Influence of V and Zn in FeCrCuMnTi High-Entropy Alloys on Microstructures and Uniaxial Compaction Behavior Prepared by Mechanical Alloying
Abstract
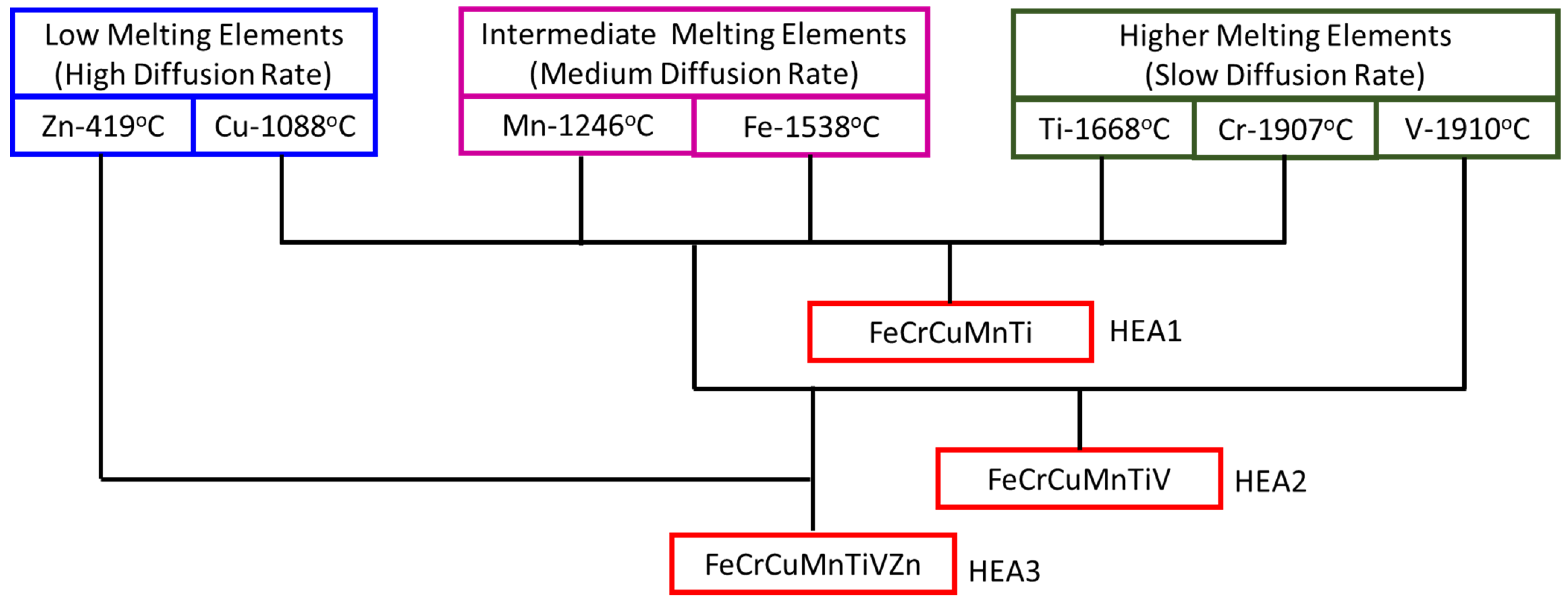
:1. Introduction
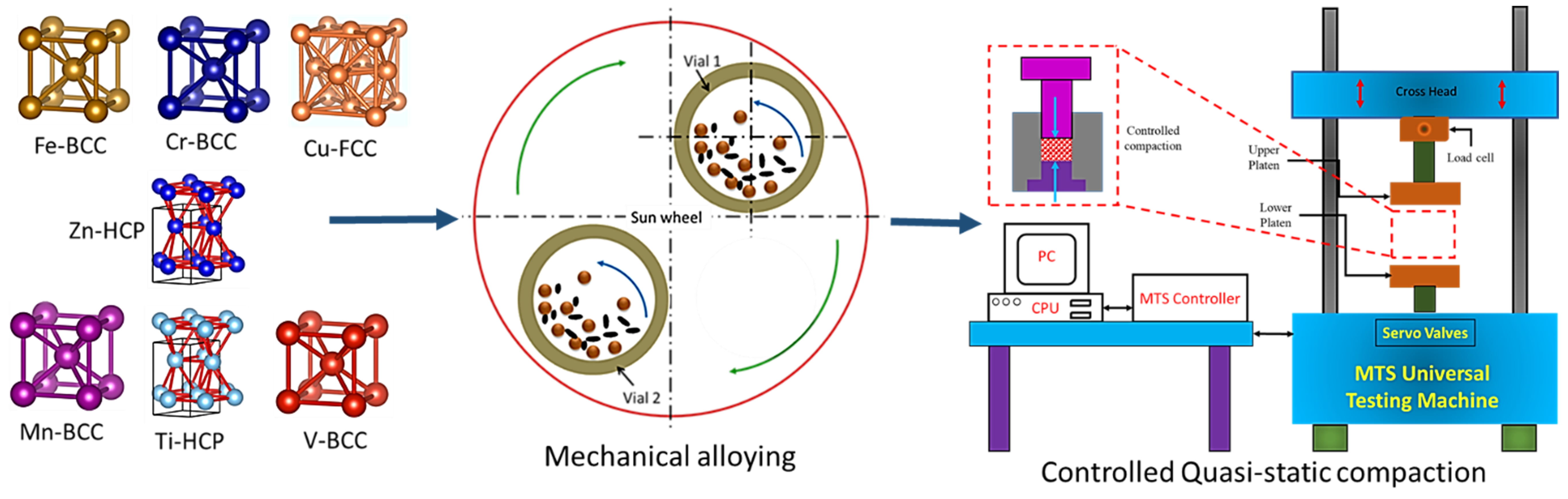
2. Materials and Methods
3. Results
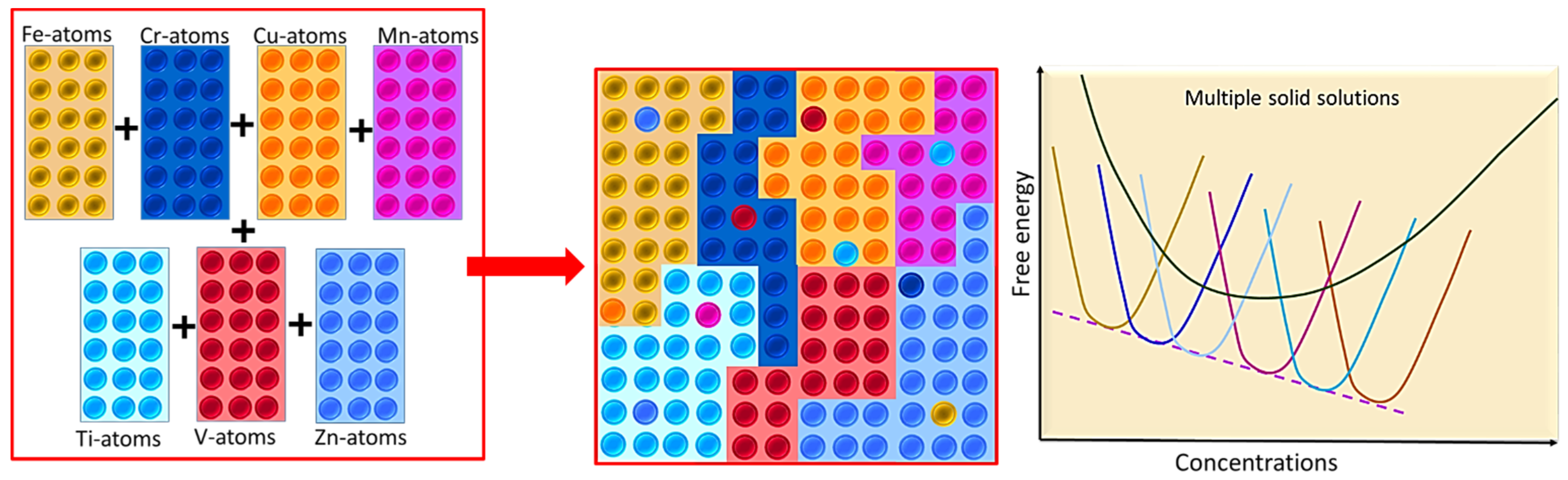
3.1. Phase Evolutions and Structural Characterization Using XRD
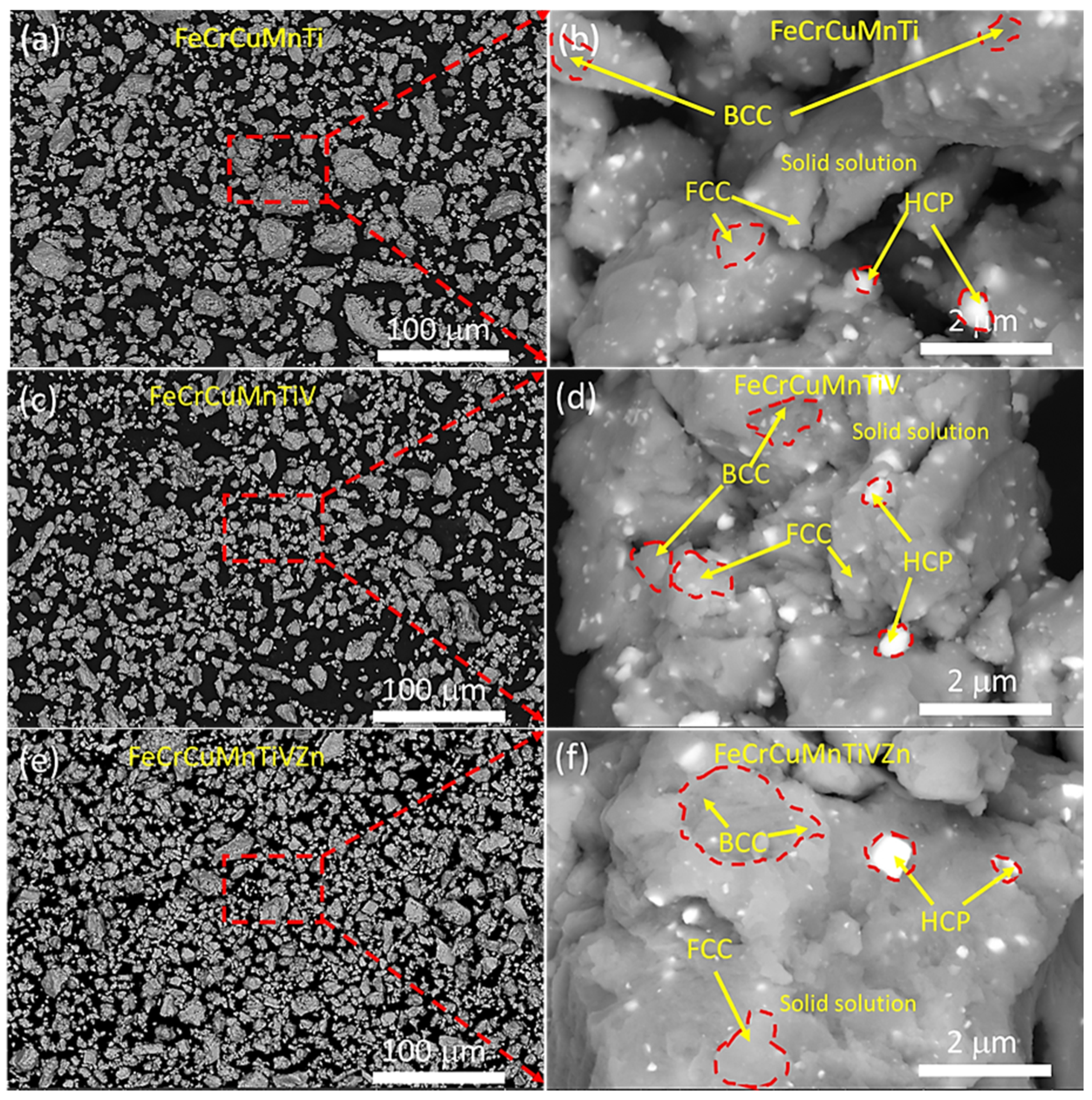
3.2. Microstructural Examinations Using HRSEM and HRTEM
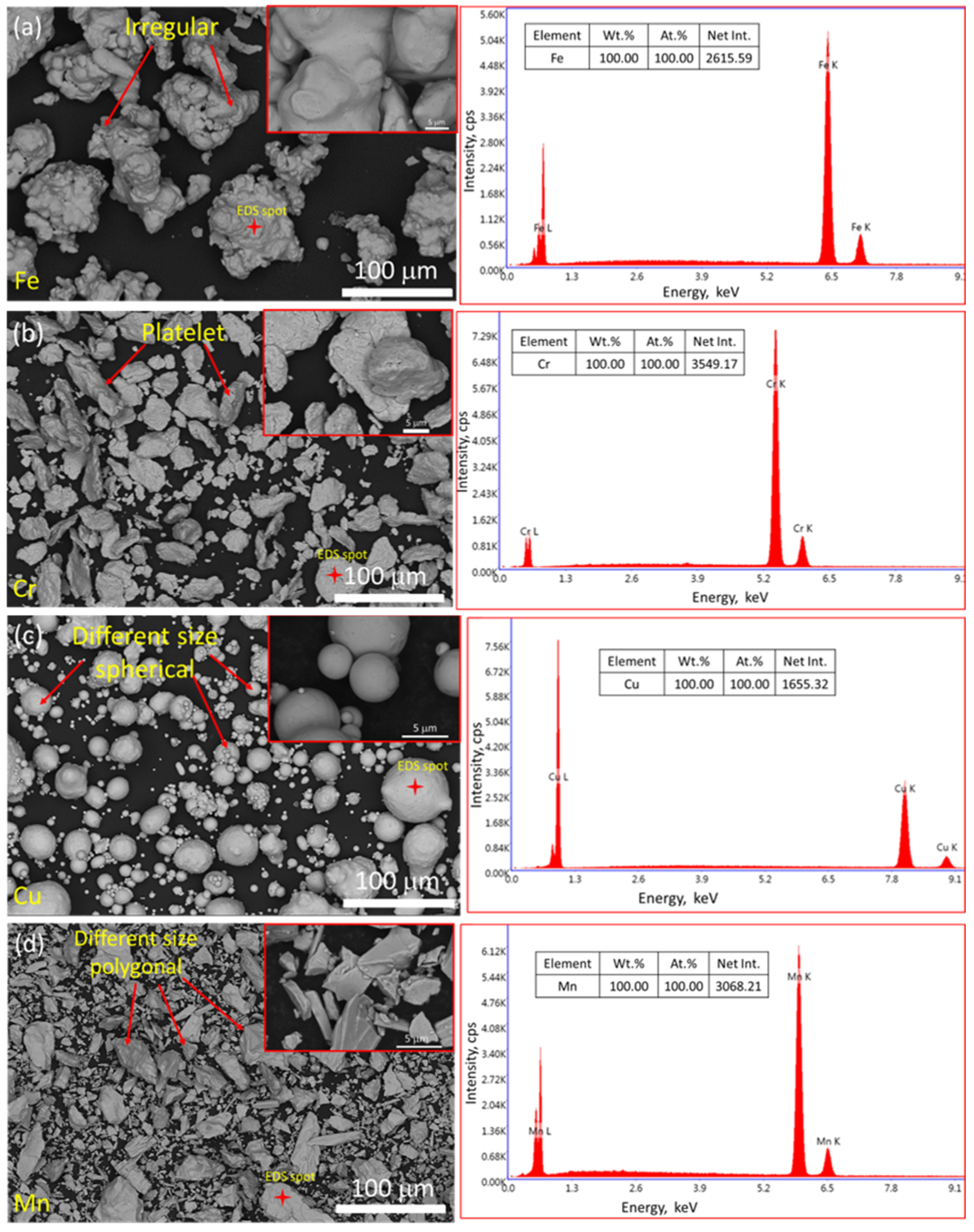
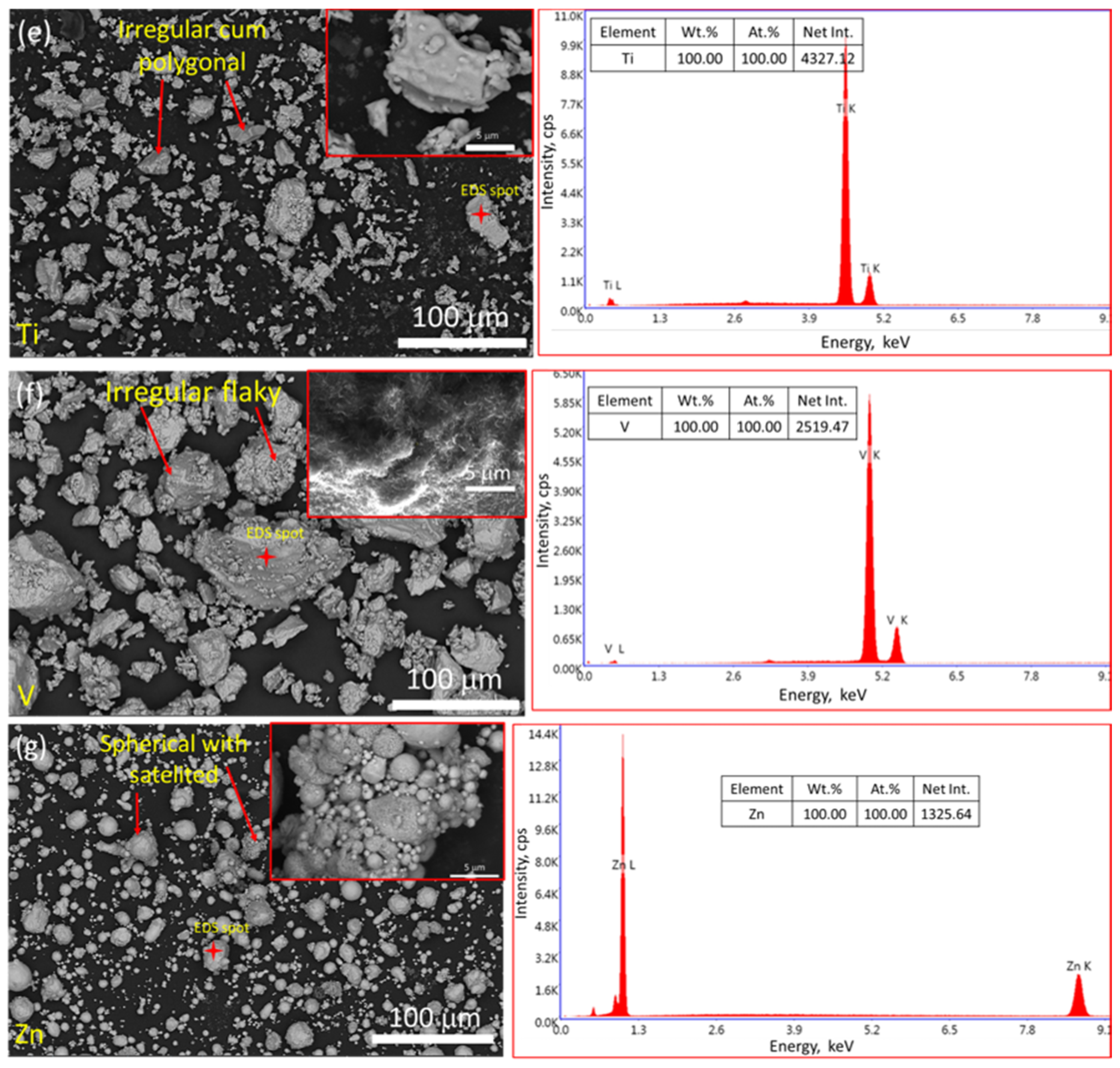
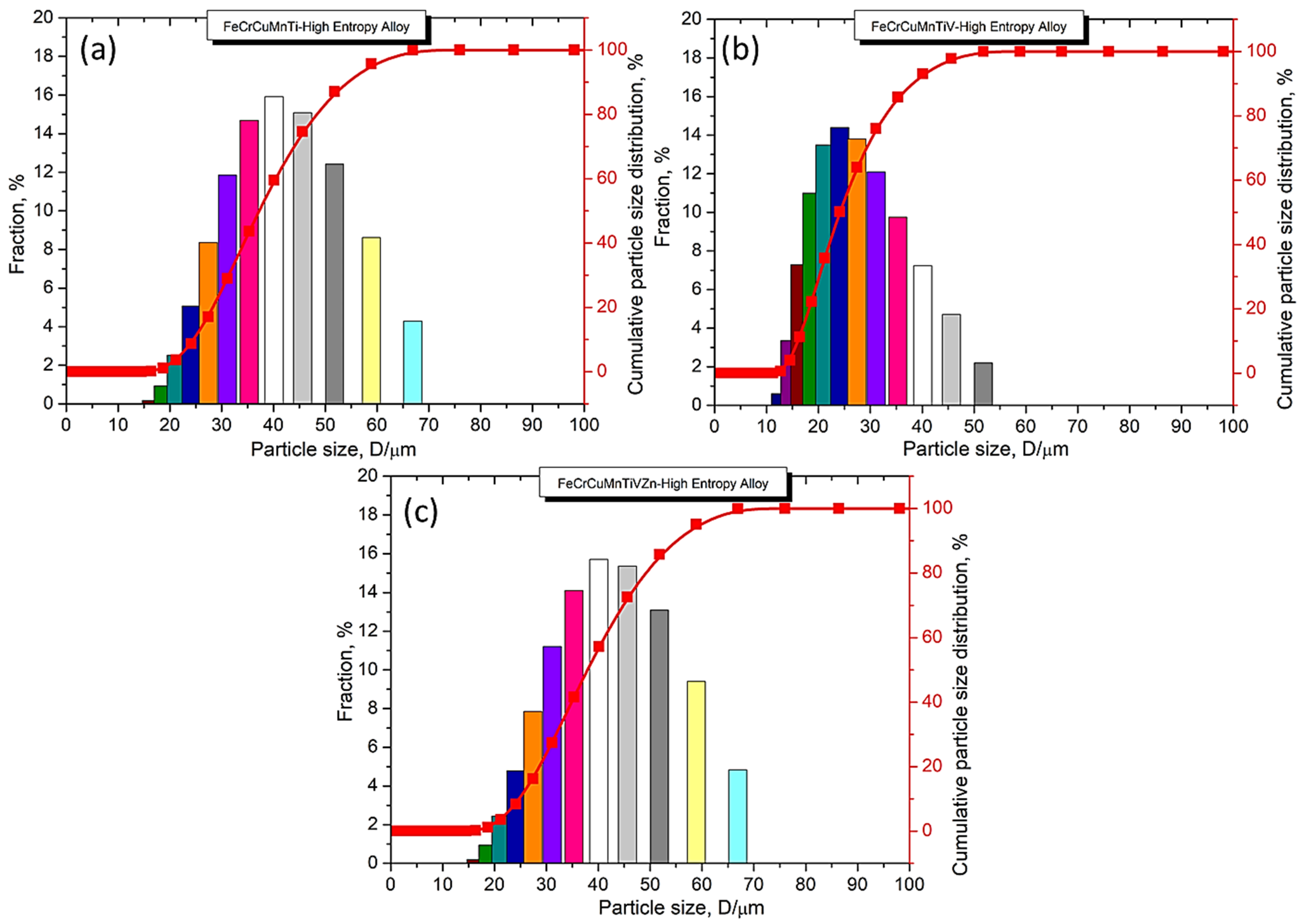
3.3. Examination of Powder Particle Size, Distribution, Apparent Density, Tap Density, and True Density
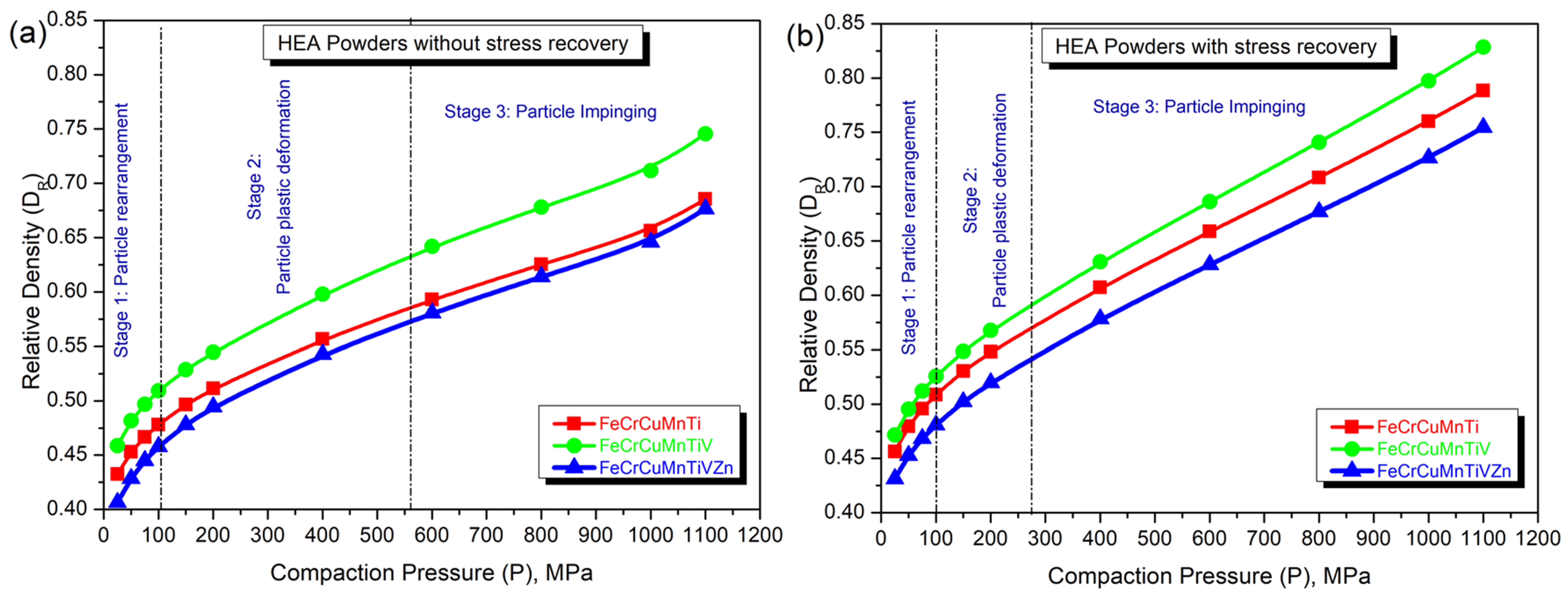
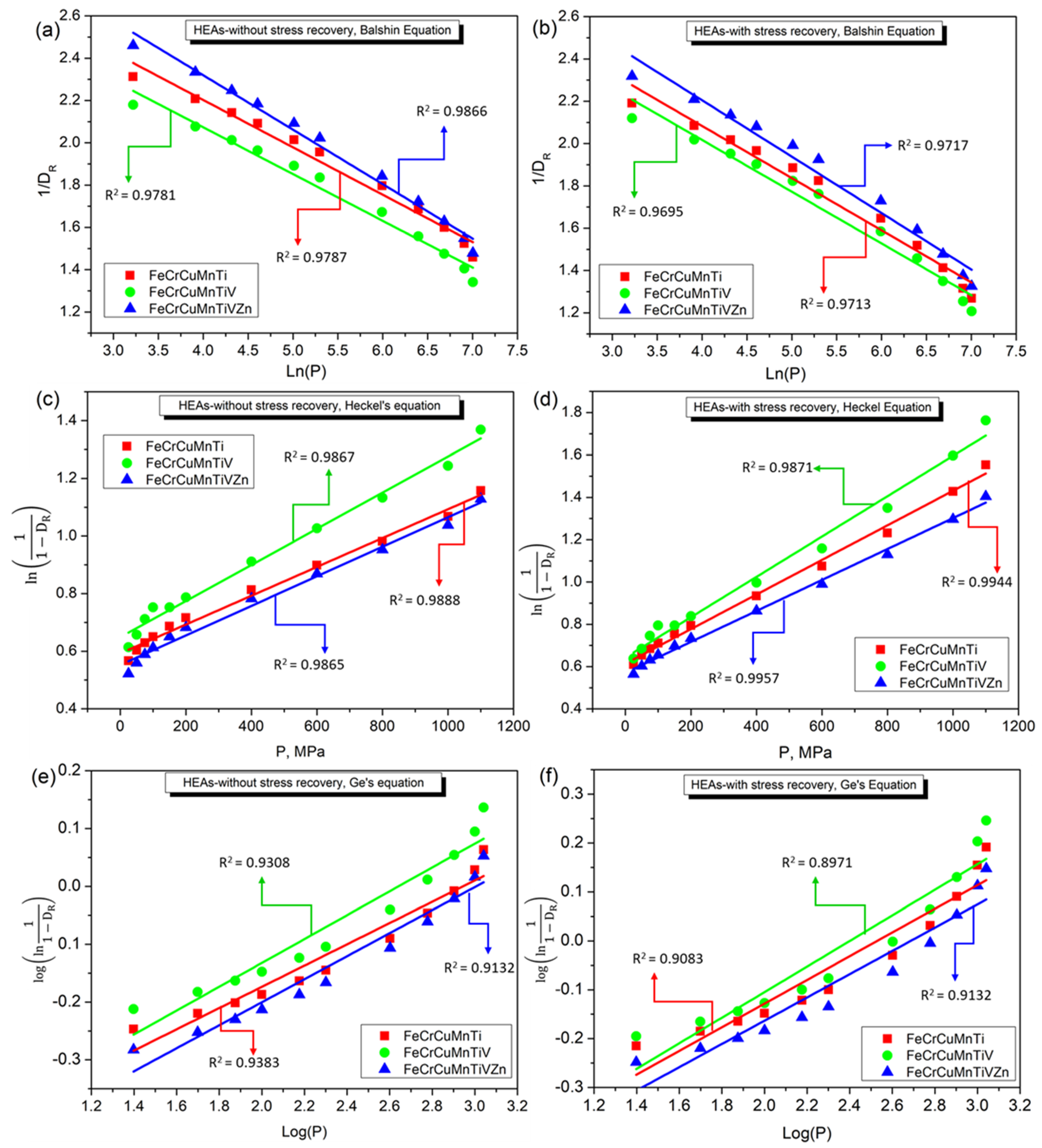
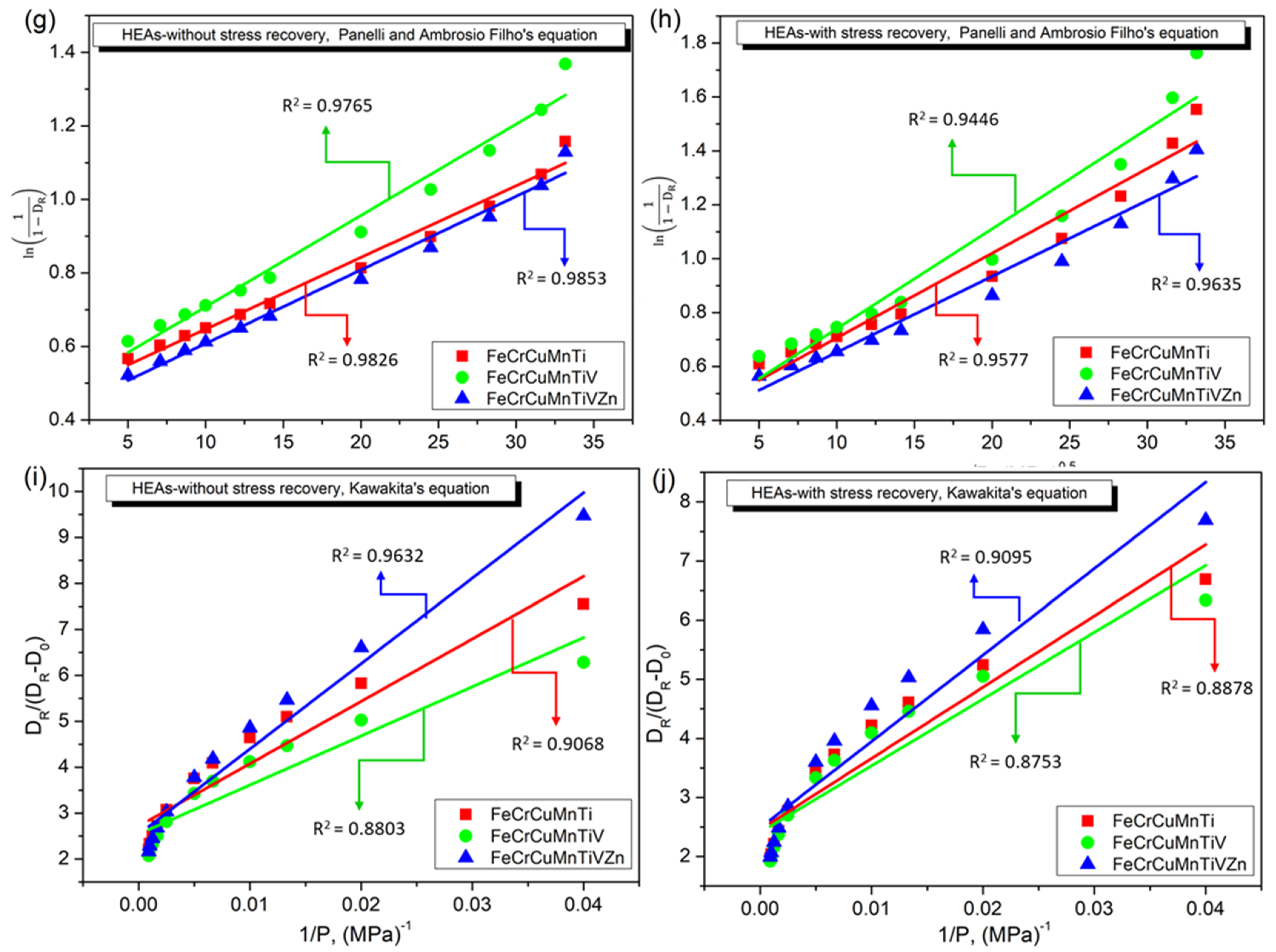
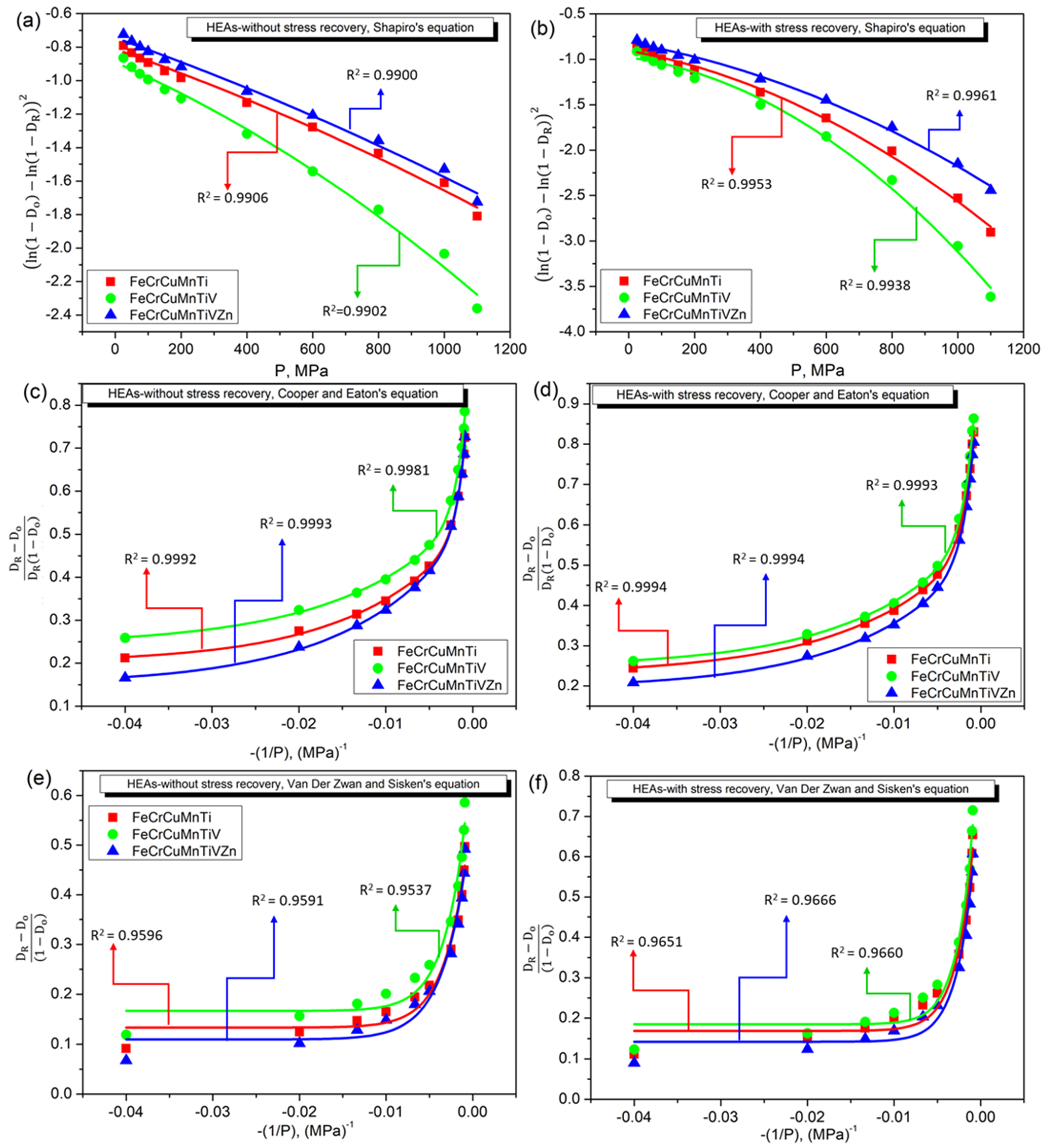
3.4. Densification Behavior of FeCrCuMnTi, FeCrCuMnTiV, and FeCrCuMnTiVZn HEAs
4. Discussion
5. Conclusions
- ❖
- Three nanostructured equiatomic FeCrCuMnTi, FeCrCuMnTiV, and FeCrCuMnTiVZn HEAs exhibited multiple solid solutions with major FCC phases, minor BCC, and HCP phases evidenced through XRD, HRSEM, and HRTEM.
- ❖
- XRD results revealed that FeCrCuMnTiVZn HEA produced more crystallite size reduction, more lattice strain, and a high value of lattice constants due to more structural refinements and high configurational entropy due to incorporation Zn.
- ❖
- A greater powder particle size reduction (24.56 ± 1.85 μm) occurred in FeCrCuMnTiV HEA due to the domination of fracturing mechanisms produced by the addition of V atoms.
- ❖
- The densification performance indicates that the FeCrCuMnTiV HEA produced a higher relative density of 0.7456 in the as-milled condition and 0.8284 in the stress-recovered conditions. The stress-relieved powder samples exhibited a higher relative density compared to the as-milled powders owing to the elimination of lattice strain.
- ❖
- Based on the applied linear and non-linear models, Balshin’s Equation (1) and Heckel’s Equation (2) linear models are well-fitted linear models. Whereas, Shapiro’s Equation (6), and Cooper and Eaton’s Equation (7) non-linear models have produced regression coefficients of more than 0.99 indicating the good accuracy of non-linear models compared to linear models.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alshataif, Y.A.; Sivasankaran, S.; Al-Mufadi, F.A.; Alaboodi, A.S.; Ammar, H.R. Synthesis, Microstructures and Mechanical Behaviour of Cr0.21Fe0.20Al0.41Cu0.18 and Cr0.14Fe0.13Al0.26Cu0.11Si0.25Zn0.11 Nanocrystallite Entropy Alloys Prepared by Mechanical Alloying and Hot-Pressing. Met. Mater. Int. 2020, 27, 139–155. [Google Scholar] [CrossRef]
- Lu, Z.P.; Wang, H.; Chen, M.W.; Baker, I.; Yeh, J.W.; Liu, C.T.; Nieh, T.G. An assessment on the future development of high-entropy alloys: Summary from a recent workshop. Intermetallics 2015, 66, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Ting, T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Ping, Z. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, M.B.; Yang, X. Advanced Technology in High-Entropy Alloys; Chemical Industry Press: Beijing, China, 2019. [Google Scholar]
- Gludovatz, B.; Hohenwarter, A.; Catoor, D.; Chang, E.H.; George, E.P.; Ritchie, R.O. A fracture-resistant high-entropy alloy for cryogenic applications. Science 2014, 345, 1153–1158. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.G.; Ma, K.H.; Wang, Q.; Shek, C.H. Compositional dependence of phase formation and mechanical properties in three CoCrFeNi-(Mn/Al/Cu) high entropy alloys. Intermetallics 2016, 79, 1–11. [Google Scholar] [CrossRef]
- Gali, A.; George, E.P. Tensile properties of high-and medium-entropy alloys. Intermetallics 2013, 39, 74–78. [Google Scholar] [CrossRef] [Green Version]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Fazakas, E.; Zadorozhnyy, V.; Louzguine-Luzgin, D. V Effect of iron content on the structure and mechanical properties of Al25Ti25Ni25Cu25 and (AlTi) 60-xNi20Cu20Fex (x = 15, 20) high-entropy alloys. Appl. Surf. Sci. 2015, 358, 549–555. [Google Scholar] [CrossRef]
- Hemphill, M.A.; Yuan, T.; Wang, G.Y.; Yeh, J.W.; Tsai, C.W.; Chuang, A.; Liaw, P.K. Fatigue behavior of Al0. 5CoCrCuFeNi high entropy alloys. Acta Mater. 2012, 60, 5723–5734. [Google Scholar] [CrossRef]
- Schuh, B.; Mendez-Martin, F.; Völker, B.; George, E.P.; Clemens, H.; Pippan, R.; Hohenwarter, A. Mechanical properties, microstructure and thermal stability of a nanocrystalline CoCrFeMnNi high-entropy alloy after severe plastic deformation. Acta Mater. 2015, 96, 258–268. [Google Scholar] [CrossRef] [Green Version]
- Sivaprasad, K.; Muthupandi, V.; Szpunar, J.A. Characterization of nanocrystalline AlCoCrCuNiFeZn high entropy alloy produced by mechanical alloying. Procedia Mater. Sci. 2014, 5, 1020–1026. [Google Scholar]
- Fang, S.; Chen, W.; Fu, Z. Microstructure and mechanical properties of twinned Al0. 5CrFeNiCo0. 3C0. 2 high entropy alloy processed by mechanical alloying and spark plasma sintering. Mater. Des. 2014, 54, 973–979. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, W.; Wu, B.; Cao, X.; Liu, L.; Fu, Z. Effects of Co and Ti on microstructure and mechanical behavior of Al0. 75FeNiCrCo high entropy alloy prepared by mechanical alloying and spark plasma sintering. Mater. Sci. Eng. A 2015, 648, 217–224. [Google Scholar] [CrossRef]
- Wang, P.; Cai, H.; Cheng, X. Effect of Ni/Cr ratio on phase, microstructure and mechanical properties of NixCoCuFeCr2− x (x = 1.0, 1.2, 1.5, 1.8 mol) high entropy alloys. J. Alloys Compd. 2016, 662, 20–31. [Google Scholar] [CrossRef]
- Avila-Rubio, M.A.; Carreño-Gallardo, C.; Herrera-Ramirez, J.M.; García-Grajeda, B.A.; Pérez-González, F.A.; Ramirez-Ramirez, J.H.; Garza-Montes-de-Oca, N.F.; Baldenebro-Lopez, F.J. Microstructure and microhardness of high entropy alloys with Zn addition: AlCoFeNiZn and AlCoFeNiMoTiZn. Adv. Powder Technol. 2021. [Google Scholar] [CrossRef]
- Pan, L.; Liu, A.; Wang, L.; Cheng, X. Microstructures and mechanical properties of FeCoNi (MoW) x high-entropy alloys. Mater. Lett. 2021, 131250. [Google Scholar] [CrossRef]
- Son, S.; Kim, S.; Kwak, J.; Gu, G.H.; Hwang, D.S.; Kim, Y.-T.; Kim, H.S. Superior antifouling properties of a CoCrFeMnNi high-entropy alloy. Mater. Lett. 2021, 300, 130130. [Google Scholar] [CrossRef]
- Zhu, T.; Zhong, Z.H.; Ren, X.L.; Song, Y.M.; Ye, F.J.; Wang, Q.Q.; Ngan, A.H.W.; Wang, B.Y.; Cao, X.Z.; Xu, Q. Influence of hydrogen behaviors on tensile properties of equiatomic FeCrNiMnCo high-entropy alloy. J. Alloys Compd. 2022, 892, 162260. [Google Scholar] [CrossRef]
- Daryoush, S.; Mirzadeh, H.; Ataie, A. Amorphization, mechano-crystallization, and crystallization kinetics of mechanically alloyed AlFeCuZnTi high-entropy alloys. Mater. Lett. 2021, 307, 131098. [Google Scholar] [CrossRef]
- Liang, C.; Wang, C.; Liang, M.; Xie, Y.; Liu, W.; Yang, J.; Li, X.; Liu, C.; Zhou, S. Effect of different aging time on the microstructure and tribological behavior of (Co1. 5FeNi) 90Ti6Al4 high entropy alloy. Vacuum 2021, 110677. [Google Scholar] [CrossRef]
- Xia, S.; Xia, Z.; Zhao, D.; Xie, Y.; Liu, X.; Wang, L. Microstructure formation mechanism and corrosion behavior of FeCrCuTiV two-phase high entropy alloy prepared by different processes. Fusion Eng. Des. 2021, 172, 112792. [Google Scholar] [CrossRef]
- Liu, D.; Jin, X.; Guo, N.; Liaw, P.K.; Qiao, J.W. Non-equiatomic FeMnCrNiAl high-entropy alloys with heterogeneous structures for strength and ductility combination. Mater. Sci. Eng. A 2021, 818, 141386. [Google Scholar] [CrossRef]
- Garlapati, M.M.; Vaidya, M.; Karati, A.; Mishra, S.; Bhattacharya, R.; Murty, B.S. Influence of Al content on thermal stability of nanocrystalline AlxCoCrFeNi high entropy alloys at low and intermediate temperatures. Adv. Powder Technol. 2020, 31, 1985–1993. [Google Scholar] [CrossRef]
- Ammar, H.R.; Sivasankaran, S.; Alaboodi, A.S.; Al-Mufadi, F.A. Synthesis, microstructural investigation and compaction behavior of Al0. 3CrFeNiCo0. 3Si0. 4 nanocrystalline high entropy alloy. Adv. Powder Technol. 2021, 32, 398–412. [Google Scholar] [CrossRef]
- Alshataif, Y.A.; Sivasankaran, S.; Al-Mufadi, F.A.; Alaboodi, A.S.; Ammar, H.R. Synthesis, structure, and mechanical response of Cr0. 26Fe0. 24Al0. 5 and Cr0. 15Fe0. 14Al0. 30Cu0. 13Si0. 28 nanocrystallite entropy alloys. Adv. Powder Technol. 2020, 31, 2161–2177. [Google Scholar] [CrossRef]
- Mane, R.B.; Panigrahi, B.B. Effect of alloying order on non-isothermal sintering kinetics of mechanically alloyed high entropy alloy powders. Mater. Lett. 2018, 217, 131–134. [Google Scholar] [CrossRef]
- Rossi, R.; Alves, M.K.; Al-Qureshi, H.A. A model for the simulation of powder compaction processes. J. Mater. Process. Technol. 2007, 182, 286–296. [Google Scholar] [CrossRef]
- Yim, D.; Jang, M.J.; Bae, J.W.; Moon, J.; Lee, C.-H.; Hong, S.-J.; Hong, S.I.; Kim, H.S. Compaction behavior of water-atomized CoCrFeMnNi high-entropy alloy powders. Mater. Chem. Phys. 2018, 210, 95–102. [Google Scholar] [CrossRef]
- Cocks, A.C.F. Constitutive modelling of powder compaction and sintering. Prog. Mater. Sci. 2001, 46, 201–229. [Google Scholar] [CrossRef]
- Smith, L.N.; Midha, P.S.; Graham, A.D. Simulation of metal powder compaction, for the development of a knowledge based powder metallurgy process advisor. J. Mater. Process. Technol. 1998, 79, 94–100. [Google Scholar] [CrossRef]
- Ali, S.; Karunanithi, R.; Prashanth, M.; Sivasankaran, S. A novel study on mechanically alloyed nanocrystalline Ti-6Al-4V alloy fabricated by spark plasma sintering. Powder Metall. 2021, 64, 149–164. [Google Scholar] [CrossRef]
- Callister, W.D.; Rethwisch, D.G.; Blicblau, A.; Bruggeman, K.; Cortie, M.; Long, J.; Hart, J.; Marceau, R.; Ryan, M.; Parvizi, R. Materials Science and Engineering: An Introduction; Wiley: New York, NY, USA, 2021; ISBN 0730382834. [Google Scholar]
- Jena, A.K.; Gupta, A.K.; Chaturvedi, M.C. A differential scanning calorimetric investigation of precipitation kinetics in the Al-1.53 wt% Cu-0.79 wt% Mg alloy. Acta Metall. 1989, 37, 885–895. [Google Scholar] [CrossRef]
- Shivam, V.; Sanjana, V.; Mukhopadhyay, N.K. Phase Evolution and Thermal Stability of Mechanically Alloyed AlCrFeCoNiZn High-Entropy Alloy. Trans. Indian Inst. Met. 2020, 73, 821–830. [Google Scholar] [CrossRef]
- Singh, A.K.; Subramaniam, A. On the formation of disordered solid solutions in multi-component alloys. J. Alloys Compd. 2014, 587, 113–119. [Google Scholar] [CrossRef]
- Balshin, M.Y. Theory of compacting. Vestnik Metalloprom 1938, 18, 124–137. [Google Scholar]
- Heckel, R.W. Density-pressure relationships in powder compaction. Trans Metall. Soc AIME 1961, 221, 671–675. [Google Scholar]
- Rong-de, G. A new powder compaction equation. Int. J. Powder Metall. 1991, 27, 211–216. [Google Scholar]
- Panelli, R.; Ambrozio Filho, F. A study of a new phenomenological compacting equation. Powder Technol. 2001, 114, 255–261. [Google Scholar] [CrossRef]
- Kawakita, K.; Lüdde, K.-H. Some considerations on powder compression equations. Powder Technol. 1971, 4, 61–68. [Google Scholar] [CrossRef]
- Shapiro, I. Compaction of powders XII. Evaluation of published compaction equations for modeling purposes. Adv. Powder Metall. Part. Mater 1995, 1, 2–97. [Google Scholar]
- Cooper, A.R.J.; Eaton, L.E. Compaction behavior of several ceramic powders. J. Am. Ceram. Soc. 1962, 45, 97–101. [Google Scholar] [CrossRef]
- DER ZWAN, V.A.N. The compaction and mechanical properties of some spray-dried ceramic materials. In Proceedings of the 10th International Conference of Ceramics, Berchtesgaden, Germany, 1–4 September 1979; p. 159. [Google Scholar]
- Zhang, Y.; Zhou, Y.J.; Lin, J.P.; Chen, G.L.; Liaw, P.K. Solid-solution phase formation rules for multi-component alloys. Adv. Eng. Mater. 2008, 10, 534–538. [Google Scholar] [CrossRef]
- Ye, Y.F.; Wang, Q.; Lu, J.; Liu, C.T.; Yang, Y. High-entropy alloy: Challenges and prospects. Mater. Today 2016, 19, 349–362. [Google Scholar] [CrossRef]
- Guo, S.; Hu, Q.; Ng, C.; Liu, C.T. More than entropy in high-entropy alloys: Forming solid solutions or amorphous phase. Intermetallics 2013, 41, 96–103. [Google Scholar] [CrossRef]
- Miedema, A.R. The electronegativity parameter for transition metals: Heat of formation and charge transfer in alloys. J. Less Common Met. 1973, 32, 117–136. [Google Scholar] [CrossRef]
- Yang, T.; Xia, S.; Liu, S.; Wang, C.; Liu, S.; Zhang, Y.; Xue, J.; Yan, S.; Wang, Y. Effects of Al addition on microstructure and mechanical properties of AlxCoCrFeNi High-entropy alloy. Mater. Sci. Eng. A 2015, 648, 15–22. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y. Prediction of high-entropy stabilized solid-solution in multi-component alloys. Mater. Chem. Phys. 2012, 132, 233–238. [Google Scholar] [CrossRef]
- Sriharitha, R.; Murty, B.S.; Kottada, R.S. Alloying, thermal stability and strengthening in spark plasma sintered AlxCoCrCuFeNi high entropy alloys. J. Alloys Compd. 2014, 583, 419–426. [Google Scholar] [CrossRef]
- George, E.P.; Raabe, D.; Ritchie, R.O. High-entropy alloys. Nat. Rev. Mater. 2019, 4, 515–534. [Google Scholar] [CrossRef]
- Singh, S.; Wanderka, N.; Murty, B.S.; Glatzel, U.; Banhart, J. Decomposition in multi-component AlCoCrCuFeNi high-entropy alloy. Acta Mater. 2011, 59, 182–190. [Google Scholar] [CrossRef]
- Shun, T.-T.; Hung, C.-H.; Lee, C.-F. Formation of ordered/disordered nanoparticles in FCC high entropy alloys. J. Alloys Compd. 2010, 493, 105–109. [Google Scholar] [CrossRef]
- Wang, F.J.; Zhang, Y. Effect of Co addition on crystal structure and mechanical properties of Ti0. 5CrFeNiAlCo high entropy alloy. Mater. Sci. Eng. A 2008, 496, 214–216. [Google Scholar] [CrossRef]


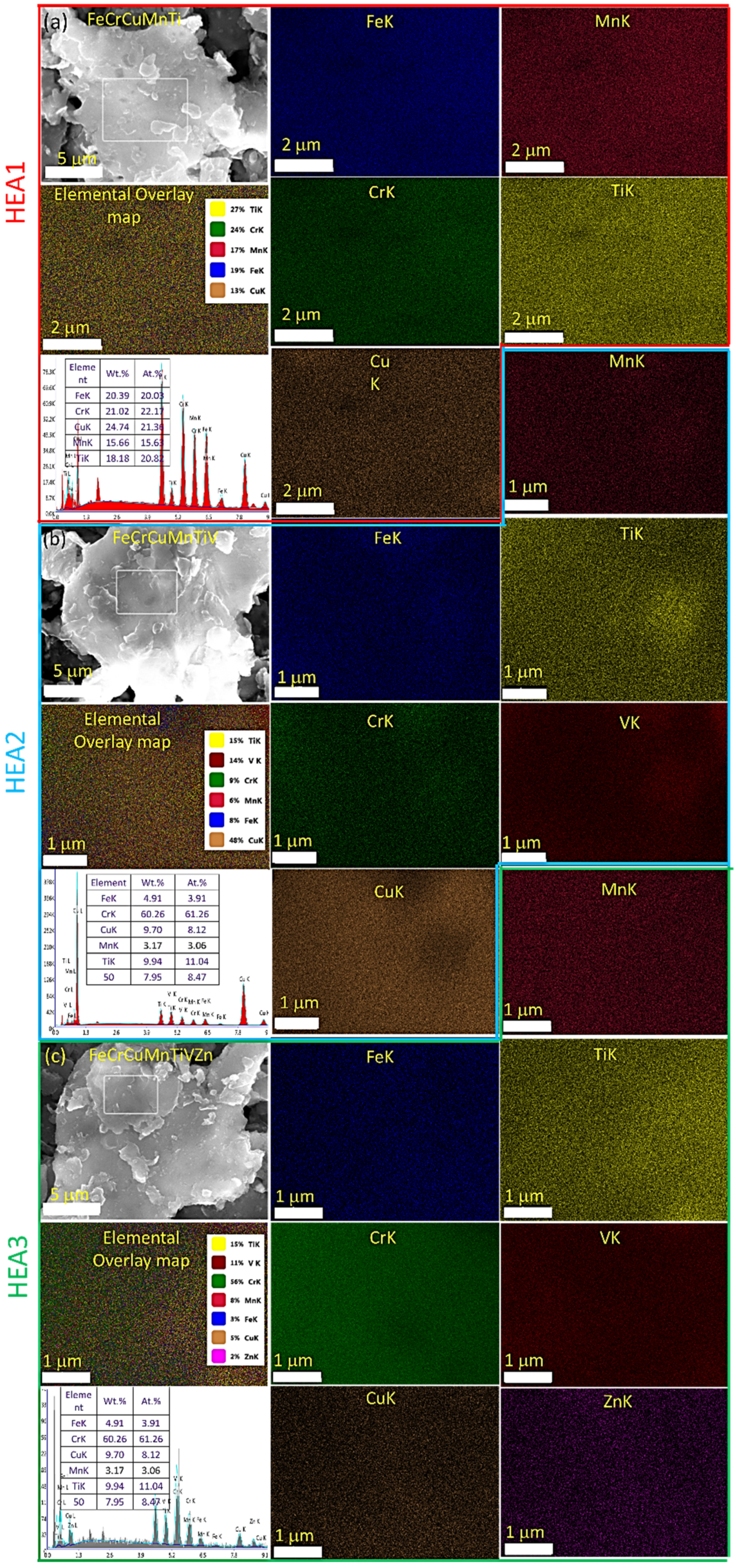
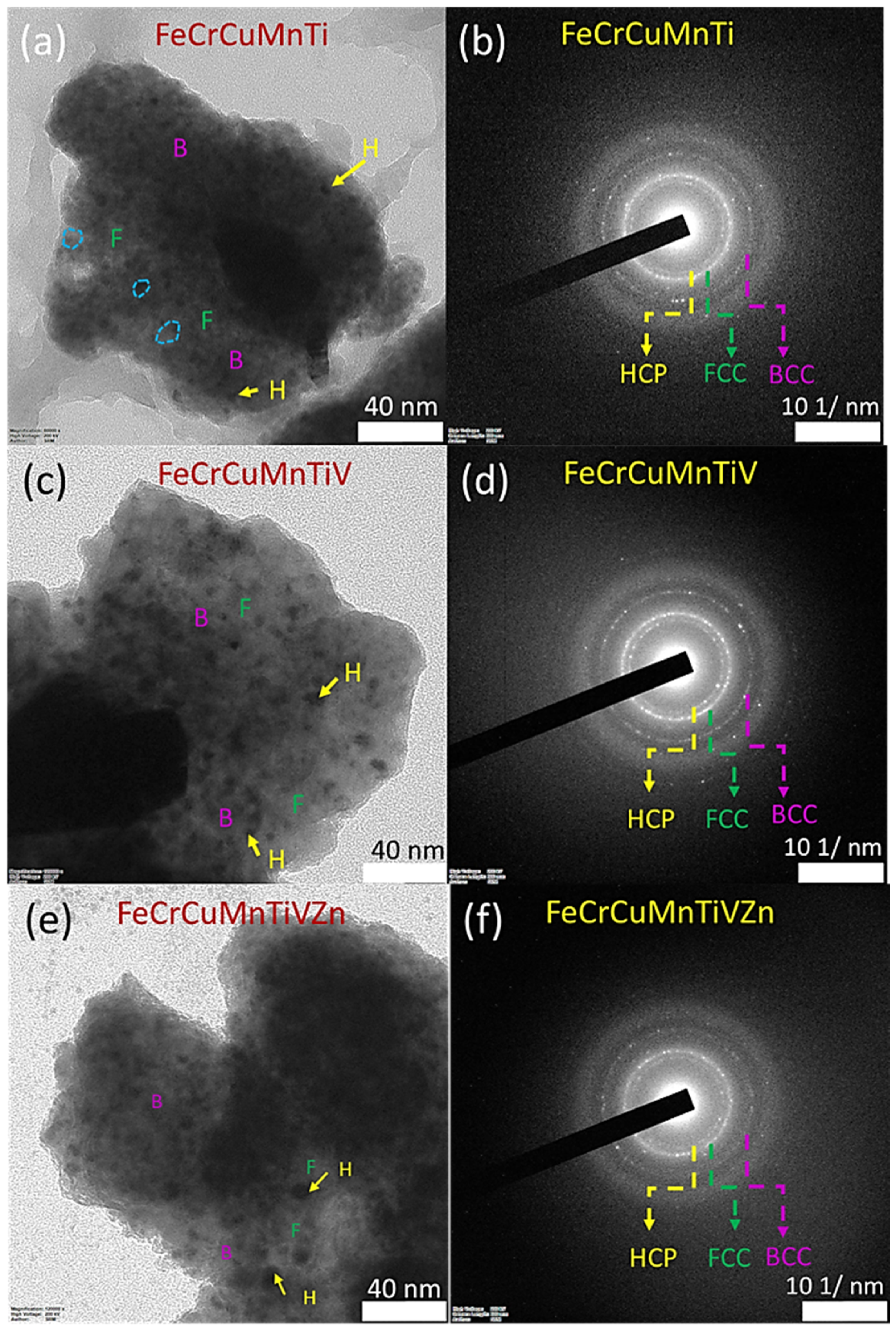

| Alloy Code | Alloy Composition (Atomic Fraction of Each Element) | LECO CS 744 and ONH 836 Analyzers (wt.%) | Crystallite Size (t), nm | Lattice Strain, % | Lattice Constant, nm | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O | C | H | N | S | BCC | FCC | HCP | BCC | FCC | HCP | BCC | FCC | HCP | |||
| ‘a’ | ‘a’ | ‘a’ | ‘c’ | |||||||||||||
| HEA1 | FeCrCuMnTi (0.2) | 0.68 | 0.45 | 0.08 | 0.09 | 0.005 | 18 | 9 | 31 | 0.97 | 0.95 | 1.5 | 0.485 | 0.361 | 0.296 | 0.483 |
| HEA2 | FeCrCuMnTiV (0.167) | 0.75 | 0.62 | 0.09 | 0.10 | 0.004 | 24 | 10 | 48 | 0.96 | 0.92 | 2.9 | 0.491 | 0.363 | 0.300 | 0.490 |
| HEA3 | FeCrCuMnTiVZn (0.143) | 0.82 | 0.74 | 0.12 | 0.09 | 0.001 | 14 | 6 | 22 | 1.1 | 0.98 | 5.6 | 0.502 | 0.365 | 0.308 | 0.503 |
| Alloy Code | Equiatomic Composition | Fe | Cr | Cu | Mn | Ti | V | Zn |
|---|---|---|---|---|---|---|---|---|
| HEA1 | FeCrCuMnTi | 20.75 ± 0.06 | 19.95 ± 0.22 | 19.61 ± 0.20 | 19.95 ± 0.05 | 19.79 ± 0.06 | - | - |
| HEA2 | FeCrCuMnTiV | 20.47 ± 0.29 | 16.52 ± 0.41 | 16.23 ± 0.65 | 15.89 ± 0.04 | 14.94 ± 0.15 | 15.95 ± 0.42 | |
| HEA3 | FeCrCuMnTiVZn | 14.27 ± 0.11 | 15.16 ± 0.39 | 13.02 ± 0.38 | 14.19 ± 0.01 | 14.59 ± 0.22 | 15.59 ± 0.50 | 13.16 ± 0.60 |
| High Entropy Alloy Powders | Powder Particle Size | Surface Area | Apparent Density | Tap Density | True Density | Packing at Apparent Density | Packing at Tapped Density (150 No. of Taps) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D10 (μm) | D50 (μm) | D90 (μm) | Davg (μm) | m2/kg | g/cm3 | g/cm3 | g/cm3 | % | % | |||||
| As-Milled | Stress Recovered | As-Milled | Stress Recovered | As-Milled | Stress Recovered | As-Milled | Stress Recovered | |||||||
| FeCrCuMnTi | 24.5 | 37 | 53.9 | 40.79 ± 1.84 | 16.35 | 2.60 ± 0.045 | 2.73 ± 0.053 | 3.30 ± 0.032 | 3.47 ± 0.028 | 6.22 ± 0.011 | 41.69 ± 0.563 | 43.68 ± 0.489 | 52.88 ± 0.657 | 55.52 ± 0.258 |
| FeCrCuMnTiV | 16 | 23.9 | 37.9 | 26.78 ± 2.89 | 24.75 | 2.62 ± 0.015 | 2.75 ± 0.023 | 3.40 ± 0.013 | 3.58 ± 0.031 | 6.52 ± 0.018 | 39.98 ± 0.687 | 41.92 ± 0.202 | 51.96 ± 0.326 | 54.57 ± 0.272 |
| FeCrCuMnTiVZn | 24.6 | 37.7 | 54.7 | 41.39 ± 1.20 | 16.14 | 2.49 ± 0.017 | 2.62 ± 0.032 | 3.08 ± 0.012 | 3.24 ± 0.024 | 6.64 ± 0.014 | 37.47 ± 0.723 | 39.39 ± 0.277 | 46.40 ± 0.468 | 49.08 ± 0.240 |
| Alloy Composition | Theoretical Density (g/cm3) | Green Density (g/cm3) | Relative Density (%) | ||
|---|---|---|---|---|---|
| No Stress Recovery | Stress Recovered | No Stress Recovery | Stress Recovered | ||
| FeCrCuMnTi | 6.25 | 4.29 | 4.93 | 68.56 | 78.85 |
| FeCrCuMnTiV | 6.56 | 4.89 | 5.43 | 74.56 | 82.84 |
| FeCrCuMnTiVZn | 6.65 | 4.50 | 5.01 | 67.65 | 75.45 |
| Compaction Equation | Parameter | FeCrCuMnTi (HEA1) | FeCrCuMnTiV (HEA2) | FeCrCuMnTiVZn (HEA3) | |||
|---|---|---|---|---|---|---|---|
| WOS | WS | WOS | WS | WOS | WS | ||
| Balshin Equation (6) | A | 3.09845 | 3.06949 | 2.95709 | 2.99541 | 3.34952 | 3.27144 |
| K | −0.22402 | −0.24659 | −0.22102 | −0.24461 | −0.25754 | −0.26668 | |
| R2 | 0.97874 | 0.97134 | 0.97807 | 0.96946 | 0.98668 | 0.97168 | |
| Heckel’s Equation (7) | A | 0.59261 | 0.61384 | 0.64873 | 0.64299 | 0.5524 | 0.57058 |
| K | 0.00050 | 0.00081 | 0.00062 | 0.00095 | 0.00051 | 0.00073 | |
| R2 | 0.98885 | 0.99439 | 0.98671 | 0.98705 | 0.98654 | 0.99573 | |
| Ge’s Equation (8) | A | −0.54121 | −0.61291 | −0.54458 | −0.62799 | −0.59842 | −0.63899 |
| K | 0.18382 | 0.24233 | 0.20618 | 0.26160 | 0.19908 | 0.23788 | |
| R2 | 0.93834 | 0.90832 | 0.93080 | 0.89714 | 0.94849 | 0.91319 | |
| Panelli and Ambrosio Filho Equation (9) | A | 0.4522 | 0.39226 | 0.45956 | 0.37035 | 0.40766 | 0.37123 |
| K | 0.01950 | 0.03141 | 0.02485 | 0.03703 | 0.02003 | 0.02817 | |
| R2 | 0.98261 | 0.95769 | 0.97648 | 0.94458 | 0.98531 | 0.96354 | |
| Kawakita’s Equation (10) | A | 2.71834 | 2.4562 | 2.54322 | 2.40788 | 2.54003 | 2.48555 |
| K | 135.8672 | 120.53758 | 107.0222 | 112.97297 | 185.8354 | 146.22189 | |
| R2 | 0.90689 | 0.88789 | 0.88034 | 0.8753 | 0.96324 | 0.9095 | |
| Shapiro Equation (11) | A | −0.81222 | −0.90605 | −0.89129 | −0.98031 | −0.74443 | −0.8176 |
| B | −6.89 × 10−4 | −6.468 × 10−4 | −8.507 × 10−4 | −4.75 × 10−4 | −6.970 × 10−4 | −6.342 × 10−4 | |
| C | −1.55 × 10−7 | −1.013 × 10−6 | −3.736 × 10−7 | −1.663 × 10−6 | −1.342 × 10−7 | −7.2824 × 10−7 | |
| R2 | 0.99059 | 0.99532 | 0.99021 | 0.99386 | 0.99002 | 0.99608 | |
| Cooper and Eaton’s Equation (12) | a1 | 0.32711 | 0.34005 | 0.4907 | 0.57359 | 0.49666 | 0.5512 |
| a2 | 0.49837 | 0.55277 | 0.3198 | 0.34613 | 0.38257 | 0.34836 | |
| k1 | 79.0513 | 72.8862 | 826.446 | 800.000 | 961.5384 | 787.4015 | |
| k2 | 961.5384 | 793.6507 | 71.9424 | 74.96251 | 77.16049 | 76.2195 | |
| R2 | 0.9992 | 0.9994 | 0.99806 | 0.99938 | 0.9993 | 0.99946 | |
| Van Der Zwan and Sisken’s Equation (13) | a | 0.46183 | 0.71326 | 0.54438 | 0.79743 | 0.46777 | 0.67931 |
| k | 384.6153 | 505.0505 | 401.6064 | 526.3157 | 348.4320 | 500.000 | |
| R2 | 0.95966 | 0.96511 | 0.95371 | 0.96607 | 0.95918 | 0.96667 | |
| Equiatomic Alloy Composition | ΔHmix, kJ/mol | Δsmix, J/mol·k | Melting Temp, °C | Ω | Atomic Radius Difference (δ), % | , J/mol | VEC | Expected Phases | Actual Observed Phases | PED (Δx) |
|---|---|---|---|---|---|---|---|---|---|---|
| FeCrCuMnTi | −1.76 | 13.38 | 1488 | 13.3930 | 1.44% | −5747.50 | 7.20 | BCC, FCC | BCC, FCC, HCP | 0.15 |
| FeCrCuMnTiV | −2.00 | 14.90 | 1588 | 13.6441 | 1.70 | −6439.21 | 6.83 | BCC, FCC | BCC, FCC, HCP | 0.14 |
| FeCrCuMnTiVZn | −2.53 | 16.18 | 1396 | 10.6883 | 1.79 | −7353.93 | 7.57 | BCC, FCC | BCC, FCC, HCP | 0.13 |
| Element (Atomic Size, nm) | Fe | Cr | Cu | Mn | Ti | V | Zn |
|---|---|---|---|---|---|---|---|
| Fe (0.14) | - | −1 | 13 | 0 | −17 | −7 | 4 |
| Cr (0.14) | - | - | 12 | 2 | −7 | −2 | 5 |
| Cu (0.135) | - | - | - | 4 | −9 | 5 | 1 |
| Mn (0.14) | - | - | - | - | −8 | −1 | −6 |
| Ti (0.14) | - | - | - | - | - | −2 | −15 |
| V (0.135) | - | - | - | - | - | - | −2 |
| Zn (0.135) | - | - | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivasankaran, S.; Al-Mufadi, F.A.; Ammar, H.R. Influence of V and Zn in FeCrCuMnTi High-Entropy Alloys on Microstructures and Uniaxial Compaction Behavior Prepared by Mechanical Alloying. Crystals 2021, 11, 1413. https://doi.org/10.3390/cryst11111413
Sivasankaran S, Al-Mufadi FA, Ammar HR. Influence of V and Zn in FeCrCuMnTi High-Entropy Alloys on Microstructures and Uniaxial Compaction Behavior Prepared by Mechanical Alloying. Crystals. 2021; 11(11):1413. https://doi.org/10.3390/cryst11111413
Chicago/Turabian StyleSivasankaran, Subbarayan, Fahad A. Al-Mufadi, and Hany R. Ammar. 2021. "Influence of V and Zn in FeCrCuMnTi High-Entropy Alloys on Microstructures and Uniaxial Compaction Behavior Prepared by Mechanical Alloying" Crystals 11, no. 11: 1413. https://doi.org/10.3390/cryst11111413






