Solubility and Crystallization of Piroxicam from Different Solvents in Evaporative and Cooling Crystallization
Abstract
:1. Introduction
2. Experimental Method
2.1. Materials and Equipment
2.2. Solubility Measurement
2.3. Cooling Crystallization and Nucleation Kinetics of Piroxicam by Metastable Zone Width Measurement
2.4. Fast Evaporative Crystallization
2.5. Slow Evaporative Crystallization
3. Results
3.1. Solubility of Piroxicam
3.2. Piroxicam Polymorphism and Characterization
3.3. Cooling Crystallization and the Effect of Solvent on Nucleation Kinetics of Piroxicam
3.4. Evaporative Crystallization with Fast and Slow Evaporation Rates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Braatz, R.D. Advanced control of crystallization processes. Annu. Rev. Control. 2002, 26, 87–99. [Google Scholar] [CrossRef]
- Datta, S.; Grant, D.J.W. Crystal structures of drugs: Advances in determination, prediction and engineering. Nat. Rev. Drug Discov. 2004, 3, 42–57. [Google Scholar] [CrossRef]
- Chen, J.; Sarma, B.; Evans, J.M.B.; Myerson, A.S. Pharmaceutical Crystallization. Cryst. Growth Des. 2011, 11, 887–895. [Google Scholar] [CrossRef] [Green Version]
- Qu, H.; Kohonen, J.; Louhi-Kultanen, M.; Reinikainen, S.P.; Kallas, J. Spectroscopic Monitoring of Carbamazepine Crystallization and Phase Transformation in Ethanol-Water Solution. Ind. Eng. Chem. Res. 2008, 47, 6991–6998. [Google Scholar] [CrossRef]
- Nagy, Z.K.; Braatz, R.D. Advances and New Directions in Crystallization Control. Annu. Rev. Chem. Biomol. Eng. 2012, 3, 55–75. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, S.A.; McGarrity, E.S.; Meekes, H.; ter Horst, J.H. Isonicotinamide self-association: The link between solvent and polymorph nucleation. Chem. Commun. 2012, 48, 4983–4985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, T.B.; Taris, A.; Rong, B.G.; Grosso, M.; Qu, H. Polymorphic behavior of isonicotinamide in cooling crystallization from various solvents. J. Cryst. Growth 2016, 450, 81–90. [Google Scholar] [CrossRef]
- Taris, A.; Hansen, T.B.; Rong, B.G.; Grosso, M.; Qu, H. Detection of Nucleation during Cooling Crystallization through Moving Window PCA Applied to in Situ Infrared Data. Org. Process. Res. Dev. 2017, 21, 966–975. [Google Scholar] [CrossRef]
- Hansen, T.B.; Qu, H. Formation of Piroxicam Polymorphism in Solution Crystallization: Effect and Interplay of Operation Parameters. Cryst. Growth Des. 2015, 15, 4694–4700. [Google Scholar] [CrossRef]
- Hansen, T.B.; Simone, E.; Nagy, Z.; Qu, H. Process Analytical Tools to Control Polymorphism and Particle Size in Batch Crystallization Processes. Org. Process Res. Dev. 2017, 21, 855–865. [Google Scholar] [CrossRef] [Green Version]
- Vrecer, F.; Srcic, S.; Smid-Korbar, J. Investigation of piroxicam polymorphism. Int. J. Pharm. 1991, 68, 35–41. [Google Scholar] [CrossRef]
- Mishnev, A.; Kiselovs, G. New Crystalline Forms of Piroxicam. Zeitschrift für Naturforschung B 2013, 68, 168–174. [Google Scholar] [CrossRef] [Green Version]
- Sheth, A.R.; Bates, S.; Muller, F.X.; Grant, D.J.W. Polymorphism in Piroxicam. Cryst. Growth Des. 2004, 4, 1091–1098. [Google Scholar] [CrossRef]
- Vrečer, F.; Vrbinc, M.; Meden, A. Characterization of piroxicam crystal modifications. Int. J. Pharm. 2003, 256, 3–15. [Google Scholar] [CrossRef]
- Maggioni, G.M.; Bezinge, L.; Mazzotti, M. Stochastic Nucleation of Polymorphs: Experimental Evidence and Mathematical Modeling. Cryst. Growth Des. 2017, 17, 6703–6711. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters a User’s Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Bustamante, P.; Peña, M.A.; Barra, J. Partial solubility parameters of piroxicam and niflumic acid. Int. J. Pharm. 1998, 174, 141–150. [Google Scholar] [CrossRef]
- Naelapää, K.; van de Streek, J.; Rantanen, J.; Bond, A.D. Complementing High-Throughput X-ray Powder Diffraction Data with Quantum–Chemical Calculations: Application to Piroxicam Form III. J. Pharm. Sci. 2012, 101, 4214–4219. [Google Scholar] [CrossRef]
- Liu, G.; Hansen, T.B.; Qu, H.; Yang, M.; Pajander, J.P.; Rantanen, J.; Christensen, L.P. Crystallization of Piroxicam Solid Forms and the Effects of Additives. Chem. Eng. Technol. 2014, 37, 1297–1304. [Google Scholar] [CrossRef]
- Cruz-Cabeza, A.J.; Reutzel-Edens, S.M.; Bernstein, J. Facts and fictions about polymorphism. Chem. Soc. Rev. 2015, 44, 8619–8635. [Google Scholar] [CrossRef]
- Sanz, A.; Jiménez-Ruiz, M.; Nogales, A.; Martín y Marero, D.; Ezquerra, T.A. Hydrogen-Bond Network Breakage as a First Step to Isopropanol Crystallization. Phys. Rev. Lett. 2004, 93, 015503. [Google Scholar] [CrossRef] [Green Version]
- Cui, P.; Zhang, X.; Yin, Q.; Gong, J. Evidence of Hydrogen-Bond Formation during Crystallization of Cefodizime Sodium from Induction-Time Measurements and in Situ Raman Spectroscopy. Ind. Eng. Chem. Res. 2012, 51, 13663–13669. [Google Scholar] [CrossRef]
- Kelly, R.C.; Naír, R.H. Solvent Effects on the Crystallization and Preferential Nucleation of Carbamazepine Anhydrous Polymorphs: A Molecular Recognition Perspective Abstract. Org. Process. Res. Dev. 2009, 13, 1291–1300. [Google Scholar] [CrossRef]
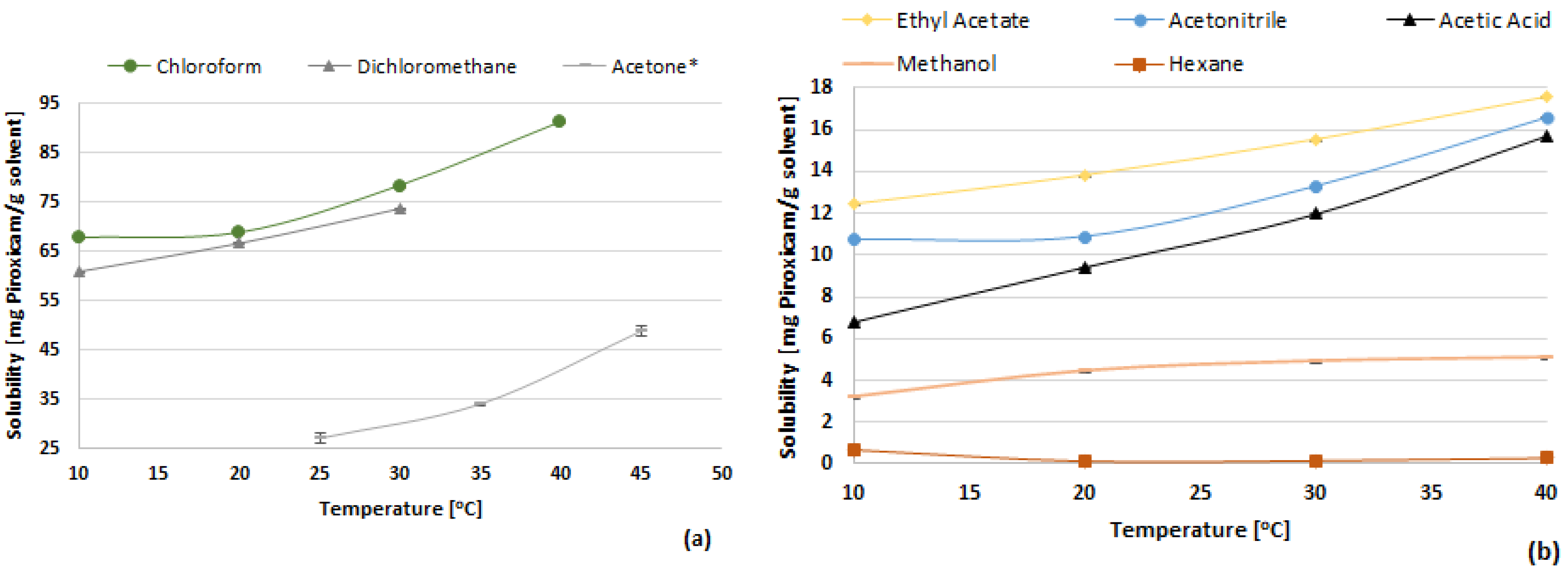





| Solvents | 10 °C | 20 °C | 30 °C | 40 °C |
|---|---|---|---|---|
| Chloroform | 67.73 ± 9.54 | 68.79 ± 0.67 | 78.40 ± 1.22 | 91.38 ± 2.38 |
| Dichloromethane | 61.00 ± 2.71 | 66.76 ± 0.76 | 73.80 ± 10.76 | |
| Ethyl acetate | 12.42 ± 0.24 | 13.82 ± 0.48 | 15.55 ± 0.47 | 17.61 ± 0.67 |
| Acetonitrile | 10.73 ± 0.78 | 10.87 ± 0.34 | 13.30 ± 0.23 | 16.61 ± 0.91 |
| Acetic acid | 6.82 | 9.43 ± 0.32 | 11.97 ± 0.24 | 15.69 ± 0.04 |
| Methanol | 3.19 ± 0.08 | 4.44 ± 1.53 | 4.91 ± 0.05 | 5.08 ± 0.38 |
| n-Hexane | 0.68 ± 0.05 | 0.14 ± 0.25 | 0.16 ± 0.11 | 0.29 ± 0.01 |
| Linear Cooling Crystallization | Fast Evaporative Crystallization | Slow Evaporative Crystallization | |||
|---|---|---|---|---|---|
| Saturated Solution at Given Temperature (Solubility Rank) | 10 °C | 30 °C | 20 °C | 30 °C | 20 °C |
| TCM (1) | - | Form I | - | Form II | Form II |
| DCM (2) | Form II | Form II | Form II | Form II | Form II |
| EtOAc (3) | Form I | Form II | Form II | Form II | Form II+I * |
| ACN (4) | Form II | Form II | Form II | Form II | Form I |
| AcOH (5) | Form I | Form I | Solvate | Solvate | - |
| MeOH (6) | - | - | Form II | Form II | Form II |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostergaard, I.; Qu, H. Solubility and Crystallization of Piroxicam from Different Solvents in Evaporative and Cooling Crystallization. Crystals 2021, 11, 1552. https://doi.org/10.3390/cryst11121552
Ostergaard I, Qu H. Solubility and Crystallization of Piroxicam from Different Solvents in Evaporative and Cooling Crystallization. Crystals. 2021; 11(12):1552. https://doi.org/10.3390/cryst11121552
Chicago/Turabian StyleOstergaard, Iben, and Haiyan Qu. 2021. "Solubility and Crystallization of Piroxicam from Different Solvents in Evaporative and Cooling Crystallization" Crystals 11, no. 12: 1552. https://doi.org/10.3390/cryst11121552
APA StyleOstergaard, I., & Qu, H. (2021). Solubility and Crystallization of Piroxicam from Different Solvents in Evaporative and Cooling Crystallization. Crystals, 11(12), 1552. https://doi.org/10.3390/cryst11121552






