Enhanced Fluorescence Detection of Interleukin 10 by Means of 1D Photonic Crystals
Abstract
:1. Introduction
2. Materials and Methods
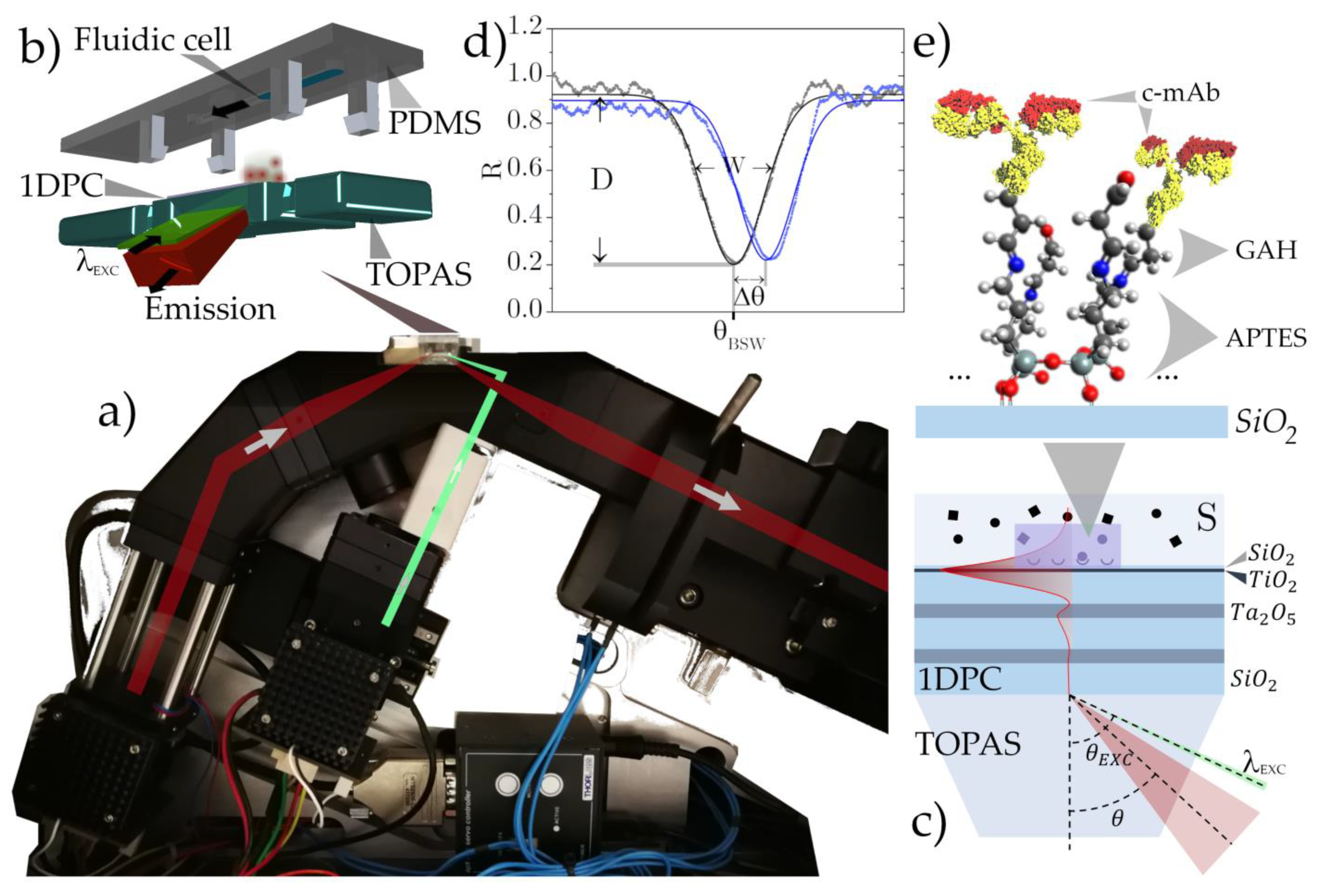
2.1. Optical Read-Out System and 1DPC Design
2.2. Surface Bioconiugation of the 1DPC
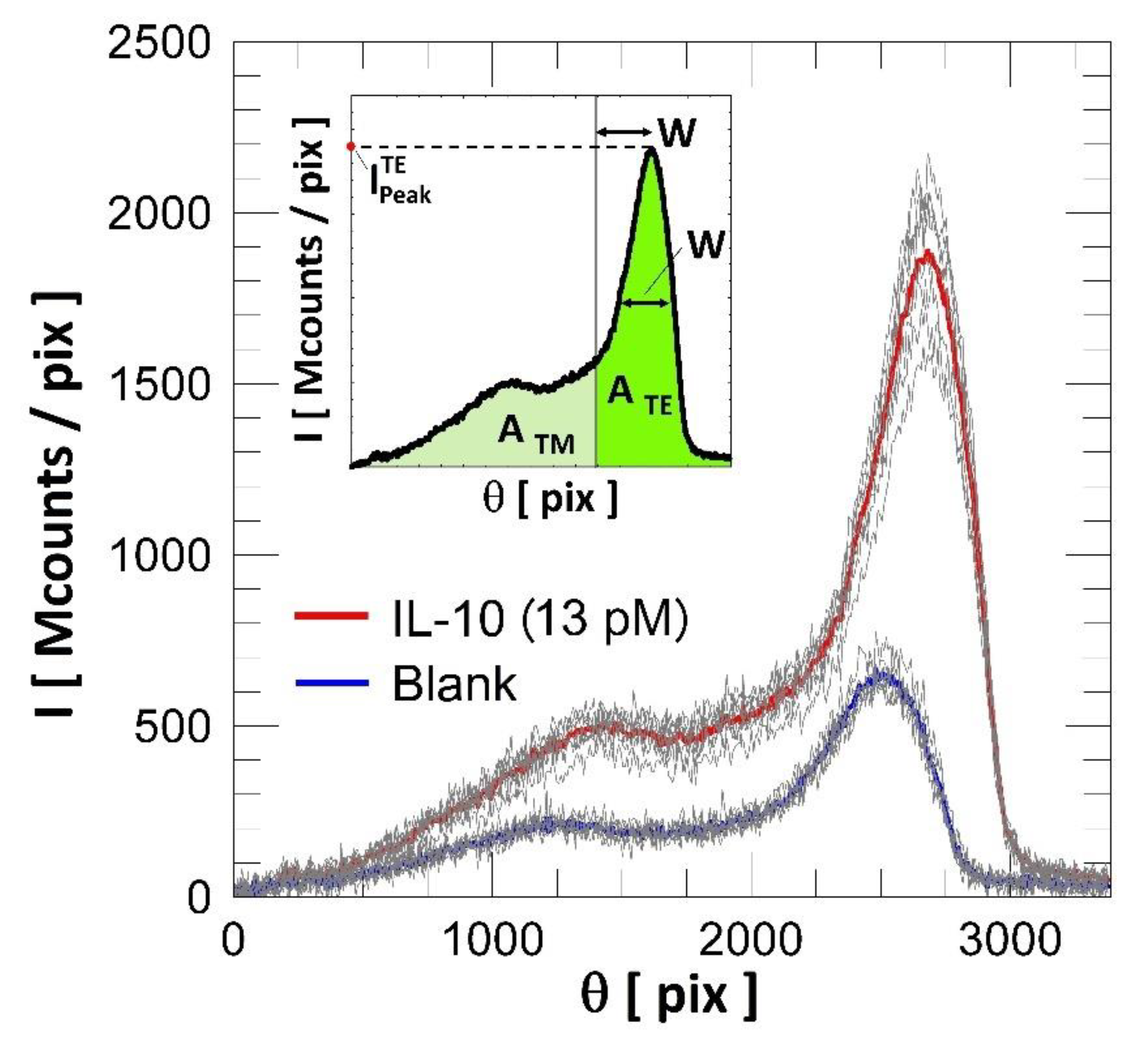
3. Results and Discussions
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, S.; Wu, D.; Wu, P.; Wang, Z.; Huang, J. Serum IL-10 Predicts Worse Outcome in Cancer Patients: A Meta-Analysis. PLoS ONE 2015, 10, e0139598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, H.; Chamberlain, T.C.; Mui, A.L.; Little, J.P. Elevated Interleukin-10 Levels in COVID-19: Potentiation of Pro-Inflammatory Responses or Impaired Anti-Inflammatory Action? Front. Immunol. 2021, 12, 677008. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, H.; Dauphars, D.J.; He, Y.-W. A Potential Role of Interleukin 10 in COVID-19 Pathogenesis. Trends Immunol. 2021, 42, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Ma, Q.; Li, C.; Liu, R.; Zhao, L.; Wang, W.; Zhang, P.; Liu, X.; Gao, G.; Liu, F.; et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg. Microbes Infect. 2020, 9, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Qin, L.; Zhang, P.; Li, K.; Liang, L.; Sun, J.; Xu, B.; Dai, Y.; Li, X.; Zhang, C.; et al. Longitudinal COVID-19 profiling associates IL-1RA and IL-10 with disease severity and RANTES with mild disease. JCI Insight 2020, 5, 139834. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer Science & Business Media: New York, NY, USA, 2013; ISBN 978-1-4757-3061-6. [Google Scholar]
- Gao, B.; Liu, H.; Gu, Z. Patterned Photonic Nitrocellulose for Pseudo-Paper Microfluidics. Anal. Chem. 2016, 88, 5424–5429. [Google Scholar] [CrossRef]
- Mathias, P.; Ganesh, N.; Cunningham, B.T. Application of Photonic Crystal Enhanced Fluorescence to a Cytokine Immunoassay. Anal. Chem. 2008, 80, 9013–9020. [Google Scholar] [CrossRef] [PubMed]
- Usuba, R.; Yokokawa, M.; Ackermann, T.N.; Llobera, A.; Fukunaga, K.; Murata, S.; Ohkohchi, N.; Suzuki, H. Photonic Lab-on-a-Chip for Rapid Cytokine Detection. ACS Sens. 2016, 1, 979–986. [Google Scholar] [CrossRef]
- Available online: www.rndsystems.com/products/human-il-10-quantikine-elisa-kit_d1000b (accessed on 1 December 2021).
- Badugu, R.; Nowaczyk, K.; Descrovi, E.; Lakowicz, J.R. Radiative decay engineering 6: Fluorescence on one-dimensional photonic crystals. Anal. Biochem. 2013, 442, 83–96. [Google Scholar] [CrossRef] [Green Version]
- Mathias, P.C.; Ganesh, N.; Chan, L.L.; Cunningham, B.T. Combined enhanced fluorescence and label-free biomolecular detection with a photonic crystal surface. Appl. Opt. 2007, 46, 2351–2360. [Google Scholar] [CrossRef]
- Rizzo, R.; Alvaro, M.; Danz, N.; Napione, L.; Descrovi, E.; Schmieder, S.; Sinibaldi, A.; Rana, S.; Chandrawati, R.; Munzert, P.; et al. Bloch surface wave enhanced biosensor for the direct detection of Angiopoietin-2 tumor biomarker in human plasma. Biomed. Opt. Express 2018, 9, 529–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzo, R.; Alvaro, M.; Danz, N.; Napione, L.; Descrovi, E.; Schmieder, S.; Sinibaldi, A.; Chandrawati, R.; Rana, S.; Munzert, P.; et al. Bloch surface wave label-free and fluorescence platform for the detection of VEGF biomarker in biological matrices. Sens. Actuators B Chem. 2018, 255, 2143–2150. [Google Scholar] [CrossRef] [Green Version]
- Sinibaldi, A.; Fieramosca, A.; Rizzo, R.; Anopchenko, A.; Danz, N.; Munzert, P.; Magistris, C.; Barolo, C.; Michelotti, F. Combining label-free and fluorescence operation of Bloch surface wave optical sensors. Opt. Lett. 2014, 39, 2947–2950. [Google Scholar] [CrossRef]
- Toma, K.; Descrovi, E.; Toma, M.; Ballarini, M.; Mandracci, P.; Giorgis, F.; Mateescu, A.; Jonas, U.; Knoll, W.; Dostálek, J. Bloch surface wave-enhanced fluorescence biosensor. Biosens. Bioelectron. 2013, 43, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Plasmonics in Biology and Plasmon-Controlled Fluorescence. Plasmonics 2006, 1, 5–33. [Google Scholar] [CrossRef] [PubMed]
- Toma, K.; Vala, M.; Adam, P.; Homola, J.; Knoll, W.; Dostálek, J. Compact surface plasmon-enhanced fluorescence biochip. Opt. Express 2013, 21, 10121–10132. [Google Scholar] [CrossRef]
- Reiner, A.T.; Fossati, S.; Dostalek, J. Chemical Biosensor platform for parallel surface plasmon-enhanced epifluorescence and surface plasmon resonance detection. Sens. Actuators B Chem. 2018, 257, 594–601. [Google Scholar] [CrossRef]
- Danz, N.; Occhicone, A.; Pflumm, C.; Munzert, P.; Michelotti, F.; Michaelis, D. Spectral analysis of organic LED emitters’ orientation in thin layers by resonant emission on dielectric stacks. Opt. Express 2021, 29, 6608–6619. [Google Scholar] [CrossRef]
- Drexhage, K. Influence of a dielectric interface on fluorescence decay time. J. Lumin. 1970, 1–2, 693–701. [Google Scholar] [CrossRef]
- Michelotti, F.; Sepe, E. Anisotropic Fluorescence Emission and Photobleaching at the Surface of One-Dimensional Photonic Crystals Sustaining Bloch Surface Waves. I. Theory. J. Phys. Chem. C 2019, 123, 21167–21175. [Google Scholar] [CrossRef]
- Sinibaldi, A.; Fieramosca, A.; Danz, N.; Munzert, P.; Occhicone, A.; Barolo, C.; Michelotti, F. Effects of Reabsorption due to Surface Concentration in Highly Resonant Photonic Crystal Fluorescence Biosensors. J. Phys. Chem. C 2018, 122, 26281–26287. [Google Scholar] [CrossRef]
- Kretschmann, E.; Raether, H. Radiative Decay of Non Radiative Surface Plasmons Excited by Light. Z. fur Nat. Sect. A J. Phys. Sci. 1968, 23, 2135–2136. [Google Scholar] [CrossRef]
- Munzert, P.; Danz, N.; Sinibaldi, A.; Michelotti, F. Surface & Coatings Technology Multilayer coatings for Bloch surface wave optical biosensors. Surf. Coat. Technol. 2017, 314, 79–84. [Google Scholar]
- Sinibaldi, A.; Rizzo, R.; Figliozzi, G.; Descrovi, E.; Danz, N.; Munzert, P.; Anopchenko, A.; Michelotti, F. A full ellipsometric approach to optical sensing with Bloch surface waves on photonic crystals. Opt. Express 2013, 21, 23331–23344. [Google Scholar] [CrossRef]
- Sepe, E.; Sinibaldi, A.; Danz, N.; Munzert, P.; Michelotti, F. Anisotropic Fluorescence Emission and Photobleaching at the Surface of One-Dimensional Photonic Crystals Sustaining Bloch Surface Waves. II. Experiments. J. Phys. Chem. C 2019, 123, 21176–21184. [Google Scholar] [CrossRef]
- Yeh, P.; Yariv, A.; Hong, C.S. Electromagnetic propagation in periodic stratified media. I. General theory. J. Opt. Soc. Am. 1977, 67, 423–438. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, R.; Danz, N.; Michelotti, F.; Maillart, E.; Anopchenko, A.; Wächter, C. Optimization of angularly resolved Bloch surface wave biosensors. Opt. Express 2014, 22, 23202–23214. [Google Scholar] [CrossRef]
- Michelotti, F.; Rizzo, R.; Sinibaldi, A.; Munzert, P.; Wächter, C.; Danz, N. Design rules for combined label-free and fluorescence Bloch surface wave biosensors. Opt. Lett. 2017, 42, 2798–2801. [Google Scholar] [CrossRef]
- Johnson, S.G.; Joannopoulos, J.D. Block-iterative frequency-domain methods for Maxwell’s equations in a plane wave basis. Opt. Express 2001, 8, 173–190. [Google Scholar] [CrossRef] [Green Version]
- Joannopoulos, J.D.; Johnson, S.G.; Winn, J.N.; Meade, R.D. Photonic Crystals: Molding the Flow of Light; Princeton University Press: Princeton, NJ, USA, 2008. [Google Scholar]
- Mahan, J.E. Physical Vapor Deposition of Thin Films; Wiley: New York, NY, USA, 2000. [Google Scholar]
- Hofstraat, J.W. Polymer Characterization by Fluorescence Spectroscopy. In Applied Polymer Science: 21st Century; Elsevier Science: Amsterdam, The Netherlands, 2000; pp. 829–850. [Google Scholar]
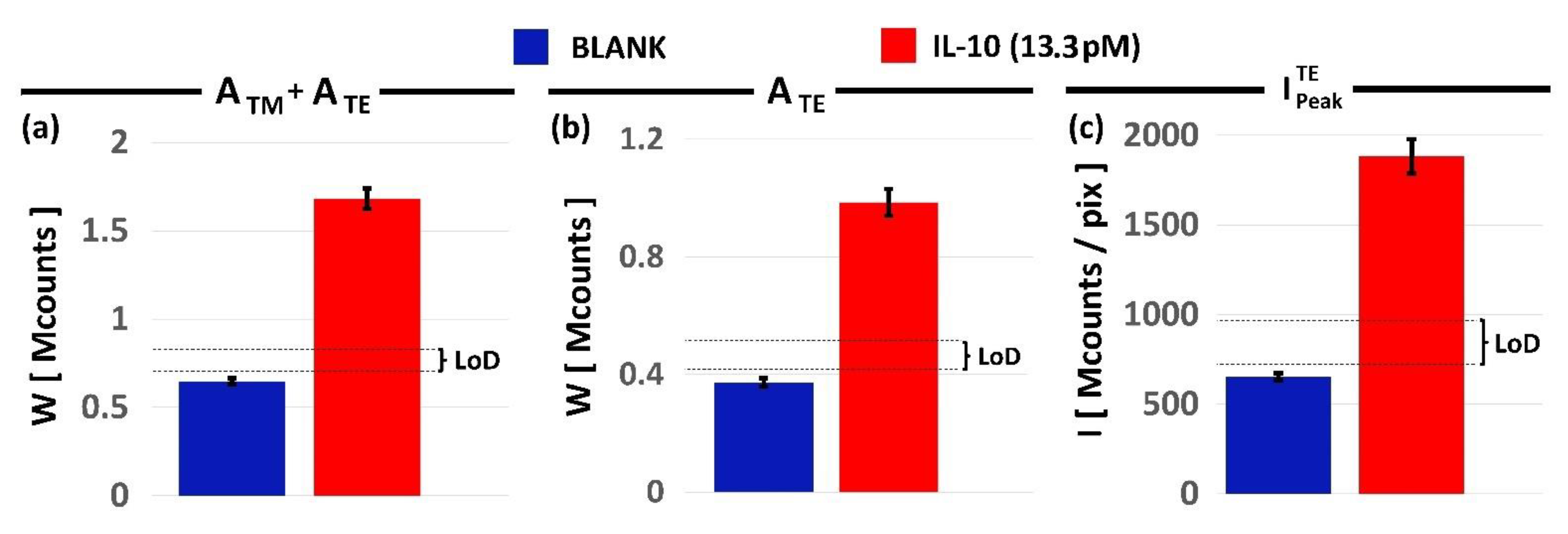
| LoD [pg/mL] | LoD [pM] | CVLoD [%] | |
|---|---|---|---|
| ATM + ATE | 113 ± 9 | 6.0 ± 0.5 | 8.3 |
| ATE | 118 ± 12 | 6.25 ± 0.65 | 10.4 |
| 110 ± 15 | 5.8 ± 0.8 | 13.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Occhicone, A.; Del Porto, P.; Danz, N.; Munzert, P.; Sinibaldi, A.; Michelotti, F. Enhanced Fluorescence Detection of Interleukin 10 by Means of 1D Photonic Crystals. Crystals 2021, 11, 1517. https://doi.org/10.3390/cryst11121517
Occhicone A, Del Porto P, Danz N, Munzert P, Sinibaldi A, Michelotti F. Enhanced Fluorescence Detection of Interleukin 10 by Means of 1D Photonic Crystals. Crystals. 2021; 11(12):1517. https://doi.org/10.3390/cryst11121517
Chicago/Turabian StyleOcchicone, Agostino, Paola Del Porto, Norbert Danz, Peter Munzert, Alberto Sinibaldi, and Francesco Michelotti. 2021. "Enhanced Fluorescence Detection of Interleukin 10 by Means of 1D Photonic Crystals" Crystals 11, no. 12: 1517. https://doi.org/10.3390/cryst11121517







