Abstract
Methanol electrochemical oxidation in a direct methanol fuel cell (DMFC) is considered to be an efficient pathway for generating renewable energy with low pollutant emissions. NiO−CuO and Ni0.95Cr0.05O2+δ thin films were synthesized using a simple dip-coating method and tested for the electro-oxidation of methanol. These synthesized electrocatalysts were characterized by X-ray diffraction spectroscopy (XRD), X-ray photoelectron spectroscopy (XPS), Scanning electron microscopy (SEM), Energy-dispersive X-ray spectroscopy (EDS), and Raman spectroscopy. Different electrochemical techniques were used to investigate the catalytic activity of these prepared electrocatalysts for methanol oxidation, including linear sweep voltammetry (LSV), electrochemical impedance spectroscopy (EIS), and chronoamperometry (CA). In the presence of 0.3 M methanol, the current densities of NiO−CuO and Ni0.95Cr0.05O2+δ thin films were found to be 12.2 mA·cm−2 and 6.5 mA·cm−2, respectively. The enhanced catalytic activity of NiO−CuO and Ni0.95Cr0.05O2+δ thin films may be a result of the synergistic effect between different metal oxides. The Chronoamperometry (CA) results of the mixed metal oxide thin films confirmed their stability in basic media. Furthermore, the findings of electrochemical impedance spectroscopy (EIS) of mixed metal oxide thin films demonstrated a lower charge transfer resistance as compared to the pure NiO, CuO, and Cr2O3 thin films.
1. Introduction
Due to the rapid increase in worldwide energy consumption and detrimental environmental emissions from conventional fossil fuel, explorations of alternative clean energy resources and economical devices for efficient energy conversions are demanded [1,2,3,4]. To manage these problems, direct methanol fuel cells (DMFCs) have attracted extensive research attention, owing to the benefits they provide of high energy conversion efficiency, low pollution emissions, high energy density, easy fuel storage, and ambient operating conditions [5]. Pt-based catalysts were commonly used in DMFCs for methanol oxidation [6,7]. Even though Pt and Pt-based catalysts are widespread and effective for catalysis, they still present significant challenges such as their high cost, restricted availability, low stability, and the fact that they are easily poisoned by CO-like intermediates produced during the methanol oxidation reaction (MOR) [8,9].
Recently, DMFCs that use transition metal oxides catalysts such as NiOx, CoOx, CuOx, CrOx, ZnOx, MnOx, and FeOx, have shown improved reaction kinetics as well as strong anti-poisoning potential [10,11,12,13,14]. Therefore, transition metal oxides and their composites have attracted considerable attention as electrocatalysts due to their low electrical resistance, low cost, easy preparation, and excellent catalytic activity [15,16,17,18]. From among these metal oxide composites, nickel-based materials are emerging as one of the most promising candidates due to their chemical stability, electrical properties, and the ability to remove intermediate COad in alkaline media [19,20,21]. Several researchers have demonstrated that the addition of non-precious metallic materials, complexes, and oxides with nickel-based catalysts has increase catalytic activity and durability [22]. Multiple investigations have documented the electrocatalytic activity of NiO, produced through various methods, for methanol electro-oxidation in alkaline media. According to Ghanem et al., mesoporous NiO/N-CNF and Ni/Ni(OH)2 have shown excellent stability and electrochemical activity for the electro-oxidation of methanol in alkaline media. Therefore, these NiO based binary systems are expected to exhibit improved electrocatalytic efficacy in methanol oxidation [23]. These catalyst’s electro-oxidation processes include the formation of a higher valence nickel oxide, which serves as a chemical oxidizing agent [13]. The electrocatalytic activity is primarily determined by the catalytic role of Ni(OH)2/NiOOH, which is an important electron transfer intermediate for the oxidation of alcohols in alkaline solutions. Moreover, increasing the number of active sites improves NiO catalytic activity [24].
Similarly, oxides of Cu and Cr have demonstrated exceptional methanol oxidation in an alkaline medium using various fabrication methods and morphologies [25,26]. As a result, the mixed metal oxides of Ni, Cu, and Cr oxides are expected to possess a high electrocatalytic activity and stability in alkaline DMFCs for methanol oxidation. In the present work, the composite and solid solution of Cu and Cr with Ni oxides is synthesized. The as-prepared mixed metal oxide electrodes have shown improved electrocatalytic properties towards MeOH oxidation and high tolerance to CO poisoning. It is also found that these oxides have good conductivity. Furthermore, their electrochemical activity is influenced by the synthesis of the catalyst material and the fabrication of the electrode. For the deposition of metal oxide thin films, several techniques have been reported including spin coating [27], electrodeposition [28], chemical vapor deposition [29,30,31] and so on. Other studies have used the dip coating approach in the presence of simple, soluble precursor solutions. This technique is low cost and requires simple processing conditions [32,33]. Furthermore, the dip coating method has advantages such as repeatability, homogeneity, safety, and purity at low temperatures. We selected the dip-coating method to fabricate electrocatalysts on the conductive substrate without the use of a binder or carbon content. This approach not only offers superior substrate adhesion, but also facilitates the electrochemical reaction cost-effectively.
In the present study, mixed metal oxide catalysts NiO−CuO and Ni0.95Cr0.05O2+δ were synthesized by a simple dip-coating method for the enhancement of electrocatalytic activity of the nickel-based catalyst for methanol electrooxidation in an alkaline medium. The catalysts were characterized by X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), Scanning electron microscopy (SEM), Raman spectroscopy, and Atomic force spectroscopy (AFM). The electrocatalytic properties of the Ni–M (M = Cr, Cu) nano-oxides for MOR were studied by linear sweep voltammetry (LSV), chronoamperometry (CA), and electrochemical impedance spectroscopy (EIS).
2. Materials and Methods
The Fluorine-doped tin oxide (FTO) coated glass substrates (surface resistivity ∼8 Ω/sq), nickel (II) acetate tetrahydrate, (Ni(CH3COO)2·4H2O), copper (II) acetate, (Cu(CH3COO)2), chromium (III) acetylacetonate (Cr(C5H7O2)3), ethanol (C2H5OH), methanol (CH3OH) and sodium hydroxide (NaOH) were bought from Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany. The reagents were of analytical grade and used without any further treatment. Deionized water was used for both synthesis and treatment under atmospheric conditions.
Synthesis of Mixed Metal Oxide Thin Films
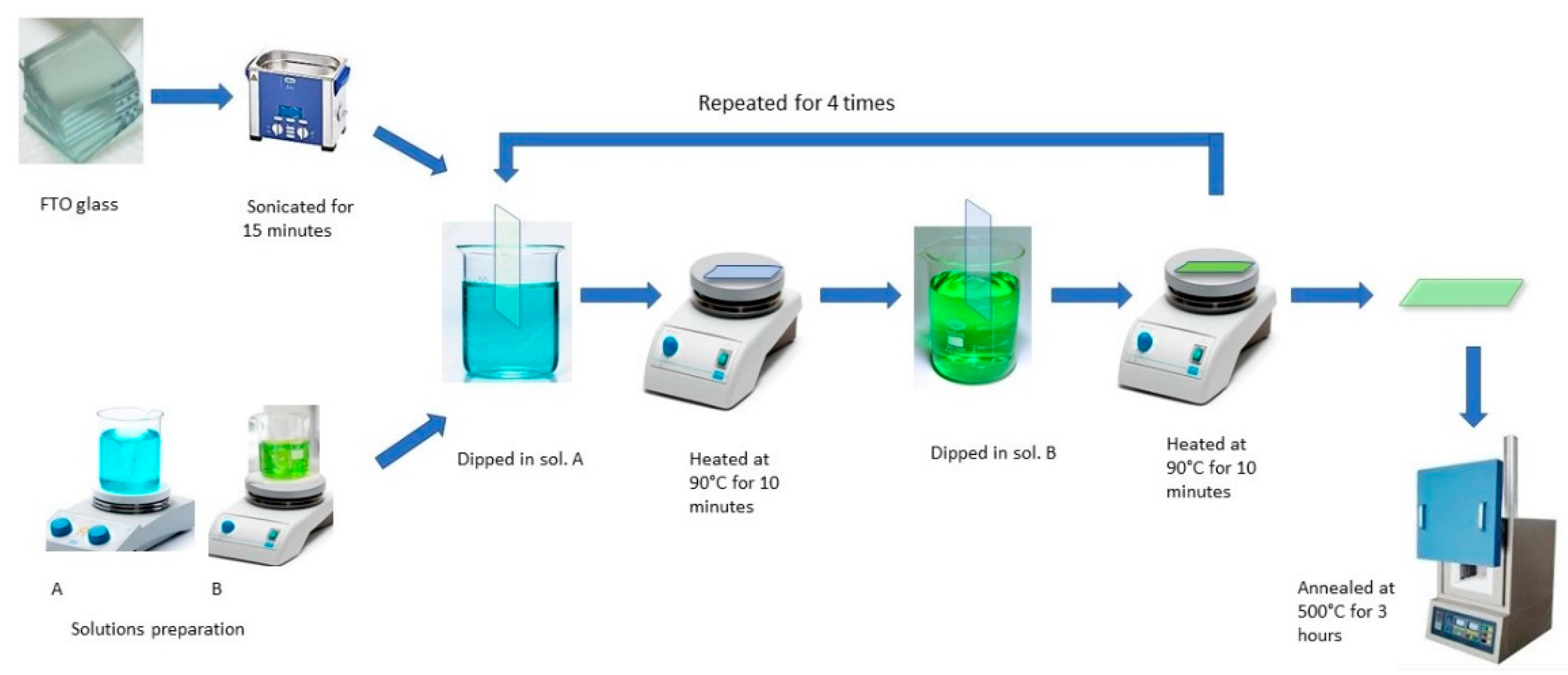
The NiO−CuO and Ni0.95Cr0.05O2+δ thin films were prepared on an FTO glass substrate using a simple dip-coating method. For a typical reaction 0.1 M Ni, Cu, and Cr precursor solutions were prepared by dissolving (Ni(CH3COO)2·4H2O), (Cu(CH3COO)2), and (Cr(C5H7O2)3), respectively, in a 5 mL mixture of methanol and ethanol (1:1) followed by stirring for 15 min. The FTO glass substrates (2 × 1 cm2) were washed thoroughly with detergent followed by ultrasonic cleaning in a 1:1 solution of ethanol and acetone for 20 min and then dried in air at room temperature before coating in precursor solutions. To prepare the NiO−CuO thin-film, the dried FTO glass substrate was first immersed in the Ni precursor solution for 30 s. After 30 s, it was removed from the precursor solution and dried by heating at 90 °C for 10 min followed by immersion in the Cu precursor solution for 30 s and heating at 90 °C for 10 min. This process of alternative dip-coating was repeated four times and finally, washing was performed with distilled water. Ni0.95Cr0.05O2+δ thin films were also prepared by the same alternative dip-coating of FTO glass substrate in 0.1 M Ni and Cr precursor solutions. Finally, coated NiO−CuO and Ni0.95Cr0.05O2+δ thin films were calcined in a muffled furnace at a temperature of 500 °C for 3 h. The overall scheme is available in Figure 1. The FTO glass substrate was preferred over glass carbon electrode (GCE) for direct coating of the catalyst from precursor via dip coating. Because, after dipping into the precursor solution, the FTO glass substrate was annealed at 500 °C, the catalyst film strongly adhered to the substrate, and an extra binder was not required for coating purpose. However, dip-coating from the precursor solution, followed by heating above 400 °C is not possible for the glassy carbon electrode (carbon material in presence of O2 above 400 °C may decompose). Furthermore, when a powdered sample is loaded on GCE the adhesion is very poor and leaves the surface of the substrate during catalytic activity. The addition of a binder (nafion) provides better adhesion, however, the binder obstructs the flow of the electron from the catalyst to the GCE, which leads to poor catalytic performance.
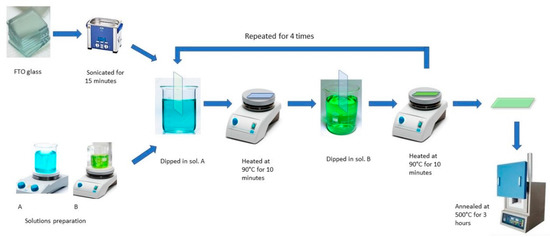
Figure 1.
Schematic diagram of dip coating method.
These fabricated NiO−CuO, Ni0.95Cr0.05O2+δ thin films were subjected to different physical and chemical characterizations. The phase and crystal structure of synthesized NiO−CuO, Ni0.95Cr0.05O2+δ thin films were assessed by X-Ray diffractometer (BRUKER D8 Advance XRD, Karlsruhe, Germany) equipped with Cu Kα radiation of λ = 1.540608 Å and operated at 2θ (20–80°). X-ray photoelectron spectroscopy (XPS) (Versa probe II, ULVAC-PHI, Inc. Chanhassen, MN, USA) studies were conducted under an ultra-high vacuum (~10−10 mbar). Surface morphology and elemental compositions of thin films were observed by FESEM (JEOL JSM-7600F, Tokyo, Japan) fitted with Oxford energy-dispersive X-ray spectroscopy (EDS, High Wycombe, UK). An atomic force microscopy (Nanosurf C3000 AFM, Nanosurf, Liestal, Switzerland) technique [AFM-NT-MDT] was used in a non-contact mode on all the fabricated films to study the surface topography and roughness using the attached NT-MDT software. Raman spectra were recorded under ambient conditions using Raman micro-spectroscopy (uRaman-532 TEC-Ci, Technospex, Singapore), equipped with a 532 nm laser and an optical microscope (Nikon Eclipse Ci L, Singapore).
Electrochemical studies of methanol oxidation were performed on GAMRY G750 (Gamry Instruments, Inc. Warminster, PA, USA) in the potentiostatic mode by using three testing techniques, including linear sweep voltammetry (LSV), chronoamperometry (CA), and electrochemical impedance spectroscopy (EIS). The NiO−CuO and Ni0.95Cr0.05O2+δ thin films operated as a working electrode, with a platinum wire that served as a counter electrode and Ag/AgCl, KCl (3 M) as a reference electrode. A supporting electrolyte was used, namely, 0.5 M NaOH. The activity of thin films for methanol electro-oxidation was investigated using an active area of 1 cm2.
3. Results and Discussion
3.1. X-ray Diffraction
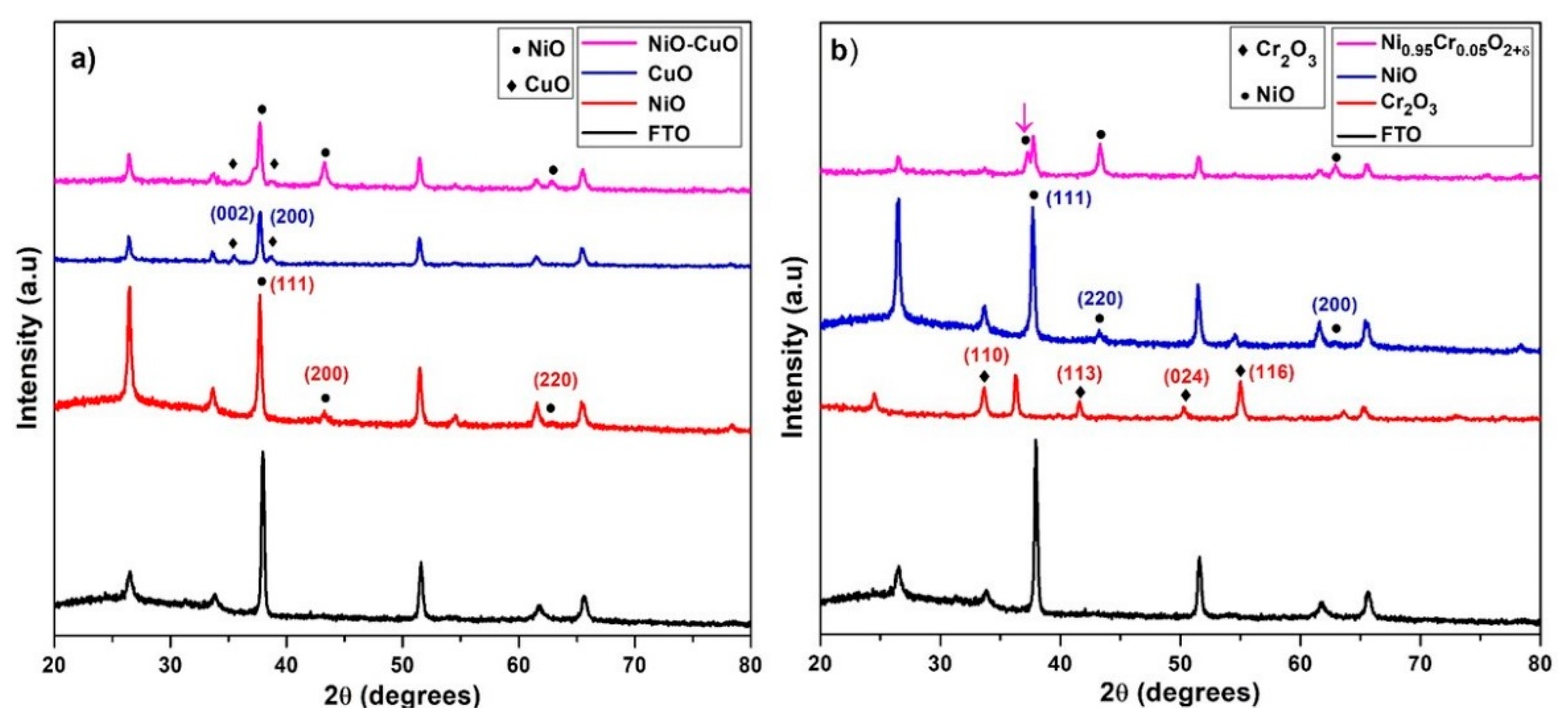
The powder X-ray diffraction measurements were used to examine the crystallographic structures and phase details of thin films. Figure 2a displays the XRD spectra of the NiO, CuO, and NiO−CuO thin films. The diffraction peaks of NiO and CuO thin films are in agreement with the standard spectrum of cubic NiO phase (JCPDS 01−073−1519) [34] and monoclinic CuO phase (JCPDS 45-0937) [35]. The XRD spectra of the NiO/CuO thin film show three diffraction peaks of NiO at 2θ = 37.7°, 43.3° and 61.6°, indexed as (111), (200), and (220) plane orientations and two distinct diffraction peaks of CuO at 2θ = 35.4° and 38.6°, which were assigned as (002) and (200) plane orientations. This suggests the existence of both crystalline NiO and CuO in NiO−CuO thin film. Using the Scherer equation, the average crystallite sizes of CuO, NiO, and NiO−CuO were estimated as 30.3, 22.25 and 32 nm respectively. Figure 2b displays X-ray diffraction patterns of NiO, Cr2O3, and Ni0.95Cr0.05O2+δ. Cr2O3 thin film patterns show diffraction peaks at 2θ = 24.5°, 33.6°, 36.3°, and 54.9° which are assigned to the rhombohedral phase Cr2O3 (110), (113), (024), and (116) lattice planes (JCPDS file 00−038−1479) [36]. Ni0.95Cr0.05O2+δ spectra show no distinct diffraction peaks of Cr2O3 besides the cubic NiO phase (JCPDS 01−073−1519) and a small shift in NiO peaks in the solid solution indicates the occupation of some nickel sites by chromium ions. It is believed that the replacement of Ni2+ ions and Cr3+ ions generated some distortions in the normal cubic structure of NiO that caused a slight shift in the XRD reflections. The incorporation of Cr3+ ions is further supported by XPS and EDS results. The average crystallite sizes of Cr2O3 and Ni0.95Cr0.05O2+δ, calculated from diffraction peaks, are found to be 38.1 and 32.2 nm respectively.
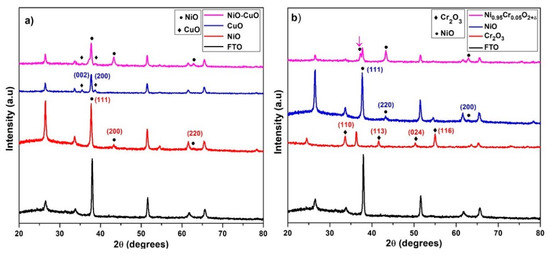
Figure 2.
XRD of (a) NiO, CuO, and NiO−CuO (b) Cr2O3, NiO and Ni0.95Cr0.05O2+δ.
3.2. X-ray Photoelectron Spectroscopy
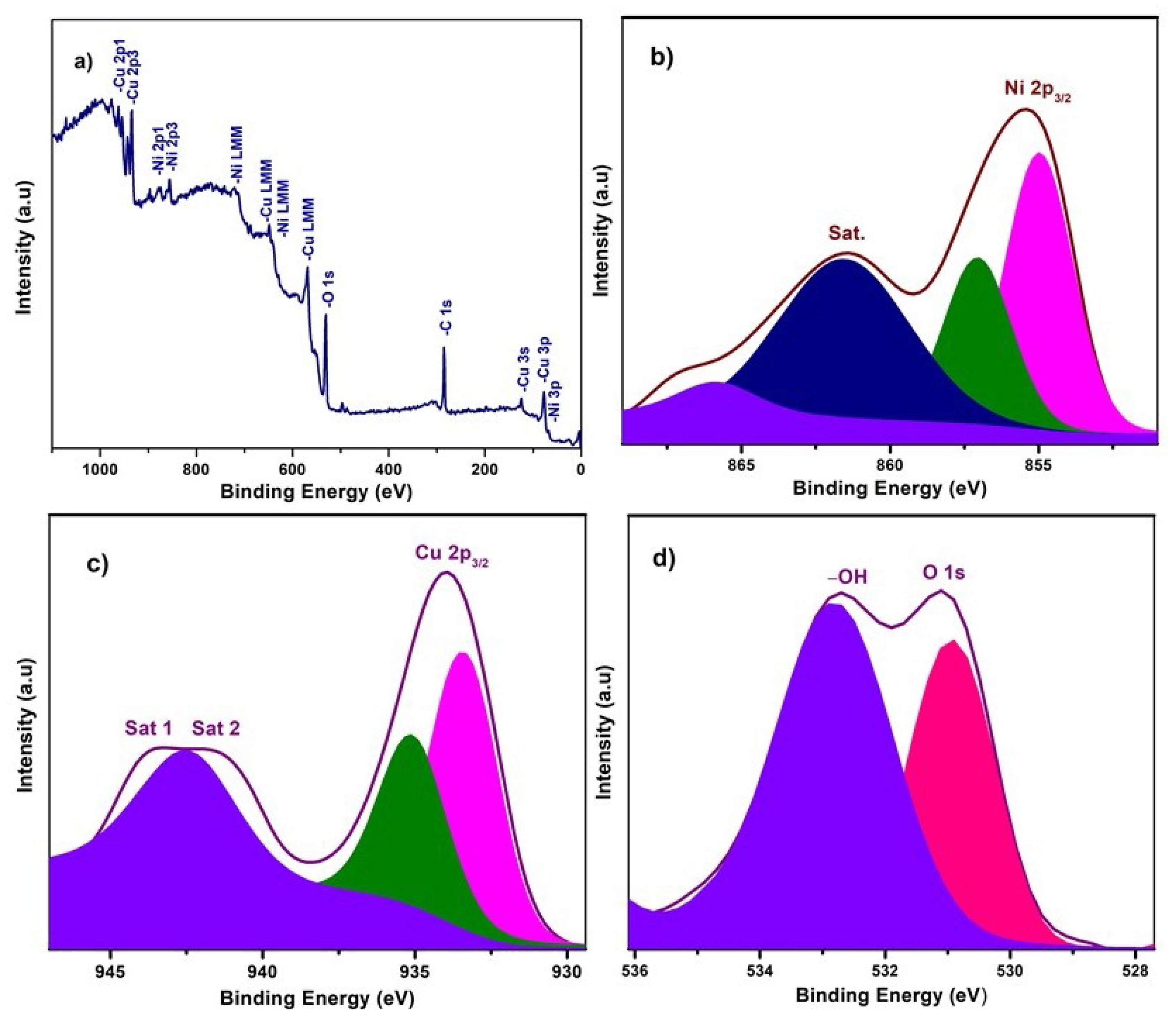
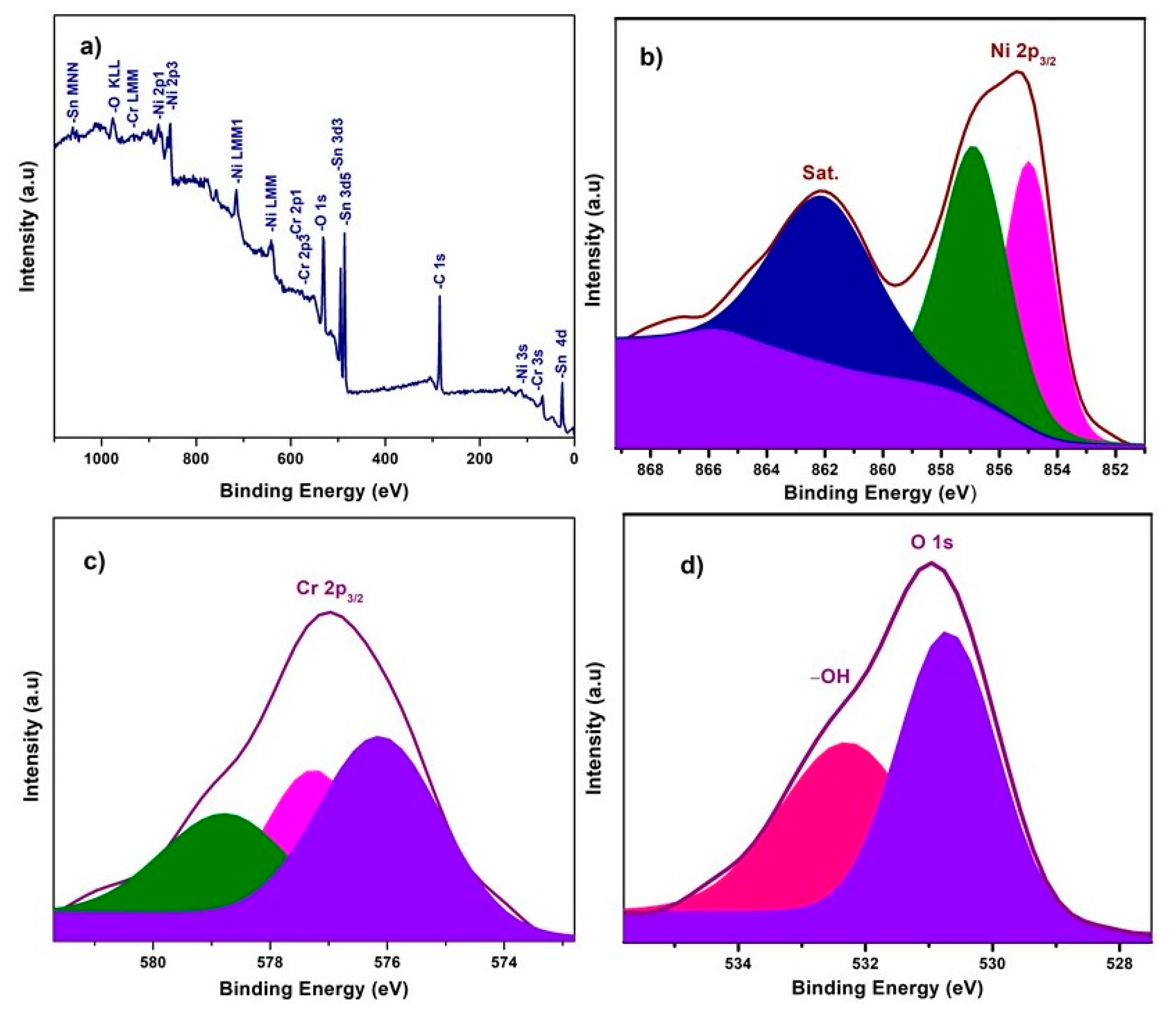
X-ray photoelectron spectroscopy (XPS) experiments were carried out to investigate the chemical composition and chemical bonding state of elements in the NiO−CuO and Ni0.95Cr0.05O2+δ thin films, shown in Figure 3 and Figure 4. In a wide scan XPS spectrum of NiO−CuO (Figure 3a), the presence of strong peaks at binding energies of 285.6, 530.4, 855.2, and 933.6 eV corresponds to C, O, Ni and Cu [37], respectively. Carbon is common in the XPS analysis due to contaminations from both the inside and outside of the vacuum chamber. As shown in Figure 3b, the deconvoluted peaks of the binding energy of 855 and 857 eV belong to Ni 2p3/2, and the satellite peak at 861.6 eV reveals the existence of NiO [38,39]. In Figure 3c, the deconvoluted peaks at 933.9 and 935.134 eV are attributed to Cu 2p3/2, and confirmed by the presence of their satellite peaks at 941.7 and 943.3 eV [32]. The O 1s spectra (Figure 3d) has two distinct peaks at the binding energies of 530.9 and 532.9 eV. The binding energy peak at 530.9 eV corresponds to Ni–O and Cu–O bonds. While the peak at 532.9 eV is a result of the residual water and absorbed oxygen species such as hydroxyl or carbonate species on surface [40]. The wide scan XPS spectrum of Ni0.95Cr0.05O2+δ thin films (Figure 4a) reveals strong peaks at binding energies of 284.9, 531.3, 486.5, 854.5, and 576.9 eV, attributed to C, O, Sn, Ni and Cr respectively. In Figure 4b, a peak is deconvoluted into two peaks with a binding energy of 855 and 857 eV, attributed to Ni 2p3/2, and their satellite peak at 862.2 eV confirms the NiO phase [39]. The XPS spectrum of Cr (Figure 4c) consists of deconvoluted peaks at a binding energy of 576.1, 577.3, and the peak of 578.7 eV reveals the existence of Cr 2p3/2. The signal with the binding energy of 576.1 eV is attributed to spinel oxides comprising Cr (Ni0.95Cr0.05O2+δ) [41]. The O 1s spectra Figure 4d has two distinct peaks at binding energies 530.7 and 532.3 eV, corresponding to the Ni–O, Cr–O bonds and –OH bonds [42] of residual water respectively. These XPS survey spectra of NiO−CuO and Ni0.95Cr0.05O2+δ thin films confirm the presence of their respective elements, which are compatible with the EDX and XRD results.
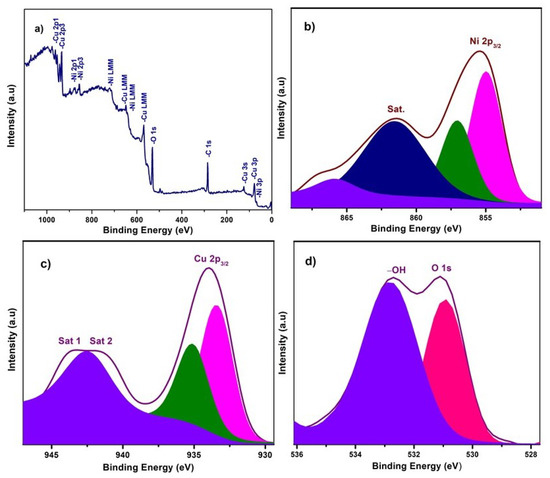
Figure 3.
(a) XPS survey scan of NiO−CuO, (b–d) high resolution XPS spectra of Ni 2p3, Cu 2p3 and O 1s, respectively, for NiO−CuO thin film.
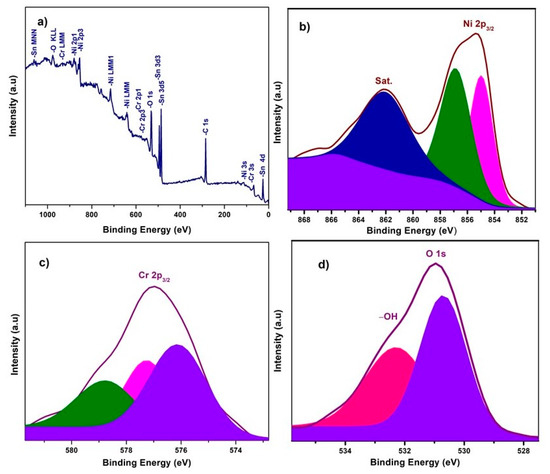
Figure 4.
(a) XPS survey scan of Ni0.95Cr0.05O2+δ, (b–d) high resolution XPS spectra of Ni 2p3, Cr 2p3 and O 1s, respectively, for Ni0.95Cr0.05O2+δ thin film.
3.3. Surface Topography
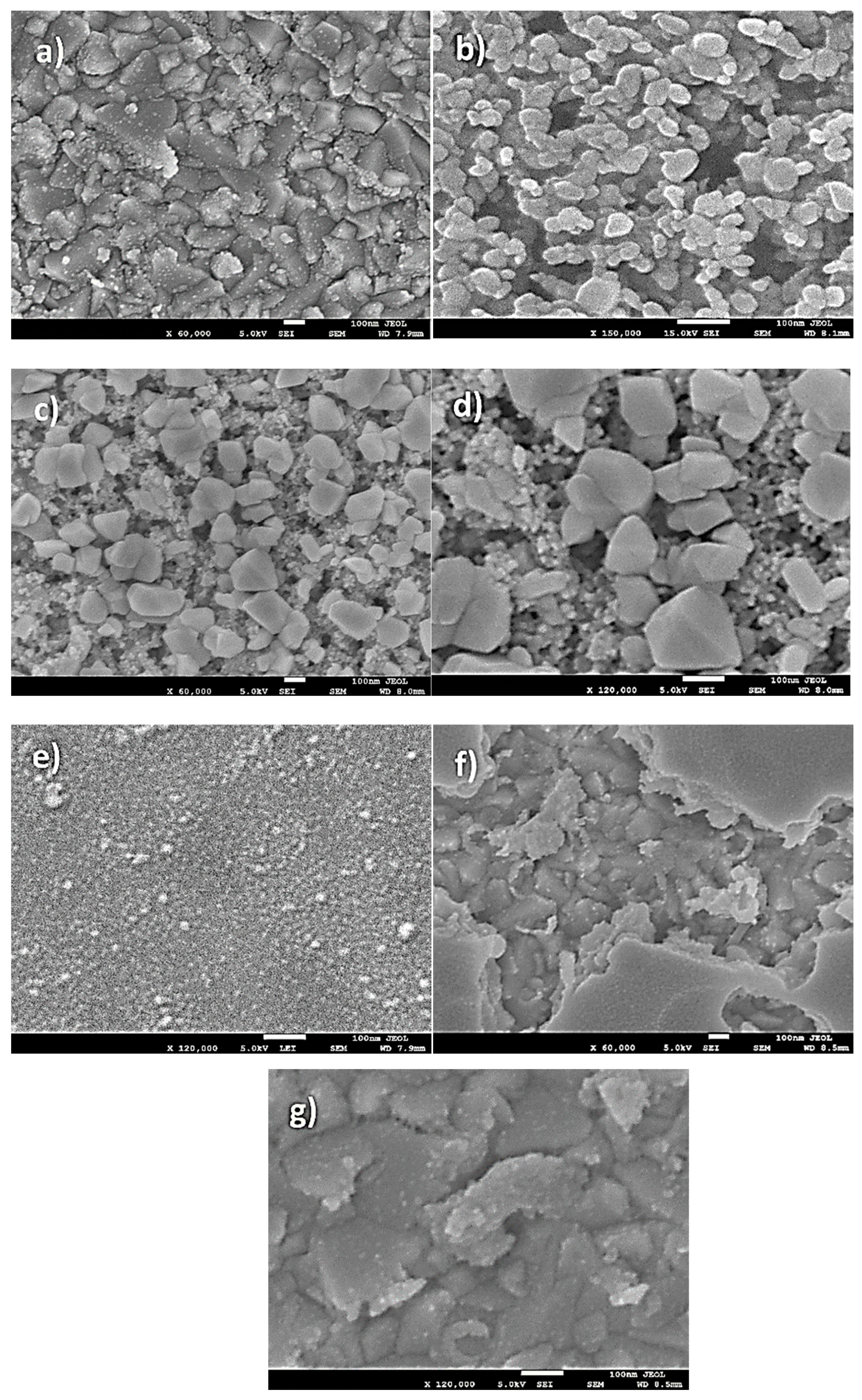
Figure 5a–d presents FESEM images of NiO, CuO, and NiO−CuO thin films (SEM images of bare FTO are added in Supplementary Materials as Figure S1). It is observed in all these SEM images that the substrate surface is evenly coated with dense and well-interconnected nano-particles. Furthermore, in the case of NiO−CuO, several nano-particles appear to be aggregated. The average particle size of NiO, CuO and NiO−CuO is found to be 141.6, 42.6 and 92.1 nm respectively. Figure 5e–g shows the surface morphology of Cr2O3 and Ni0.95Cr0.05O2+δ thin films. SEM images of Cr2O3 and Ni0.95Cr0.05O2+δ display the uniform deposition of irregular nanoparticles, with an average particle size of 14.2 and 77.9 nm respectively. For comparison, the FESEM of pure FTO is incorporated as Figure S1 in the Supplementary Materials. The EDS results of the NiO−CuO thin film reveals a 1:1 atomic ratio of the Ni and Cu elements as-synthesized. The EDS results of the Ni0.95Cr0.05O2+δ thin film also verifies Cr doping in the NiO lattice as the atomic percentage of Cr to Ni is calculated to be 5%. The elemental mapping (Figure S2 in Supplementary Materials) shows uniform distribution of both the metals in NiO−CuO composite thin films.
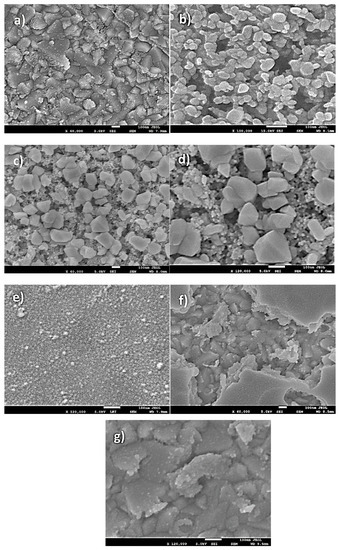
Figure 5.
SEM images of (a) NiO (b) CuO (c) NiO−CuO at magnification of ×60,000 (d) NiO−CuO at ×120,000 magnification (e) Cr2O3, (f) Ni0.95Cr0.05O2+δ at magnification of ×60,000 and (g) Ni0.95Cr0.05O2+δ at ×120,000 magnification.
3.4. Raman Spectroscopy
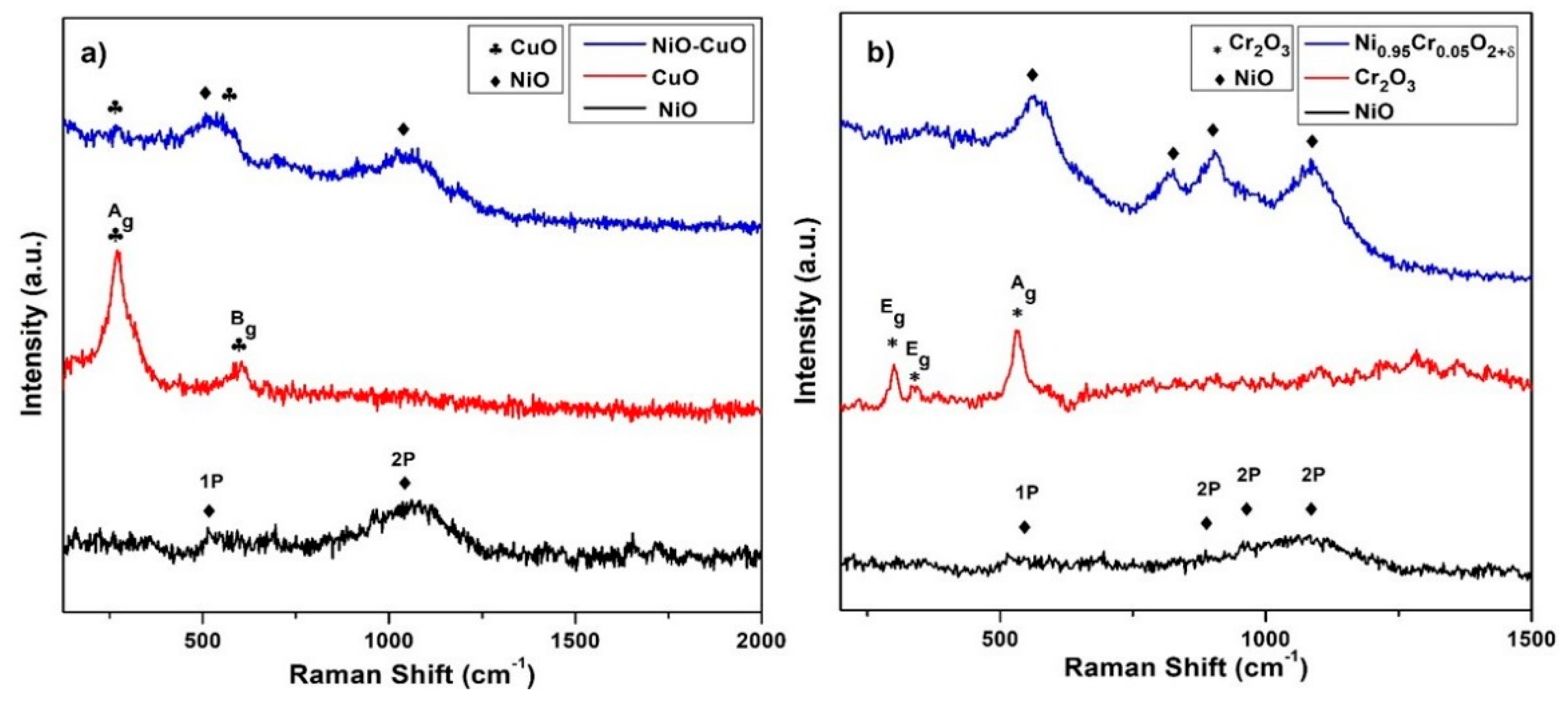
The Raman spectra of NiO, CuO, and NiO−CuO thin films are shown in Figure 6a. Raman spectra of the pure NiO thin film reveal two broad bands at 520 cm−1 and 1064 cm−1 which are ascribed to NiO scattering of a first-order phonon (1P) and second-order phonon (2P), respectively [43]. CuO shows two Raman active modes at 270 cm−1 and 603 cm−1, allocated as the Ag and Bg modes [44]. Additionally, NiO−CuO shows distinct characteristic bands of both NiO and CuO, confirming the presence of both metal oxides as shown in the XRD results. Figure 6b shows the Raman spectra of NiO, Cr2O3, and Ni0.95Cr0.05O2+δ. Three Raman bands of Cr2O3 are observed at 300 cm−1, 342 cm−1, and 534 cm−1 assigned as Eg, Eg, and Ag vibration modes [45]. In the Ni0.95Cr0.05O2+δ spectrum, only vibrational modes of NiO appear. The sharpening of all of the Raman peaks of NiO in the Ni0.95Cr0.05O2+δ spectrum suggests that doping results in increased crystallinity of the films, which correlates with the XRD results.
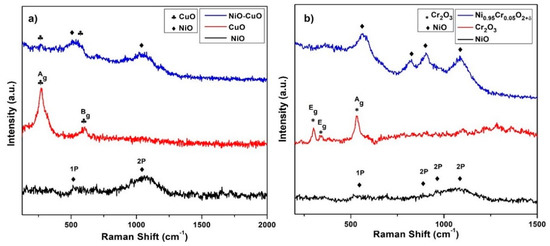
Figure 6.
Raman spectra of (a) NiO, CuO, and NiO−CuO (b) NiO, Cr2O3, and Ni0.95Cr0.05O2+δ.
3.5. Surface Roughness Measurements
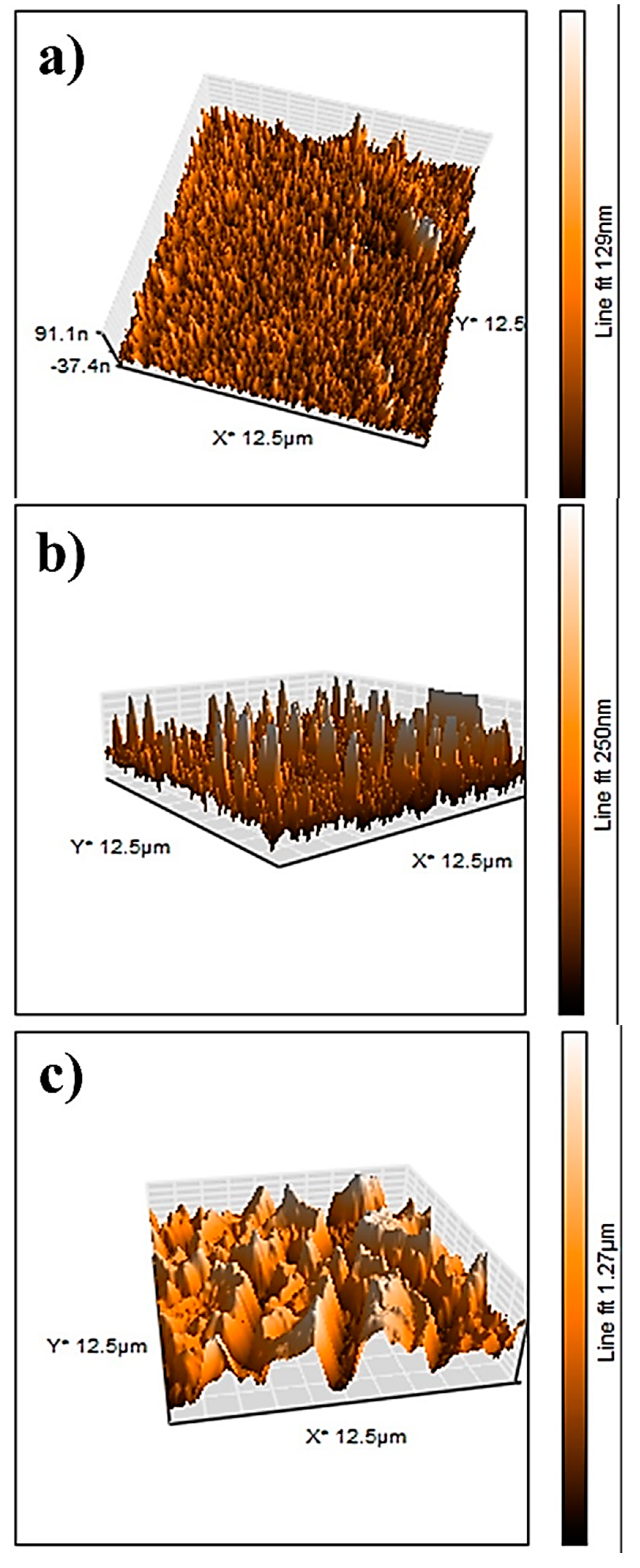
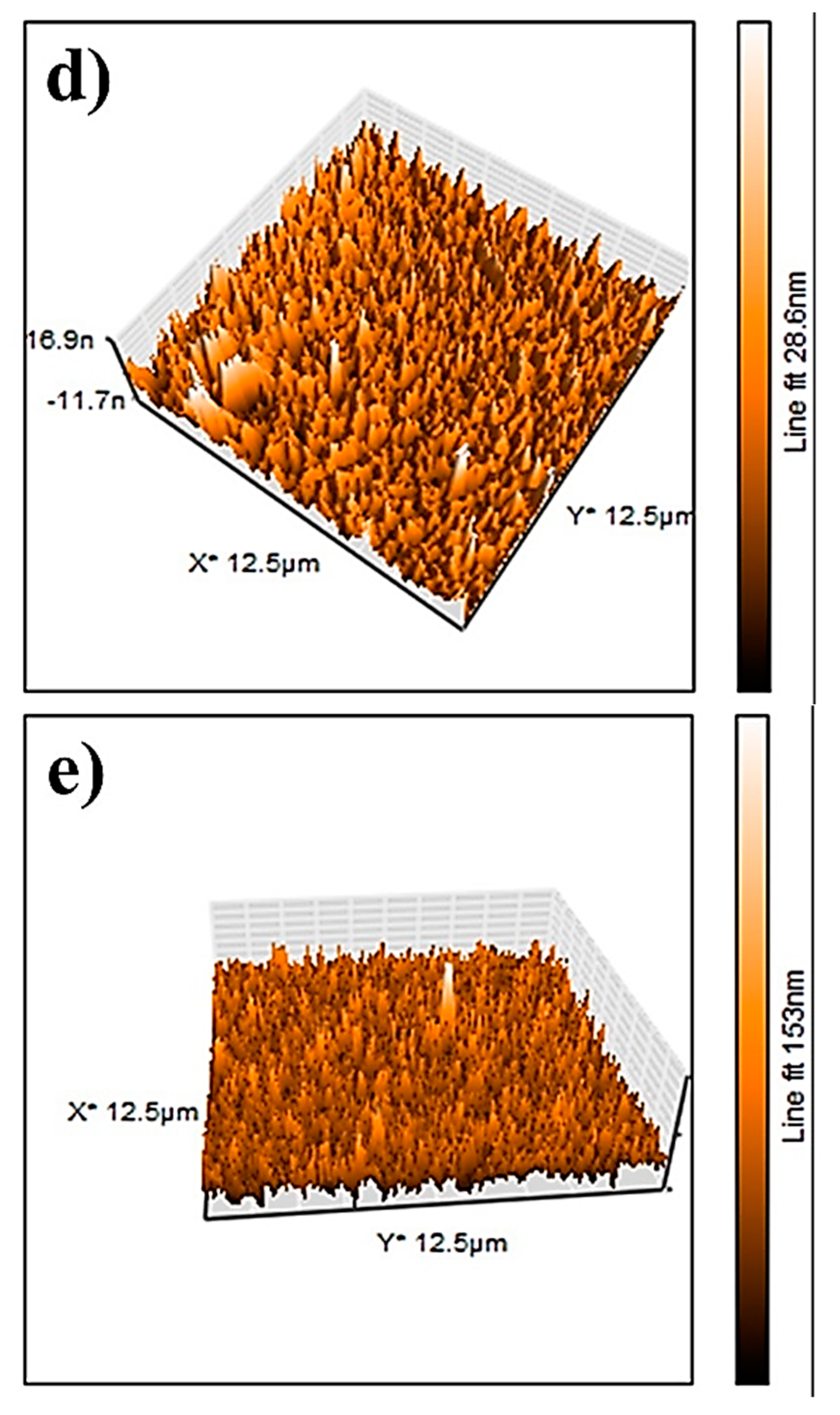
The AFM results of the NiO, CuO, NiO−CuO, Cr2O3, and Ni0.95Cr0.05O2+δ thin films are shown in Figure 7a–e. The root-mean-square-roughness (RMS) values of NiO, CuO, NiO−CuO, Cr2O3, and Ni0.95Cr0.05O2+δ thin films are found to be 17.3, 40.2, 257, 3, and 12.4 nm, respectively. These results reveal that the surface roughness of all the films are high, however, the roughness of the NiO−CuO and Ni0.95Cr0.05O2+δ thin films are higher compared to the NiO, CuO, and Cr2O3 thin films. It is believed that high surface roughness facilitates deeper electrode-electrolyte interaction and enhances the active surface area for the catalytic oxidation of methanol.
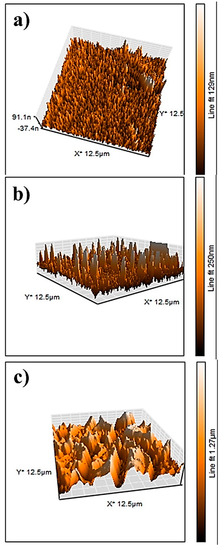
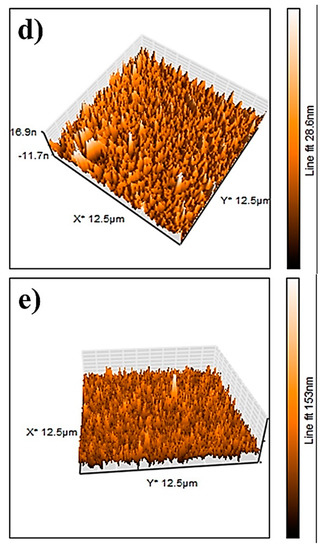
Figure 7.
Three-dimensional AFM−images of (a) NiO, (b) CuO, (c) NiO−CuO, (d) Cr2O3, and (e) Ni0.95Cr0.05O2+δ.
3.6. Electrochemical Oxidation of Methanol
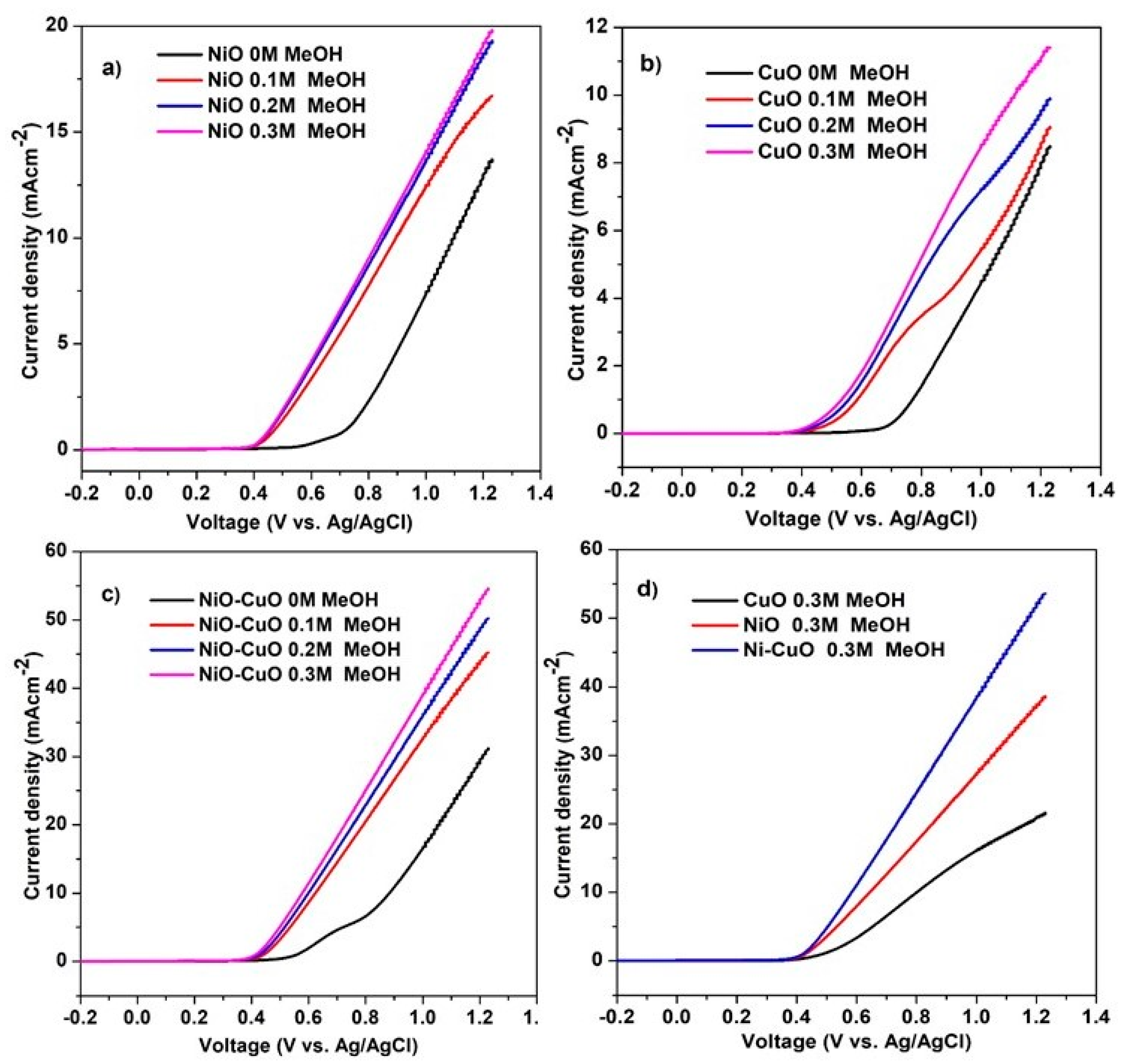
The electrooxidation of methanol by NiO−CuO and Ni0.95Cr0.05O2+δ thin films were investigated in 0.5 M NaOH, with different concentrations of methanol, ranging from 0 M to 0.3 M at a scan rate of 50 mVs−1. It is believed that the anodic current density and the onset potential are the major factors in determining a catalyst’s electrochemical oxidation potential. The LSV curves of the NiO, CuO, and NiO−CuO thin films in Figure 8a–c demonstrate that the current density of catalysts continually increases during methanol oxidation as the concentration of methanol increases, for up to 0.3 M. Moreover, the onset potential of catalysts for methanol electro-oxidation appeared at 0.43 V for the addition of methanol. Figure 8d reveals that all the tested catalysts exhibit catalytic activity for methanol oxidation, but the electrocatalytic activity of NiO−CuO thin film is higher than of pure NiO and pure CuO thin film. The current densities of NiO, CuO, and NiO−CuO thin films are 4.2 mA·cm−2, 2 mA/0.5 cm−2, and 6.1 mA/0.5 cm−2 (J = 12.2 mA·cm−2) at 0.6 V vs Ag/AgCl in the presence of 0.3 M methanol, respectively. The enhancement of methanol oxidation for the NiO−CuO catalyst can be attributed to the synergistic role of both metal oxides. This synergistic effect is observed when both metal oxides are mixed due to electronic interactions. The highest current density and lowest onset potential value were found in NiO−CuO, due to the presence of the electro-active species alpha-Ni(OH)2 and the higher absorption ability of CuO, indicating that the splitting and oxidation of methanol molecules is easier at a lower potential.
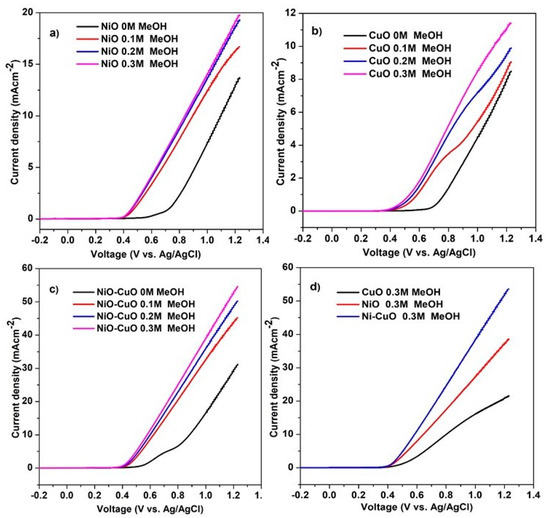
Figure 8.
LSV of (a) NiO, (b) CuO, (c) NiO−CuO and (d) comparison of NiO, CuO, and NiO−CuO catalysts at different methanol concentrations in 0.5 M NaOH at a scan rate of 50 mVs−1.
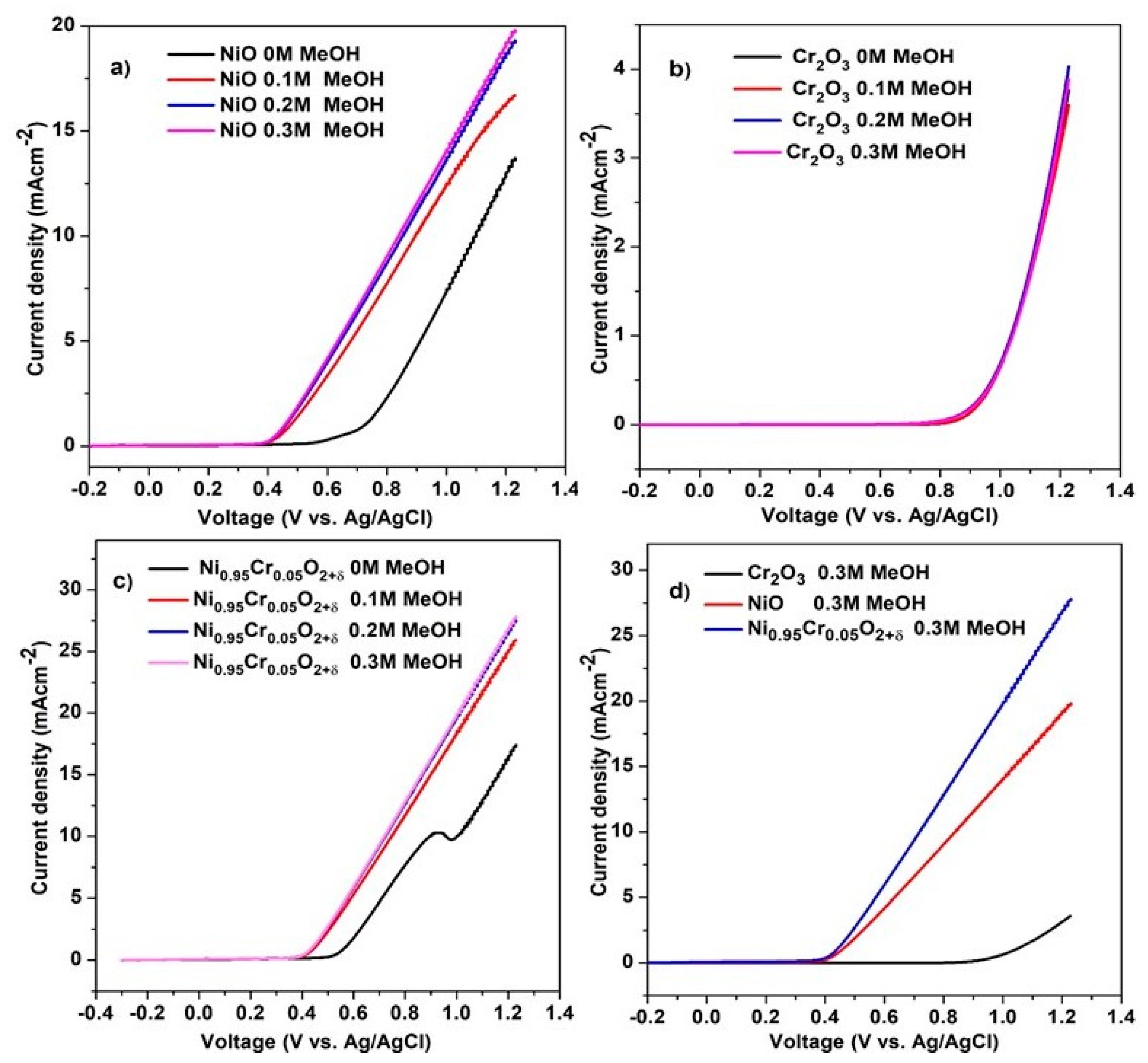
Figure 9a–c shows LSV results of NiO, Cr2O3, and Ni0.95Cr0.05O2+δ thin films at different methanol concentrations in 0.5 M NaOH, recorded at a scan rate of 50 mVs−1. Ni0.95Cr0.05O2+δ shows the onset potential of 0.39 V through the addition of methanol, and that the current produced during the electrooxidation of methanol increased significantly as the concentration of methanol increased. The current density of Ni0.95Cr0.05O2+δ thin film is 6.5 mA·cm−2 vs. 0.6 V in the presence of 0.3 M methanol. The higher current density of Ni0.95Cr0.05O2+δ than NiO and Cr2O3 is due to the combined effect of the Cr and NiO.
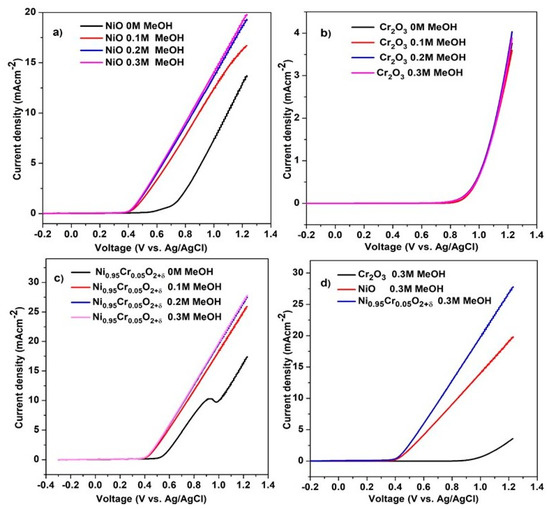
Figure 9.
LSV of (a) NiO, (b) Cr2O3, (c) Ni0.95Cr0.05O2+δ and (d) comparison of NiO, Cr2O3, and Ni0.95Cr0.05O2+δ catalysts at different methanol concentrations in 0.5 M NaOH at a scan rate of 50 mVs−1.
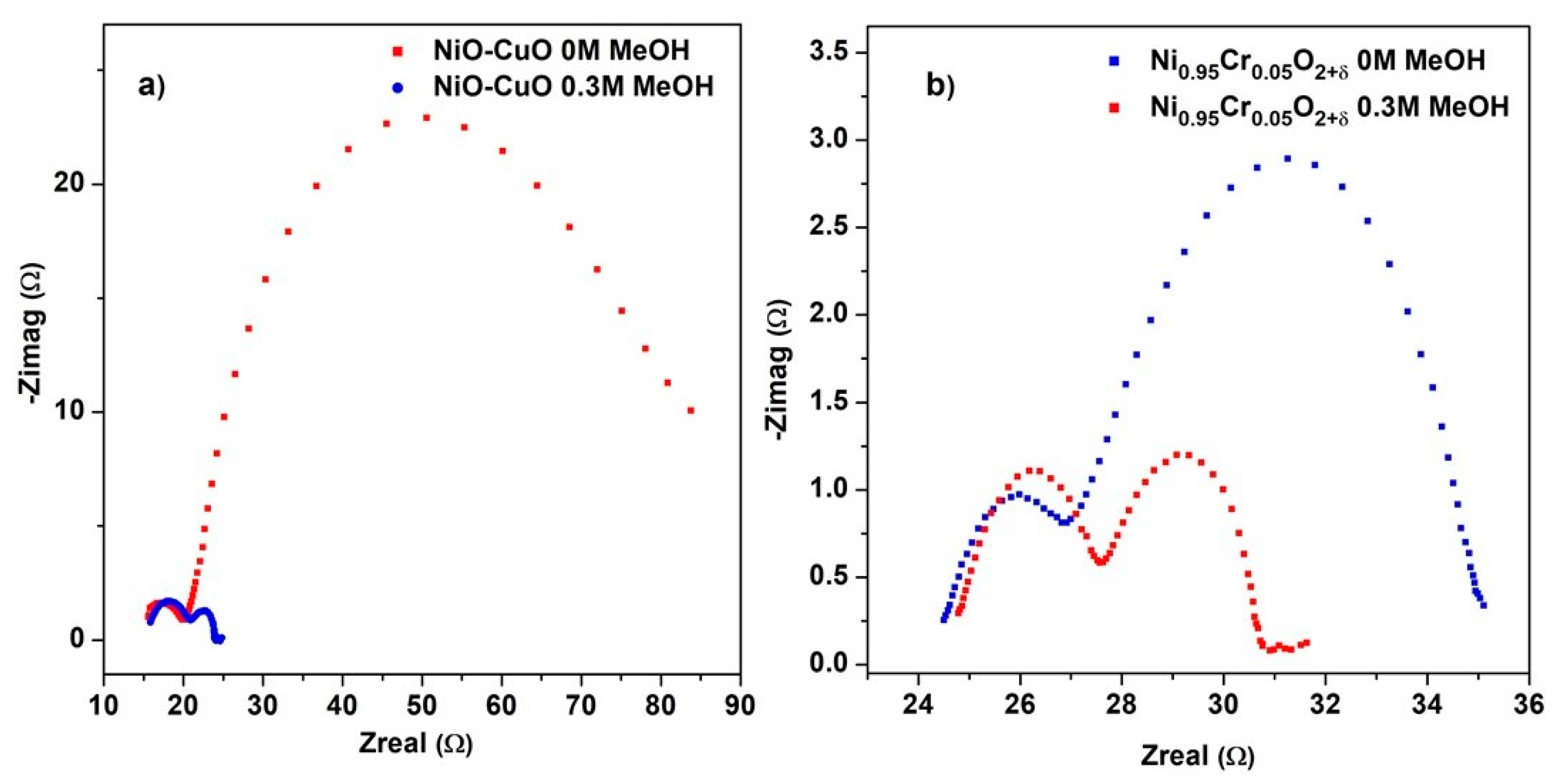
Electrochemical impedance spectroscopy (EIS) is useful for determining the parameters that influence an electrode’s efficiency, such as its charge transfer and diffusion properties. Figure 10a,b displays the Nyquist plot of NiO−CuO and Ni0.95Cr0.05O2+δ thin-film measured at the potential of 0.6 V in a frequency range of 100 KHz to 1 Hz in 0.5 M NaOH before and after the addition of 0.3 M methanol. The Nyquist plot displays two semicircles, one in the high-frequency region related to solution resistance and a second in the low-frequency region related to charging transfer resistance (Rct). Rct indicates the rate of charge exchange at the electrochemical interface between an aqueous solution and composite ions of the electrolyte. For the EIS plots of both NiO−CuO and Ni0.95Cr0.05O2+δ thin films, a smaller diameter of the second semicircle can be observed after the addition of methanol, which suggests fast electron-charge transfer. After the addition of methanol, the value of Rct calculated for NiO−CuO and Ni0.95Cr0.05O2+δ thin film is 3.18 Ω/cm2 and 2.85 Ω/cm2 respectively. These small Rct values in methanol indicate low charge transfer resistance, improved conductivity, and a higher catalytic activity for NiO−CuO and Ni0.95Cr0.05O2+δ electrocatalysts.
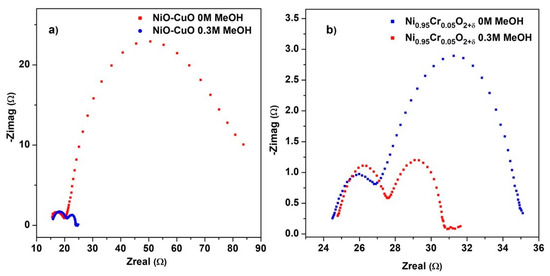
Figure 10.
EIS plot of (a) NiO−CuO and (b) Ni0.95Cr0.05O2+δ in 0.5 M NaOH before and after addition of 0.3 M methanol.
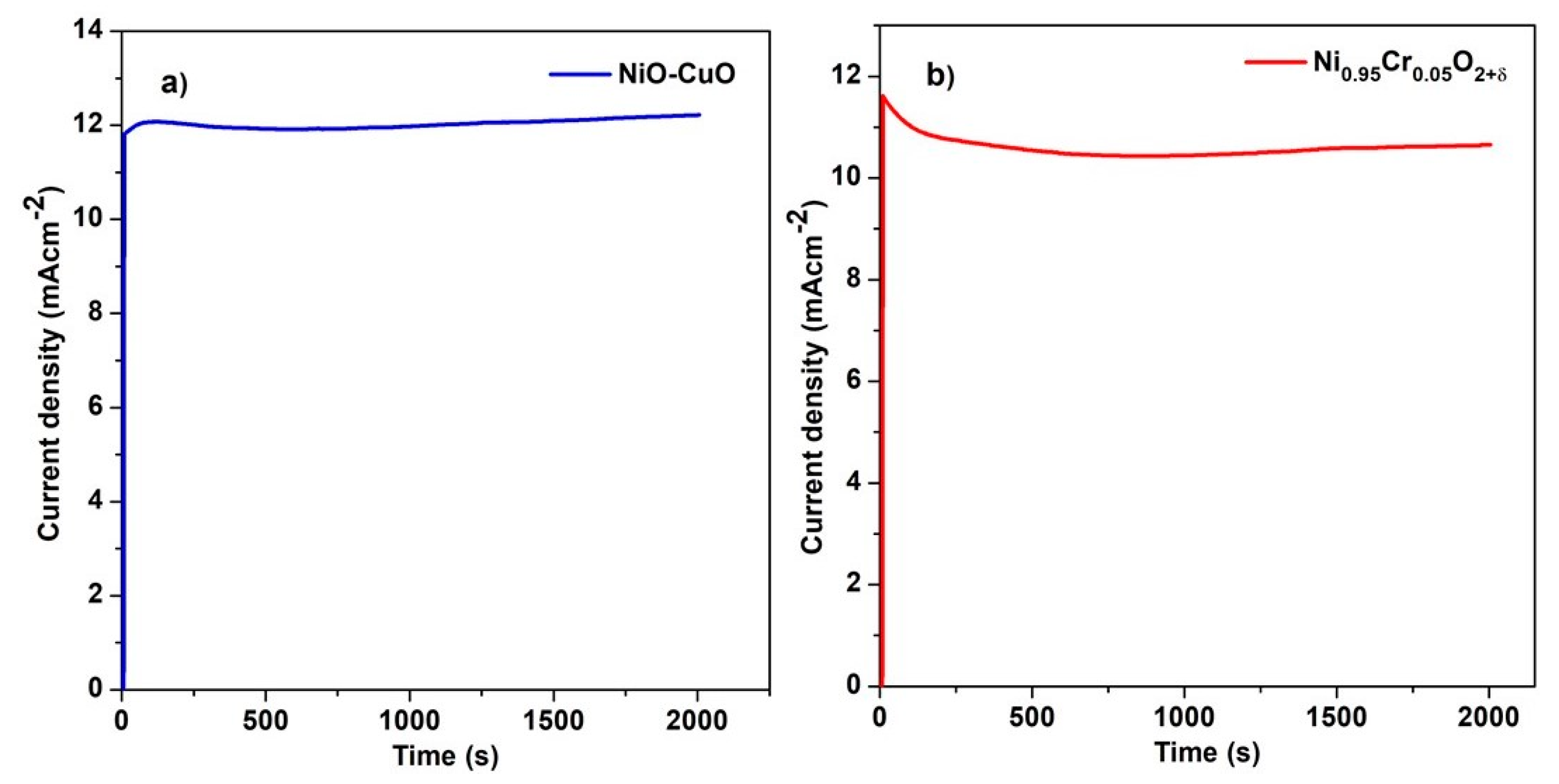
A chronoamperometric test was carried out to characterize the stability of the methanol oxidation reaction for the NiO−CuO (Figure 11a) and Ni0.95Cr0.05O2+δ (Figure 11b) thin films in 0.5 M NaOH and 0.3 M methanol. Figure 11a,b shows that both samples displayed some current decay in the first few seconds, after which a relatively steady state was reached. This decay may be due to the adsorption of reaction intermediates such as CO. Furthermore, this decrease in current density may be due to a decrease in the methanol concentration near the electrode surface due to the rapid oxidation of methanol at the start. Subsequently, these changes occur under the mass transfer control process. NiO−CuO and Ni0.95Cr0.05O2+δ thin films have shown a stability of 95% and 89%, respectively, at a potential of 0.6 V for 2000 s. Moreover, NiO−CuO and Ni0.95Cr0.05O2+δ electrodes have maintained catalytic activity and stability toward methanol oxidation over a long period.
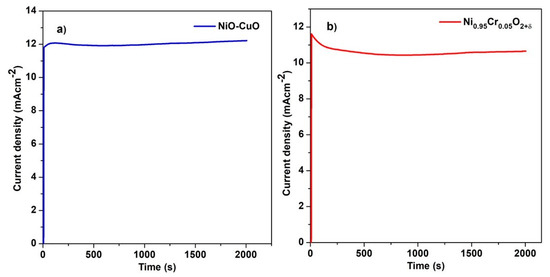
Figure 11.
Chronoamperometric graph of (a) NiO−CuO and (b) Ni0.95Cr0.05O2+δ in 0.5 M NaOH and 0.3 M methanol.
4. Conclusions
In summary, NiO−CuO and Ni0.95Cr0.05O2+δ thin films were fabricated on FTO glass substrates via a facile dip-coating method followed by calcination at 500 °C. The films were appropriately characterized through different analytical techniques such XRD, XPS, FESEM, EDX. The uniformly distributed films on FTO glass substrate were then examined for their electrocatalytic oxidation of methanol. It was observed that the efficiency of methanol oxidation was significantly higher for the NiO−CuO and Ni0.95Cr0.05O2+δ thin films as compared to pure NiO and CuO, while Cr2O3 was found to be inactive for methanol electro-oxidation. All the results of the NiO−CuO and Ni0.95Cr0.05O2+δ thin films showed better electrocatalytic activity, lower charge transfer resistance, good stability, and resistance to the poisoning effect.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cryst11111398/s1: FESEM image of bare FTO glass substrate and EDX elemental mapping NiO−CuO composite film are available as Figures S1 and S2, respectively in Supplementary Materials.
Author Contributions
Conceptualization, M.A.M.; methodology, R.L. and M.A.M.; software, R.L. and A.J.; validation, M.A.M. and J.I.; formal analysis, M.A.M., A.J. and S.S.; investigation, R.L., M.A.M. and J.I.; data curation, R.L. and M.A.M.; writing—original draft preparation, R.L.; writing—review and editing, M.A.M., J.I. and S.S.; supervision, M.A.M., A.K., methodology and data analysis, investigations and manuscript editing, S.W. All authors have read and agreed to the published version of the manuscript.
Funding
HEC (Pakistan), NRPU research grant #20−12197/NRPU/RGM/R&D/HEC/2020 and King Khalid University; grant # KKU/RCAMS/0010/21. The APC was funded by King Khalid University Saudi Arabia.
Data Availability Statement
Not applicable.
Acknowledgments
Authors acknowledge support and funding provided by HEC (Pakistan), NRPU research grant #20−12197/NRPU/RGM/R&D/HEC/2020 and authors also acknowledge support and funding of King Khalid University; grant # KKU/RCAMS/0010/21.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, P.; Zhou, Y.; Hu, M.; Chen, J. Well-dispersed NiO nanoparticles supported on nitrogen-doped carbon nanotube for methanol electrocatalytic oxidation in alkaline media. Appl. Surf. Sci. 2017, 392, 562–571. [Google Scholar] [CrossRef]
- Ni, B.; He, P.; Liao, W.; Chen, S.; Gu, L.; Gong, Y.; Wang, K.; Zhuang, J.; Song, L.; Zhou, G.; et al. Surface Oxidation of AuNi Heterodimers to Achieve High Activities toward Hydrogen/Oxygen Evolution and Oxygen Reduction Reactions. Small 2018, 14, 1703749. [Google Scholar] [CrossRef] [PubMed]
- Ni, B.; Wang, K.; He, T.; Gong, Y.; Gu, L.; Zhuang, J.; Wang, X. Mimic the Photosystem II for Water Oxidation in Neutral Solution: A Case of Co3O4. Adv. Energy Mater. 2018, 8, 1702313. [Google Scholar] [CrossRef]
- Bing Ni, Q.Z.; Ouyang, C.; Zhang, S.; Yu, B.; Zhuang, J.; Gu, L.; Wang, X. The Synthesis of Sub-Nano-Thick Pd Nanobelt–Based Materials for Enhanced Hydrogen Evolution Reaction Activity. CCS Chem. 2020, 2, 13. [Google Scholar] [CrossRef]
- Li, J.-S.; Li, S.-L.; Tang, Y.-J.; Li, K.; Zhou, L.; Kong, N.; Lan, Y.-Q.; Bao, J.-C.; Dai, Z.-H. Heteroatoms ternary-doped porous carbons derived from MOFs as metal-free electrocatalysts for oxygen reduction reaction. Sci. Rep. 2014, 4, 5130. [Google Scholar] [CrossRef] [Green Version]
- Tate, G.L.; Mehrabadi, B.A.T.; Xiong, W.; Kenvin, A.; Monnier, J.R. Synthesis of Highly Active Pd@Cu–Pt/C Methanol Oxidation Electrocatalysts via Continuous, Co-Electroless Deposition. Nanomaterials 2021, 11, 793. [Google Scholar] [CrossRef]
- Artal, R.; Serrà, A.; Michler, J.; Philippe, L.; Gómez, E. Electrodeposition of Mesoporous Ni-Rich Ni-Pt Films for Highly Efficient Methanol Oxidation. Nanomaterials 2020, 10, 1435. [Google Scholar] [CrossRef]
- Gamil, S.; El Rouby, W.M.; Antuch, M.; Zedan, I.T. Nanohybrid layered double hydroxide materials as efficient catalysts for methanol electrooxidation. RSC Adv. 2019, 9, 13503–13514. [Google Scholar] [CrossRef] [Green Version]
- Barakat, N.A.M.; El-Newehy, M.; Al-Deyab, S.S.; Kim, H.Y. Cobalt/copper-decorated carbon nanofibers as novel non-precious electrocatalyst for methanol electrooxidation. Nanoscale Res. Lett. 2014, 9, 2. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Tian, Z.; Shen, P. Oxide (CeO2, NiO, Co3O4 and Mn3O4)-promoted Pd/C electrocatalysts for alcohol electrooxidation in alkaline media. Electrochim. Acta 2008, 53, 2610–2618. [Google Scholar] [CrossRef]
- Roy, A.; Jadhav, H.S.; Cho, M.; Seo, J.G. Electrochemical deposition of self-supported bifunctional copper oxide electrocatalyst for methanol oxidation and oxygen evolution reaction. J. Ind. Eng. Chem. 2019, 76, 515–523. [Google Scholar] [CrossRef]
- Pawar, S.; Pawar, B.; Inamdar, A.; Kim, J.; Jo, Y.; Cho, S.; Mali, S.; Hong, C.; Kwak, J.; Kim, H. In-situ synthesis of Cu(OH)2 and CuO nanowire electrocatalysts for methanol electro-oxidation. Mater. Lett. 2017, 187, 60–63. [Google Scholar] [CrossRef]
- Hassan, H.; Hamid, Z.A. Electrodeposited Ni–Cr2O3 nanocomposite anodes for ethanol electrooxidation. Int. J. Hydrog. Energy 2011, 36, 5117–5127. [Google Scholar] [CrossRef]
- Noor, T.; Pervaiz, S.; Iqbal, N.; Nasir, H.; Zaman, N.; Sharif, M.; Pervaiz, E. Nanocomposites of NiO/CuO Based MOF with rGO: An Efficient and Robust Electrocatalyst for Methanol Oxidation Reaction in DMFC. Nanomaterials 2020, 10, 1601. [Google Scholar] [CrossRef] [PubMed]
- Askari, M.B.; Salarizadeh, P.; Seifi, M.; Rozati, S.M. Ni/NiO coated on multi-walled carbon nanotubes as a promising electrode for methanol electro-oxidation reaction in direct methanol fuel cell. Solid State Sci. 2019, 97, 106012. [Google Scholar] [CrossRef]
- Su, D.S.; Sun, G. Nonprecious-Metal Catalysts for Low-Cost Fuel Cells. Angew. Chem. Int. Ed. 2011, 50, 11570–11572. [Google Scholar] [CrossRef]
- Mansoor, M.A.; Munawar, K.; Lim, S.P.; Huang, N.M.; Mazhar, M.; Akhtar, M.J.; Siddique, M. Iron–manganese–titanium (1:1:2) oxide composite thin films for improved photocurrent efficiency. New J. Chem. 2017, 41, 7322–7330. [Google Scholar] [CrossRef]
- Ahmed, S.; Mansoor, M.A.; Basirun, W.J.; Sookhakian, M.; Huang, N.M.; Mun, L.K.; Sohnel, T.; Arifin, Z.; Mazhar, M. The synthesis and characterization of a hexanuclear copper-yttrium complex for deposition of semiconducting CuYO2-0.5Cu2O composite thin films. New J. Chem. 2015, 39, 1031–1037. [Google Scholar] [CrossRef]
- Wang, J.; Teschner, D.; Yao, Y.; Huang, X.; Willinger, M.; Shao, L.; Schlögl, R. Fabrication of nanoscale NiO/Ni heterostructures as electrocatalysts for efficient methanol oxidation. J. Mater. Chem. A 2017, 5, 9946–9951. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Ni, Y.; Zhai, M. Simple solution-combustion synthesis of Ni-NiO@ C nanocomposites with highly electrocatalytic activity for methanol oxidation. J. Phys. Chem. Solids 2018, 112, 119–126. [Google Scholar] [CrossRef]
- Calderón, J.C.; Rios Ráfales, M.; Nieto-Monge, M.J.; Pardo, J.I.; Moliner, R.; Lázaro, M.J. Oxidation of CO and Methanol on Pd-Ni Catalysts Supported on Different Chemically-Treated Carbon Nanofibers. Nanomaterials 2016, 6, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, H.; Tammam, R.H. Preparation of Ni-metal oxide nanocomposites and their role in enhancing the electro-catalytic activity towards methanol and ethanol. Solid State Ion. 2018, 320, 325–338. [Google Scholar] [CrossRef]
- Al-Enizi, A.; Ghanem, M.A.; El-Zatahry, A.; Al-Salem, S. Nickel oxide/nitrogen doped carbon nanofibers catalyst for methanol oxidation in alkaline media. Electrochim. Acta 2014, 137, 774–780. [Google Scholar] [CrossRef]
- Kim, J.-W.; Park, S.-M. Electrochemical oxidation of ethanol at nickel hydroxide electrodes in alkaline media studied by electrochemical impedance spectroscopy. J. Korean Electrochem. Soc. 2005, 8, 117–124. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.; Luo, J.; Liu, Y.; Yang, H.; Ouyang, R.; Miao, Y. Synthesis of bimetallic Ni–Cr nano-oxides as catalysts for methanol oxidation in NaOH solution. J. Nanosci. Nanotechnol. 2015, 15, 3743–3749. [Google Scholar] [CrossRef]
- Theres, G.S.; Velayutham, G.; Krishnan, P.S.; Shanthi, K. Synergistic impact of Ni–Cu hybrid oxides deposited on ordered mesoporous carbon scaffolds as non-noble catalyst for methanol oxidation. J. Mater. Sci. 2019, 54, 1502–1519. [Google Scholar] [CrossRef]
- Jlassi, M.; Sta, I.; Hajji, M.; Ezzaouia, H. Optical and electrical properties of nickel oxide thin films synthesized by sol–gel spin coating. Mater. Sci. Semicond. Process. 2014, 21, 7–13. [Google Scholar] [CrossRef]
- Sun, S.; Xu, Z.J. Composition dependence of methanol oxidation activity in nickel–cobalt hydroxides and oxides: An optimization toward highly active electrodes. Electrochim. Acta 2015, 165, 56–66. [Google Scholar] [CrossRef]
- An, W.-J.; Thimsen, E.; Biswas, P. Aerosol-chemical vapor deposition method for synthesis of nanostructured metal oxide thin films with controlled morphology. J. Phys. Chem. Lett. 2010, 1, 249–253. [Google Scholar] [CrossRef]
- Daraz, U.; Ansari, T.M.; Arain, S.A.; Mansoor, M.A.; Mazhar, M. Study of solvent effect on structural and photoconductive behavior of ternary chalcogenides InBiS3-In2S3-Bi2S3 composite thin films deposited via AACVD. Main Group Met. Chem. 2019, 42, 102–112. [Google Scholar] [CrossRef]
- Munawar, K.; Mansoor, M.A.; Olmstead, M.M.; Yusof, F.B.; Misran, M.B.; Basirun, W.J.; Mazhar, M. Pyrochlore-structured Y2Ti2O7–2TiO2 composite thin films for photovoltaic applications. J. Aust. Ceram. Soc. 2019, 55, 921–932. [Google Scholar] [CrossRef]
- Amri, A.; Hasan, K.; Taha, H.; Rahman, M.M.; Herman, S.; Awaltanova, E.; Kabir, H.; Yin, C.-Y.; Ibrahim, K.; Bahri, S.; et al. Surface structural features and optical analysis of nanostructured Cu-oxide thin film coatings coated via the sol-gel dip coating method. Ceram. Int. 2019, 45, 12888–12894. [Google Scholar] [CrossRef]
- Meenakshi, L.J.; Aswathy, B.R.; Manoj, P.K. Effect of Annealing Temperature on Structural and Optical Properties of Nickel Oxide Thin Films by Dip Coating Method. In Proceedings oof the AIP Conference, Kollam, India, 12–14 December 2019. [Google Scholar] [CrossRef]
- Ghalmi, Y.; Habelhames, F.; Sayah, A.; Bahloul, A.; Nessark, B.; Shalabi, M.; Nunzi, J.M. Capacitance performance of NiO thin films synthesized by direct and pulse potentiostatic methods. Ionics 2019, 25, 6025–6033. [Google Scholar] [CrossRef]
- Akgul, F.A.; Akgul, G.; Yildirim, N.; Unalan, H.E.; Turan, R. Influence of thermal annealing on microstructural, morphological, optical properties and surface electronic structure of copper oxide thin films. Mater. Chem. Phys. 2014, 147, 987–995. [Google Scholar] [CrossRef]
- Al-Saadi, T.M.; Hameed, N.A. Synthesis and structural characterization of Cr2O3 nanoparticles prepared by using Cr(NO3)3·9H2O and triethanolamine under microwave irradiation. Adv. Phys. Theor. Appl. Synth. 2015, 44, 139–148. [Google Scholar]
- Naeem, R.; Yahya, R.; Mansoor, M.A.; Teridi, M.A.M.; Sookhakian, M.; Mumtaz, A.; Mazhar, M. Photoelectrochemical water splitting over mesoporous CuPbI3 films prepared by electrophoretic technique. Mon. Chem.-Chem. Mon. 2017, 148, 981–989. [Google Scholar] [CrossRef]
- Lu, Q.; Huang, R.; Wang, L.; Wu, Z.; Li, C.; Luo, Q.; Zuo, S.; Li, J.; Peng, D.; Han, G.L.; et al. Thermal annealing and magnetic anisotropy of NiFe thin films on n+-Si for spintronic device applications. J. Magn. Magn. Mater. 2015, 394, 253–259. [Google Scholar] [CrossRef]
- Kalita, C.; Sarkar, R.D.; Verma, V.; Bharadwaj, S.K.; Kalita, M.C.; Boruah, P.K.; Das, M.R.; Saikia, P. Bayesian Modeling Coherenced Green Synthesis of NiO Nanoparticles Using Camellia sinensis for Efficient Antimicrobial Activity. BioNanoScience 2021, 11, 825–837. [Google Scholar] [CrossRef]
- Liu, W.; Lu, C.; Wang, X.; Liang, K.; Tay, B.K. In situ fabrication of three-dimensional, ultrathin graphite/carbon nanotube/NiO composite as binder-free electrode for high-performance energy storage. J. Mater. Chem. A 2015, 3, 624–633. [Google Scholar] [CrossRef]
- Li, N.; Wang, N. The effect of duplex Surface mechanical attrition and nitriding treatment on corrosion resistance of stainless steel 316L. Sci. Rep. 2018, 8, 8454. [Google Scholar] [CrossRef] [Green Version]
- Mansoor, M.A.; McKee, V.; Yusof, F.B.; Lim, S.P.; Zubir, M.N.M.; Ming, H.N.; Mazhar, M. Lanthanum–titanium oxide composite from a single molecular cluster: Non-enzymatic mesoporous electrochemical nitrite ion sensor. Polyhedron 2018, 156, 332–341. [Google Scholar] [CrossRef]
- Qiu, Z.; He, D.; Wang, Y.; Zhao, X.; Zhao, W.; Wu, H. High performance asymmetric supercapacitors with ultrahigh energy density based on hierarchical carbon nanotubes@ NiO core–shell nanosheets and defect-introduced graphene sheets with hole structure. RSC Adv. 2017, 7, 7843–7856. [Google Scholar] [CrossRef] [Green Version]
- Guha, S.; Peebles, D.; Wieting, J.T. Raman and infrared studies of cupric oxide. Bull. Mater. Sci. 1991, 14, 539–543. [Google Scholar] [CrossRef]
- Li, Q.; Gou, Y.; Wang, T.-G.; Gu, T.; Yu, Q.; Wang, L. Study on Local Residual Stress in a Nanocrystalline Cr2O3 Coating by Micro-Raman Spectroscopy. Coatings 2019, 9, 500. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).