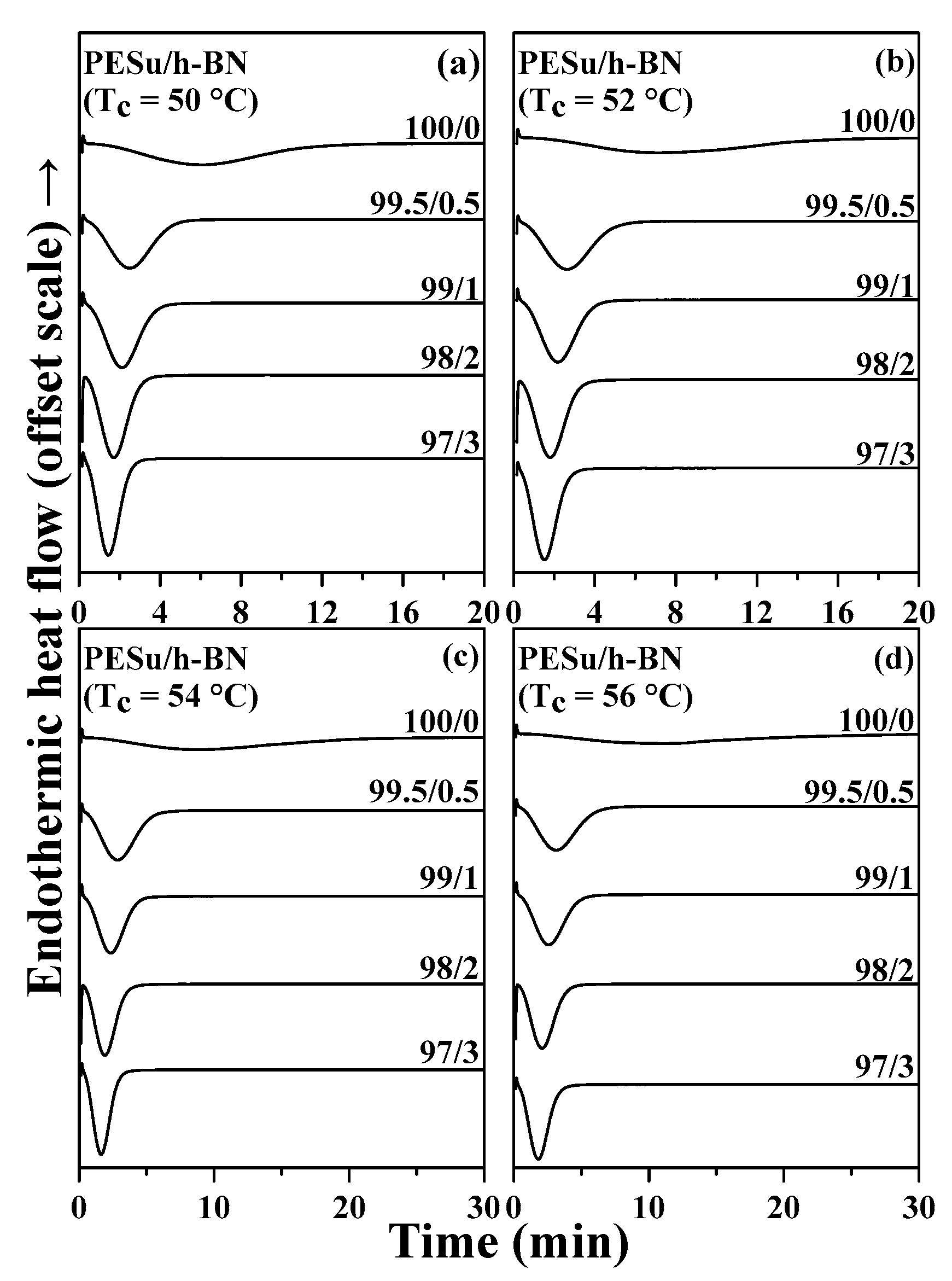
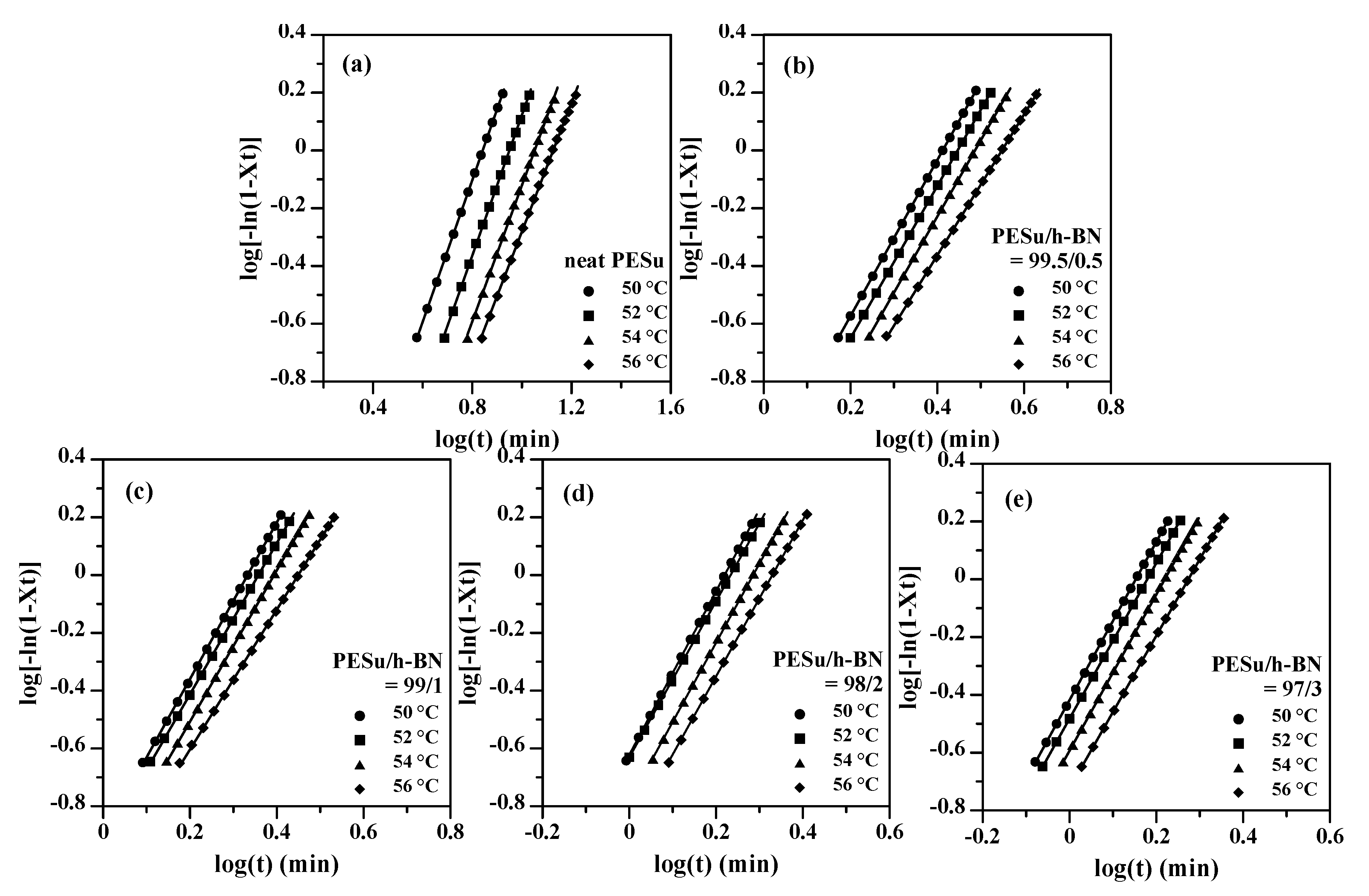


| PESu/h-BN (wt.%) | Tc (°C) | n | k (min−n) |
|---|---|---|---|
| 100/0 | 50 | 2.45 | 0.0086 |
| 52 | 2.43 | 0.0049 | |
| 54 | 2.35 | 0.0034 | |
| 56 | 2.23 | 0.0031 | |
| 99.5/0.5 | 50 | 2.70 | 0.0772 |
| 52 | 2.63 | 0.0667 | |
| 54 | 2.63 | 0.0522 | |
| 56 | 2.44 | 0.0459 | |
| 99/1 | 50 | 2.71 | 0.1253 |
| 52 | 2.62 | 0.1159 | |
| 54 | 2.62 | 0.0931 | |
| 56 | 2.42 | 0.0676 | |
| 98/2 | 50 | 2.83 | 0.2389 |
| 52 | 2.70 | 0.2340 | |
| 54 | 2.74 | 0.1652 | |
| 56 | 2.70 | 0.1285 | |
| 97/3 | 50 | 2.74 | 0.3817 |
| 52 | 2.69 | 0.3293 | |
| 54 | 2.71 | 0.2550 | |
| 56 | 2.64 | 0.1888 |
| PESu/h-BN (wt.%) | Xt (%) | F(T) |
|---|---|---|
| 100/0 | 20 | 23.37 |
| 40 | 31.84 | |
| 60 | 40.08 | |
| 80 | 49.37 | |
| 99.5/0.5 | 20 | 15.83 |
| 40 | 23.22 | |
| 60 | 31.83 | |
| 80 | 44.70 | |
| 99/1 | 20 | 14.75 |
| 40 | 20.84 | |
| 60 | 29.88 | |
| 80 | 42.64 | |
| 98/2 | 20 | 12.27 |
| 40 | 17.66 | |
| 60 | 24.05 | |
| 80 | 35.22 | |
| 97/3 | 20 | 11.64 |
| 40 | 17.14 | |
| 60 | 23.61 | |
| 80 | 33.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, L.-T.; Tseng, H.-Y.; Wu, T.-Y. Crystallization Behaviors of Composites Comprising Biodegradable Polyester and Functional Nucleation Agent. Crystals 2021, 11, 1260. https://doi.org/10.3390/cryst11101260
Lee L-T, Tseng H-Y, Wu T-Y. Crystallization Behaviors of Composites Comprising Biodegradable Polyester and Functional Nucleation Agent. Crystals. 2021; 11(10):1260. https://doi.org/10.3390/cryst11101260
Chicago/Turabian StyleLee, Li-Ting, Hsiang-Yun Tseng, and Tzi-Yi Wu. 2021. "Crystallization Behaviors of Composites Comprising Biodegradable Polyester and Functional Nucleation Agent" Crystals 11, no. 10: 1260. https://doi.org/10.3390/cryst11101260
APA StyleLee, L.-T., Tseng, H.-Y., & Wu, T.-Y. (2021). Crystallization Behaviors of Composites Comprising Biodegradable Polyester and Functional Nucleation Agent. Crystals, 11(10), 1260. https://doi.org/10.3390/cryst11101260