One Pot Ultrasonic Assisted {[Ni(tptz)Cl(H2O)2][Ni(tptz)(H2O)3]}-3Cl.5H2O Complex Formation Using Triazine Ligand, XRD/HSA-Interactions, and Spectral and Thermal Investigation
Abstract
:1. Introduction
2. Experimental Section
2.1. Measurements
2.2. Computational Crystal Data
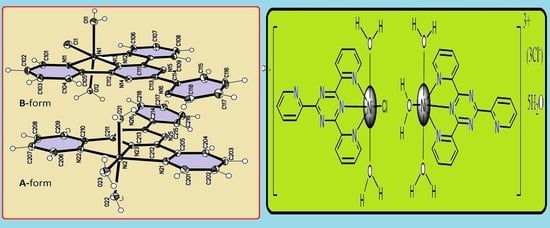
2.3. Synthesis of the {[Ni(tptz)Cl(H2O)2][Ni(tptz)(H2O)3]}3Cl.5H2O Complex
3. Results and Discussion
3.1. Synthesis, CHN-Analysis, EDX and SEM
3.2. XRD-Investigations
3.3. HSA and 2D-FP
3.4. UV-Vis. and IR Investigation
3.5. TGA Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barakat, A.; El-Faham, A.; Haukka, M.; Al-Majid, A.; Soliman, S. s-Triazine pincer ligands: Synthesis of their metalcomplexes, coordination behavior, and applications. Appl. Organomet. Chem. 2021, 35, e6317. [Google Scholar] [CrossRef]
- Hadadzadeh, H.; Maghami, M.; Simpson, J.; Khalaji, A.; Abdi, K. Nickel(II) polypyridyl Complexes of 2,4,6-Tris(2-pyridyl)-1,3,5-triazine. J. Chem. Crystallogr. 2012, 42, 656–667. [Google Scholar] [CrossRef]
- Cheng, D.-Y.; Xu, W.; Zheng, Y.-Q. Aquaoxalato (2,4,6-tri-2-pyridyl-1,3,5-triazine) cobalt (II) tetrahydrate. Acta Crystallogr. Sect. E Struct. Rep. Online 2006, 62, m2561–m2563. [Google Scholar] [CrossRef]
- Soliman, S.; Lasri, J.; Haukka, M.; Elmarghany, A.; Al-Majid, A.; El-Faham, A.; Barakat, A. Synthesis, X-ray structure, Hirshfeld analysis, and DFT studies of a new Pd(II) complex with an anionic s-triazine NNO donor ligand. J. Mol. Struct. 2020, 1217, 128463–128472. [Google Scholar] [CrossRef]
- Therrien, B. Coordination chemistry of 2,4,6-tri(pyridyl)-1,3,5-triazine ligands. J. Organomet. Chem. 2011, 696, 637–651. [Google Scholar] [CrossRef] [Green Version]
- Stang, P.J.; Olenyuk, B. Self-assembly, symmetry, and molecular architecture: Coordination as the motif in the rational design of supramolecular metallacyclic polygons and polyhedra. Acc. Chem. Res. 1997, 30, 502–518. [Google Scholar] [CrossRef]
- Glaser, T.; Lügger, T.; Fröhlich, R. Synthesis, Crystal Structures, and Magnetic Properties of a Mono-and a Dinuclear Copper (II) Complex of the 2,4,6-Tris (2-pyridyl)-1,3,5-triazine Ligand. Eur. J. Inorg. Chem. 2004, 2004, 394–400. [Google Scholar] [CrossRef]
- Zibaseresht, R.; Hartshorn, R.M. The nickel (II)/2,4,6-tris (2-pyridyl)-1,3,5-triazine system: Synthesis and crystallographic characterization of a series of complexes. Aust. J. Chem. 2005, 58, 345–353. [Google Scholar] [CrossRef]
- Zheng, Y.-Q.; Xu, W.; Lin, F.; Fang, G.-S. Syntheses and crystal structures of copper (II) complexes derived from 2,4,6-tris (2-pyridyl)-1,3,5-triazine. J. Coord. Chem. 2006, 59, 1825–1834. [Google Scholar] [CrossRef]
- Xie, H.-Z.; Pan, W.-J. Aquaoxalato (2,4,6-tri-2-pyridyl-1,3,5-triazine) zinc (II) tetrahydrate. Acta Crystallogr. Sect. E Struct. Rep. Online 2007, 63, m1231–m1232. [Google Scholar] [CrossRef]
- Witter, A.E.; Luther, G.W., III. Spectrophotometric measurement of seawater carbohydrate concentrations in neritic and oceanic waters from the US Middle Atlantic Bight and the Delaware estuary. Mar. Chem. 2002, 77, 143–156. [Google Scholar] [CrossRef]
- Gupta, N.; Grover, N.; Neyhart, G.A.; Singh, P.; Thorp, H.H. Synthesis and properties of new DNA cleavage agents based on oxoruthenium (IV). Inorg. Chem. 1993, 32, 310–316. [Google Scholar] [CrossRef]
- Ha, K. Dibromido (2,4,6-tri-2-pyridyl-1,3,5-triazine-κ3N2,N1,N6) manganese (II). Acta Crystallogr. Sect. E Struct. Rep. Online 2011, 67, m1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadda, T.B.; Akkurt, M.; Baba, M.F.; Daoudi, M.; Bennani, B.; Kerbal, A.; Chohan, Z.H. Anti-tubercular activity of ruthenium (II) complexes with polypyridines. J. Enzym. Inhib. Med. Chem. 2009, 24, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Najafpour, M.M.; Hołyńska, M.; Amini, M.; Kazemi, S.H.; Lis, T.; Bagherzadeh, M. Two new silver (I) complexes with 2,4,6-tris (2-pyridyl)-1,3,5-triazine (tptz): Preparation, characterization, crystal structure and alcohol oxidation activity in the presence of oxone. Polyhedron 2010, 29, 2837–2843. [Google Scholar] [CrossRef]
- Sharma, S.; Chandra, M.; Pandey, D.S. New Multifunctional Complexes [Ru (κ3-L)(EPh3) 2Cl]+[E= P, As; L= 2,4,6-Tris (2-pyridyl)-1,3,5-triazine] Containing both Group V and Polypyridyl Ligands. Eur. J. Inorg. Chem. 2004, 2004, 3555–3563. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, A.K.; Pandey, R.; Pandey, D.S. Bio-catalysts and catalysts based on ruthenium (II) polypyridyl complexes imparting diphenyl-(2-pyridyl)-phosphine as a co-ligand. J. Organomet. Chem. 2011, 696, 3454–3464. [Google Scholar] [CrossRef]
- Dias, V.L.N.; Fernandes, E.N.; da Silva, L.M.S.; Marques, E.P.; Zhang, J.; Marques, A.L.B. Electrochemical reduction of oxygen and hydrogen peroxide catalyzed by a surface copper (II)–2,4,6-tris (2-piridil)-1,3,5-triazine complex adsorbed on a graphite electrode. J. Power Sources 2005, 142, 10–17. [Google Scholar] [CrossRef]
- Li, S.; Zhang, L.; Kim, J.; Pan, M.; Shi, Z.; Zhang, J. Synthesis of carbon-supported binary FeCo–N non-noble metal electrocatalysts for the oxygen reduction reaction. Electrochim. Acta 2010, 55, 7346–7353. [Google Scholar] [CrossRef] [Green Version]
- De Silva, C.R.; Li, F.; Huang, C.; Zheng, Z. Europium β-diketonates for red-emitting electroluminescent devices. Thin Solid Film 2008, 517, 957–962. [Google Scholar] [CrossRef]
- Anandan, S.; Latha, S.; Murugesan, S.; Madhavan, J.; Muthuraaman, B.; Maruthamuthu, P. Synthesis, characterization and fabrication of solar cells making use of [Ru (dcbpy)(tptz) X] X (where X= Cl−, SCN−, CN−) complexes. Sol. Energy 2005, 79, 440–448. [Google Scholar] [CrossRef]
- Rubino, S.; Portanova, P.; Albanese, A.; Calvaruso, G.; Orecchio, S.; Fontana, G.; Stocco, G.C. Mono-and polynuclear complexes of Pt (II) with polypyridyl ligands: Synthesis, spectroscopic and structural characterization and cytotoxic activity. J. Inorg. Biochem. 2007, 101, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Rubino, S.; Portanova, P.; Girasolo, A.; Calvaruso, G.; Orecchio, S.; Stocco, G.C. Synthetic, structural and biochemical studies of polynuclear platinum (II) complexes with heterocyclic ligands. Eur. J. Med. Chem. 2009, 44, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Sharma, S.; Chandra, M.; Pandey, D.S. Helical racemate architecture based on osmium (II)-polypyridyl complexes: Synthesis and structural characterisation. J. Organomet. Chem. 2005, 690, 3105–3110. [Google Scholar] [CrossRef]
- Patel, R.N.; Singh, N.; Shukla, K.K.; Gundla, V.L.N.; Chauhan, U.K. Synthesis, characterization and biological activity of ternary copper (II) complexes containing polypyridyl ligands. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2006, 63, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Marzano, C.; Pellei, M.; Tisato, F.; Santini, C. Copper complexes as anticancer agents. Anti-Cancer Agents Med. Chem. 2009, 9, 185–211. [Google Scholar] [CrossRef] [PubMed]
- Hindo, S.S.; Frezza, M.; Tomco, D.; Heeg, M.J.; Hryhorczuk, L.; McGarvey, B.R.; Dou, Q.P.; Verani, C.N. Metals in anticancer therapy: Copper (II) complexes as inhibitors of the 20S proteasome. Eur. J. Med. Chem. 2009, 44, 4353–4361. [Google Scholar] [CrossRef] [Green Version]
- Patel, M.N.; Parmar, P.A.; Gandhi, D.S. Square pyramidal copper (II) complexes with forth generation fluoroquinolone and neutral bidentate ligand: Structure, antibacterial, SOD mimic and DNA-interaction studies. Bioorg. Med. Chem. 2010, 18, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer 17; University of Western: Perth, Australia, 2017. [Google Scholar]
- Burla, M.C.; Caliandro, R.; Camalli, M.; Carrozzini, B.; Cascarano, G.L.; De Caro, L.; Giacovazzo, C.; Polidori, G.; Spagna, R. SIR2004: An improved tool for crystal structure determination and refinement. J. Appl. Cryst. 2005, 38, 381–388. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. SHELX-97, Release 97-2; University of Göttingen: Göttingen, Germany, 1998. [Google Scholar]
- Guerraoui, A.; Djedouani, A.; Jeanneau, E.; Boumaza, A.; Alsalme, A.; Zarrouk, A.; Salih, K.S.M.; Warad, I. Crystal structure and spectral of new hydrazine-pyran-dione derivative: DFT enol-hydrazone tautomerization via zwitterionic intermediate, hirshfeld analysis and optical activity studies. J. Mol. Struct. 2020, 1220, 128728–128738. [Google Scholar] [CrossRef]
- Tabti, S.; Djedouani, A.; Aggoun, D.; Warad, I.; Rahmouni, S.; Romdhane, S.; Fouzi, H. New Cu (II), Co (II) and Ni (II) complexes of chalcone derivatives: Synthesis, X-ray crystal structure, electrochemical properties and DFT computational studies. J. Mol. Struct. 2018, 1155, 11–20. [Google Scholar] [CrossRef]
- Hema, M.K.; Karthik, C.S.; Warad, I.; Lokanath, N.K.; Zarrouk, A.; Kumara, K.; Pampa, K.J.; Mallu, P. Regular square planer bis-(4,4,4-trifluoro-1-(thiophen-2-yl)butane-1,3-dione)/copper(II) complex: Trans/cis-DFT isomerization, crystal structure, thermal, solvatochromism, hirshfeld surface and DNA-binding analysis. J. Mol. Struct. 2018, 1157, 69–77. [Google Scholar] [CrossRef]
- Warad, I.; Musameh, S.; Badran, I.; Nassar, N.N.; Brandao, P.; Tavares, C.J.; Barakat, A. Synthesis, solvatochromism and crystal structure of trans -[Cu(Et2NCH2CH2NH2)2.H2O](NO3)2 complex: Experimental with DFT combination. J. Mol. Struct. 2017, 11148, 328–338. [Google Scholar] [CrossRef] [Green Version]
- Aouad, M.R.; Messali, M.; Rezki, N.; Al-Zaqri, N.; Warad, I. Single proton intramigration in novel 4-phenyl-3-((4-phenyl-1H-1,2,3-triazol-1-yl)methyl)-1H-1,2,4-triazole-5(4H)-thione: XRD-crystal interactions, physicochemical, thermal, Hirshfeld surface, DFT realization of thiol/thione tautomerism. J. Mol. Liq. 2018, 264, 621–630. [Google Scholar] [CrossRef]
- Badran, I.; Abdallah, L.; Mubarakeh, R.; Warad, I. Effect of alkyl derivation on the chemical and antibacterial properties of newly synthesized Cu(II)-diamine complexes. Moroc. J. Chem. 2019, 7, 161–170. [Google Scholar]
- Hema, M.K.; Karthik, C.S.; Lokanath, N.K.; Mallu, P.; Zarrouk, A.; Salih, K.S.M.; Warad, I. Synthesis of novel Cubane [Ni4(O∩O)4(OCH3)4(OOH)4] cluster: XRD/HSA-interactions, spectral, DNA-binding, docking and subsequent thermolysis to NiO nanocrystals. J. Mol. Liq. 2020, 315, 113756–113760. [Google Scholar] [CrossRef]
- Al-Zaqri, N.; Salih, K.S.M.; Awwadi, F.F.; Alsalme, A.; Alharthi, F.A.; Alsyahi, A.; Ali, A.A.; Zarrouk, A.; Aljohani, M.; Chetouni, A.; et al. Synthesis, physicochemical, thermal, and XRD/HSA interactions of mixed [Cu(Bipy)(Dipn)](X)2 complexes: DNA binding and molecular docking evaluation. J. Coord. Chem. 2020, 73, 3236–3248. [Google Scholar] [CrossRef]
- Badran, I.; Tighadouini, S.; Radi, S.; Zarrouk, A.; Warad, I. Experimental and first-principles study of a new hydrazine derivative for DSSC applications. J. Mol. Struct. 2021, 1229, 129799. [Google Scholar] [CrossRef]
- Saleemh, F.; Musameh, S.; Sawafta, A.; Brandao, P.; Tavares, C.J.; Ferdov, S.; Barakat, A.; Ali, A.A.; Al-Noaimi, M.; Warad, I. Diethylenetriamine/diamines/copper (II) complexes [Cu(dien)(NN)]Br2: Synthesis, solvatochromism, thermal, electrochemistry, single crystal, Hirshfeld surface analysis and antibacterial activity. Arab. J. Chem. 2017, 10, 845–854. [Google Scholar] [CrossRef] [Green Version]
- Boshaala, A.; Abrahem, A.; Almughery, A.; Al-Zaqri, N.; Zarrouk, A.; Lgaz, H.; Warad, I. Spectroscopic Insight into Tetrahedrally Distorted Square Planar Copper(II) Complex: XRD/HSA, Physicochemical, DFT, and Thermal Investigations. Crystals 2021, 11, 1179. [Google Scholar] [CrossRef]
- Hijji, Y.; Rajan, R.; Ben Yahia, H.; Mansour, S.; Zarrouk, A.; Warad, I. One-Pot Microwave-Assisted Synthesis of Water-Soluble Pyran-2,4,5-triol Glucose Amine Schiff Base Derivative: XRD/HSA Interactions, Crystal Structure, Spectral, Thermal and a DFT/TD-DFT. Crystals 2021, 11, 117. [Google Scholar] [CrossRef]
- Alsimaree, A.; Alsenani, N.; Alatawi, O.; AlObaid, A.; Knight, J.G.; Messali, M.; Zarrouk, A.; Warad, I. π-Extended Boron Difluoride [N∩NBF2] Complex, Crystal Structure, Liquid NMR, Spectral, XRD/HSA Interactions: A DFT and TD-DFT Study. Crystals 2021, 11, 606. [Google Scholar] [CrossRef]
- Titi, A.; Almutairi, S.; Touzani, R.; Messali, M.; Tillardd, M.; Hammouti, B.; El Kodadi, M.; Eddikee, D.; Zarrouk, A.; Warad, I. A new mixed pyrazole-diamine/Ni(II) complex, Crystal structure, physicochemical, thermal and antibacterial investigation. J. Mol. Struc. 2021, 1236, 130304–130310. [Google Scholar] [CrossRef]
- Barakat, A.; Al-Noaimi, M.; Suleiman, M.; Aldwayyann, A.; Hammouti, B.; Ben Hadda, T.; Haddad, S.; Boshaala, A.; Warad, I. One step Synthesis of NiO nanoparticles via solid-state thermal decomposition at low-temperature of novel aqua(2,9-dimethyl-1,10-phenanthroline)NiCl2 complex. I. Int. J. Mol. Sci. 2013, 14, 23941–23954. [Google Scholar] [CrossRef] [Green Version]






| Empirical Formula | C36H44Cl4N12Ni2O10 |
|---|---|
| CCDC | 1,497,101 |
| Formula weight | 1064.05 |
| Temperature | 130(2) K |
| Wavelength | 0.71073 Å |
| Crystal system | Monoclinic |
| Space group | P2(1)/n |
| Unit cell dimensions | a = 17.139(2) Å |
| b = 14.6181(19) Å | |
| c = 17.983(2) Å β = 95.576(4)° | |
| Volume | 4484.2(10) Å3 |
| Z | 4 |
| Density (calculated) | 1.576 mg/m3 |
| Absorption coefficient | 1.147 mm−1 |
| F(000) | 2192 |
| Crystal size | 0.22 × 0.18 × 0.14 mm3 |
| Theta range for data collection | 1.57 to 27.88°. |
| Reflections collected | 37,446 |
| Independent reflections | 699 [R(int) = 0.0835] |
| Completeness to theta = 27.88° | 0.999 |
| Absorption correction | Semi-empirical from equivalents |
| Max. and min. transmission | 0.8560 and 0.7865 |
| Refinement method | Full-matrix least squares on F2 |
| Data/restraints/parameters | 10,699/30/657 |
| Goodness-of-fit on F2 | 0.725 |
| Final R indices [I > 2 sigma(I)] | R1 = 0.00427, wR2 = 0.028 |
| Largest diff. peak and hole | 1.071 and −0.476 e.Å−3 |
| Bond Type | Bond Length (Å) | Angles Type | Angle Value (°) | Angles Type | Angle Value (°) |
|---|---|---|---|---|---|
| Ni(1)-N(13) | 2.000(2) | N(13)-Ni(1)-O(11) | 90.27(10) | N(12)-C(110)-C(111) | 114.1(3) |
| Ni(1)-O(11) | 2.056(3) | N(13)-Ni(1)-O(12) | 92.90(10) | C(109)-C(110)-C(111) | 122.2(3) |
| Ni(1)-O(12) | 2.072(2) | O(11)-Ni(1)-O(12) | 174.40(11) | N(15)-C(111)-N(13) | 123.8(3) |
| Ni(1)-N(11) | 2.164(3) | N(13)-Ni(1)-N(11) | 76.30(10) | N(15)-C(111)-C(110) | 121.7(3) |
| Ni(1)-N(12) | 2.189(3) | O(11)-Ni(1)-N(11) | 87.51(10) | N(13)-C(111)-C(110) | 114.6(3) |
| Ni(1)-Cl(1) | 2.3792(9) | O(12)-Ni(1)-N(11) | 88.76(10) | N(13)-C(112)-N(14) | 124.1(3) |
| Ni(2)-N(23) | 1.988(2) | N(13)-Ni(1)-N(12) | 76.15(10) | N(13)-C(112)-C(105) | 114.2(3) |
| Ni(2)-O(23) | 2.038(2) | O(11)-Ni(1)-N(12) | 93.47(10) | N(14)-C(112)-C(105) | 121.8(3) |
| Ni(2)-O(22) | 2.047(2) | O(12)-Ni(1)-N(12) | 91.76(10) | N(14)-C(113)-N(15) | 125.9(3) |
| Ni(2)-O(21) | 2.078(3) | N(11)-Ni(1)-N(12) | 152.43(10) | N(14)-C(113)-C(114) | 118.2(3) |
| Ni(2)-N(22) | 2.146(3) | N(13)-Ni(1)-Cl(1) | 175.97(8) | N(15)-C(113)-C(114) | 116.0(3) |
| Ni(2)-N(21) | 2.181(3) | O(11)-Ni(1)-Cl(1) | 87.70(7) | N(16)-C(114)-C(115) | 123.1(3) |
| N(11)-C(101) | 1.331(4) | O(12)-Ni(1)-Cl(1) | 89.41(7) | N(16)-C(114)-C(113) | 116.2(3) |
| N(11)-C(105) | 1.356(4) | N(11)-Ni(1)-Cl(1) | 107.08(7) | C(115)-C(114)-C(113) | 120.7(3) |
| N(12)-C(106) | 1.332(4) | N(12)-Ni(1)-Cl(1) | 100.48(7) | C(114)-C(115)-C(116) | 118.3(3) |
| N(12)-C(110) | 1.360(4) | N(23)-Ni(2)-O(23) | 178.94(11) | C(117)-C(116)-C(115) | 119.6(3) |
| N(13)-C(112) | 1.324(4) | N(23)-Ni(2)-O(22) | 94.06(10) | C(116)-C(117)-C(118) | 118.1(3) |
| N(13)-C(111) | 1.338(4) | O(23)-Ni(2)-O(22) | 86.15(11) | N(16)-C(118)-C(117) | 123.6(3) |
| N(14)-C(112) | 1.335(4) | N(23)-Ni(2)-O(21) | 91.83(10) | N(21)-C(201)-C(202) | 123.0(3) |
| N(14)-C(113) | 1.348(4) | O(23)-Ni(2)-O(21) | 87.86(11) | C(203)-C(202)-C(201) | 119.2(3) |
| N(15)-C(111) | 1.328(4) | O(22)-Ni(2)-O(21) | 172.09(11) | C(202)-C(203)-C(204) | 119.0(3) |
| N(15)-C(113) | 1.347(4) | N(23)-Ni(2)-N(22) | 76.92(10) | C(205)-C(204)-C(203) | 118.4(3) |
| N(16)-C(118) | 1.334(4) | O(23)-Ni(2)-N(22) | 102.05(11) | N(21)-C(205)-C(204) | 123.5(3) |
| N(16)-C(114) | 1.352(4) | O(22)-Ni(2)-N(22) | 88.00(10) | N(21)-C(205)-C(212) | 114.1(3) |
| N(21)-C(201) | 1.337(4) | O(21)-Ni(2)-N(22) | 88.19(10) | C(204)-C(205)-C(212) | 122.4(3) |
| N(21)-C(205) | 1.364(4) | N(23)-Ni(2)-N(21) | 76.26(10) | N(22)-C(206)-C(207) | 122.7(3) |
| N(22)-C(206) | 1.338(4) | O(23)-Ni(2)-N(21) | 104.77(10) | C(208)-C(207)-C(206) | 119.2(3) |
| N(22)-C(210) | 1.358(4) | O(22)-Ni(2)-N(21) | 92.66(10) | C(207)-C(208)-C(209) | 119.1(3) |
| N(23)-C(212) | 1.333(4) | O(21)-Ni(2)-N(21) | 93.87(10) | C(210)-C(209)-C(208) | 118.2(3) |
| N(23)-C(211) | 1.334(4) | N(22)-Ni(2)-N(21) | 153.15(10) | N(22)-C(210)-C(209) | 123.5(3) |
| N(24)-C(211) | 1.329(4) | C(101)-N(11)-C(105) | 117.7(3) | N(22)-C(210)-C(211) | 113.9(3) |
| N(24)-C(213) | 1.344(4) | C(101)-N(11)-Ni(1) | 128.1(2) | C(209)-C(210)-C(211) | 122.6(3) |
| N(25)-C(212) | 1.325(4) | C(105)-N(11)-Ni(1) | 114.2(2) | N(24)-C(211)-N(23) | 123.7(3) |
| N(25)-C(213) | 1.349(4) | C(106)-N(12)-C(110) | 117.4(3) | N(24)-C(211)-C(210) | 122.3(3) |
| N(26)-C(218) | 1.337(4) | C(106)-N(12)-Ni(1) | 128.6(2) | N(23)-C(211)-C(210) | 114.0(3) |
| N(26)-C(214) | 1.349(4) | C(110)-N(12)-Ni(1) | 114.0(2) | N(25)-C(212)-N(23) | 123.9(3) |
| C(101)-C(102) | 1.386(4) | C(112)-N(13)-C(111) | 117.5(3) | N(25)-C(212)-C(205) | 122.1(3) |
| C(102)-C(103) | 1.377(4) | C(112)-N(13)-Ni(1) | 121.2(2) | N(23)-C(212)-C(205) | 114.0(3) |
| C(103)-C(104) | 1.385(4) | C(111)-N(13)-Ni(1) | 121.1(2) | N(24)-C(213)-N(25) | 125.5(3) |
| C(104)-C(105) | 1.383(4) | C(112)-N(14)-C(113) | 114.2(3) | N(24)-C(213)-C(214) | 118.0(3) |
| C(105)-C(112) | 1.480(4) | C(111)-N(15)-C(113) | 114.5(3) | N(25)-C(213)-C(214) | 116.4(3) |
| C(106)-C(107) | 1.396(5) | C(118)-N(16)-C(114) | 117.4(3) | N(26)-C(214)-C(215) | 122.8(3) |
| C(107)-C(108) | 1.370(4) | C(201)-N(21)-C(205) | 117.0(3) | N(26)-C(214)-C(213) | 116.4(3) |
| C(108)-C(109) | 1.391(4) | C(201)-N(21)-Ni(2) | 129.2(2) | C(215)-C(214)-C(213) | 120.8(3) |
| C(109)-C(110) | 1.383(4) | C(205)-N(21)-Ni(2) | 113.8(2) | C(216)-C(215)-C(214) | 118.5(3) |
| C(110)-C(111) | 1.477(4) | C(206)-N(22)-C(210) | 117.3(3) | C(217)-C(216)-C(215) | 119.0(3) |
| C(113)-C(114) | 1.489(4) | C(206)-N(22)-Ni(2) | 128.4(2) | C(216)-C(217)-C(218) | 118.9(3) |
| C(114)-C(115) | 1.376(5) | C(210)-N(22)-Ni(2) | 114.3(2) | N(26)-C(218)-C(217) | 123.5(3) |
| C(115)-C(116) | 1.391(4) | C(212)-N(23)-C(211) | 117.3(3) | ||
| C(116)-C(117) | 1.378(5) | C(212)-N(23)-Ni(2) | 121.9(2) | ||
| C(117)-C(118) | 1.386(5) | C(211)-N(23)-Ni(2) | 120.8(2) | ||
| C(201)-C(202) | 1.389(4) | C(211)-N(24)-C(213) | 114.8(3) | ||
| C(202)-C(203) | 1.378(4) | C(212)-N(25)-C(213) | 114.7(3) | ||
| C(203)-C(204) | 1.391(4) | C(218)-N(26)-C(214) | 117.3(3) | ||
| C(204)-C(205) | 1.376(4) | N(11)-C(101)-C(102) | 122.7(3) | ||
| C(205)-C(212) | 1.479(4) | C(103)-C(102)-C(101) | 119.1(3) | ||
| C(206)-C(207) | 1.384(4) | C(102)-C(103)-C(104) | 119.3(3) | ||
| C(207)-C(208) | 1.382(4) | C(105)-C(104)-C(103) | 118.0(3) | ||
| C(208)-C(209) | 1.385(4) | N(11)-C(105)-C(104) | 123.1(3) | ||
| C(209)-C(210) | 1.379(4) | N(11)-C(105)-C(112) | 114.1(3) | ||
| C(210)-C(211) | 1.481(4) | C(104)-C(105)-C(112) | 122.8(3) | ||
| C(213)-C(214) | 1.483(4) | N(12)-C(106)-C(107) | 122.2(3) | ||
| C(214)-C(215) | 1.390(4) | C(108)-C(107)-C(106) | 119.8(3) | ||
| C(215)-C(216) | 1.385(4) | C(107)-C(108)-C(109) | 119.2(3) | ||
| C(216)-C(217) | 1.376(4) | C(110)-C(109)-C(108) | 117.7(3) | ||
| C(217)-C(218) | 1.377(4) | N(12)-C(110)-C(109) | 123.7(3) |
| νHO-H | νO-H | νC-H | νC=N | νC=C | νNi-O | νNi-N | νNi-Cl |
|---|---|---|---|---|---|---|---|
| 3440 | 3290 | 3040 | 1510 | 1350 | 550 | 450 | 240 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlObaid, A.A.; Boshaala, A.; Ben Amer, Y.O.; Abrahem, A.F.; Al-Zaqri, N.; Suleiman, M.; Zarrouk, A.; Al-Maharik, N.; Khamees, H.A.; Warad, I. One Pot Ultrasonic Assisted {[Ni(tptz)Cl(H2O)2][Ni(tptz)(H2O)3]}-3Cl.5H2O Complex Formation Using Triazine Ligand, XRD/HSA-Interactions, and Spectral and Thermal Investigation. Crystals 2021, 11, 1474. https://doi.org/10.3390/cryst11121474
AlObaid AA, Boshaala A, Ben Amer YO, Abrahem AF, Al-Zaqri N, Suleiman M, Zarrouk A, Al-Maharik N, Khamees HA, Warad I. One Pot Ultrasonic Assisted {[Ni(tptz)Cl(H2O)2][Ni(tptz)(H2O)3]}-3Cl.5H2O Complex Formation Using Triazine Ligand, XRD/HSA-Interactions, and Spectral and Thermal Investigation. Crystals. 2021; 11(12):1474. https://doi.org/10.3390/cryst11121474
Chicago/Turabian StyleAlObaid, Abeer A., Ahmed Boshaala, Younis O. Ben Amer, Abrahem F. Abrahem, Nabil Al-Zaqri, Mohammed Suleiman, Abdelkader Zarrouk, Nawaf Al-Maharik, Hussien A. Khamees, and Ismail Warad. 2021. "One Pot Ultrasonic Assisted {[Ni(tptz)Cl(H2O)2][Ni(tptz)(H2O)3]}-3Cl.5H2O Complex Formation Using Triazine Ligand, XRD/HSA-Interactions, and Spectral and Thermal Investigation" Crystals 11, no. 12: 1474. https://doi.org/10.3390/cryst11121474
APA StyleAlObaid, A. A., Boshaala, A., Ben Amer, Y. O., Abrahem, A. F., Al-Zaqri, N., Suleiman, M., Zarrouk, A., Al-Maharik, N., Khamees, H. A., & Warad, I. (2021). One Pot Ultrasonic Assisted {[Ni(tptz)Cl(H2O)2][Ni(tptz)(H2O)3]}-3Cl.5H2O Complex Formation Using Triazine Ligand, XRD/HSA-Interactions, and Spectral and Thermal Investigation. Crystals, 11(12), 1474. https://doi.org/10.3390/cryst11121474