Population Balance and CFD Simulation of Particle Aggregation and Growth in a Continuous Confined Jet Mixer for Hydrothermal Synthesis of Nanocrystals
Abstract
:1. Introduction
2. Coupled CFD–PBM Model
2.1. Mathematical Formulation
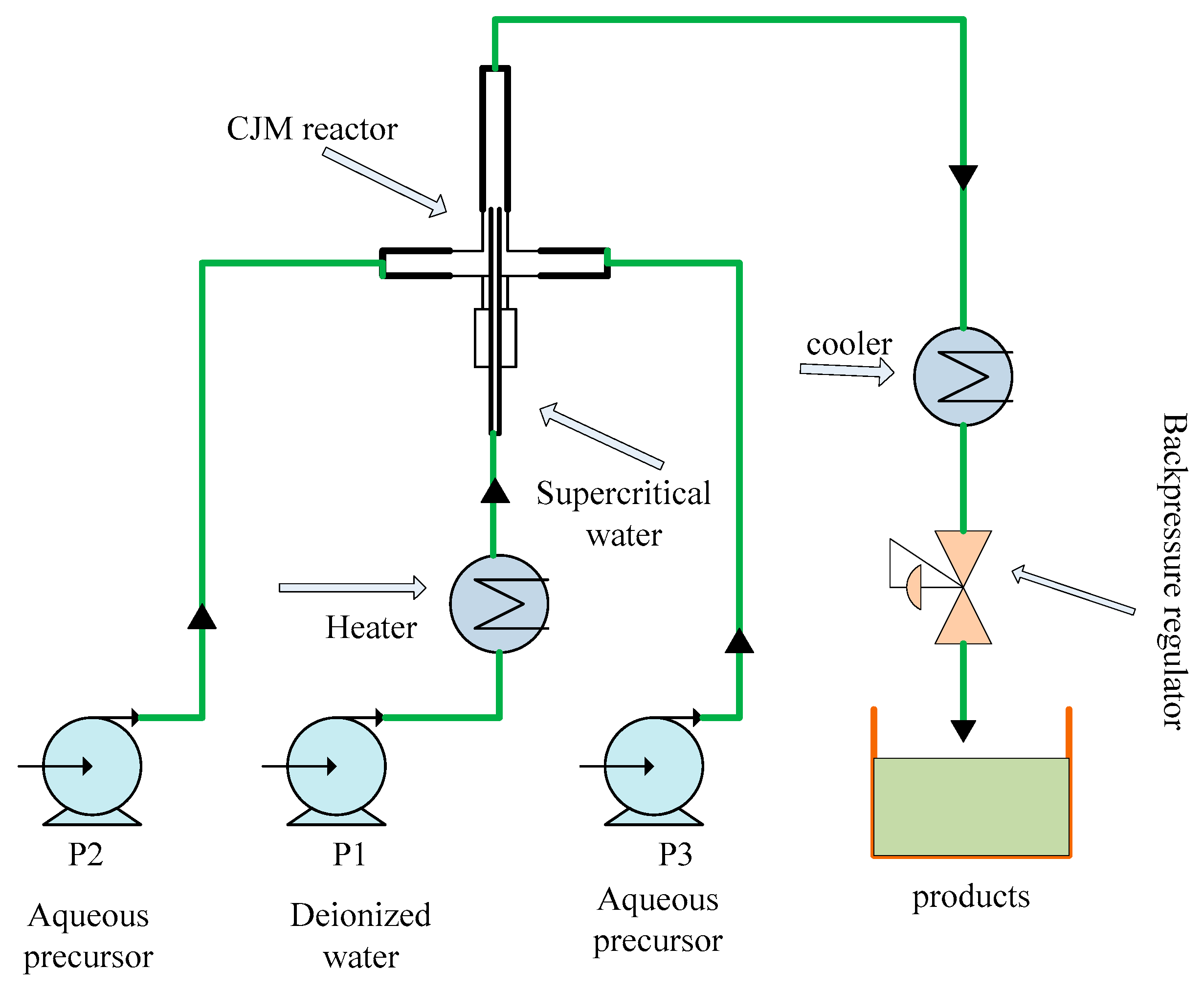
2.1.1. Meshing
2.1.2. Thermodynamic and Material Property
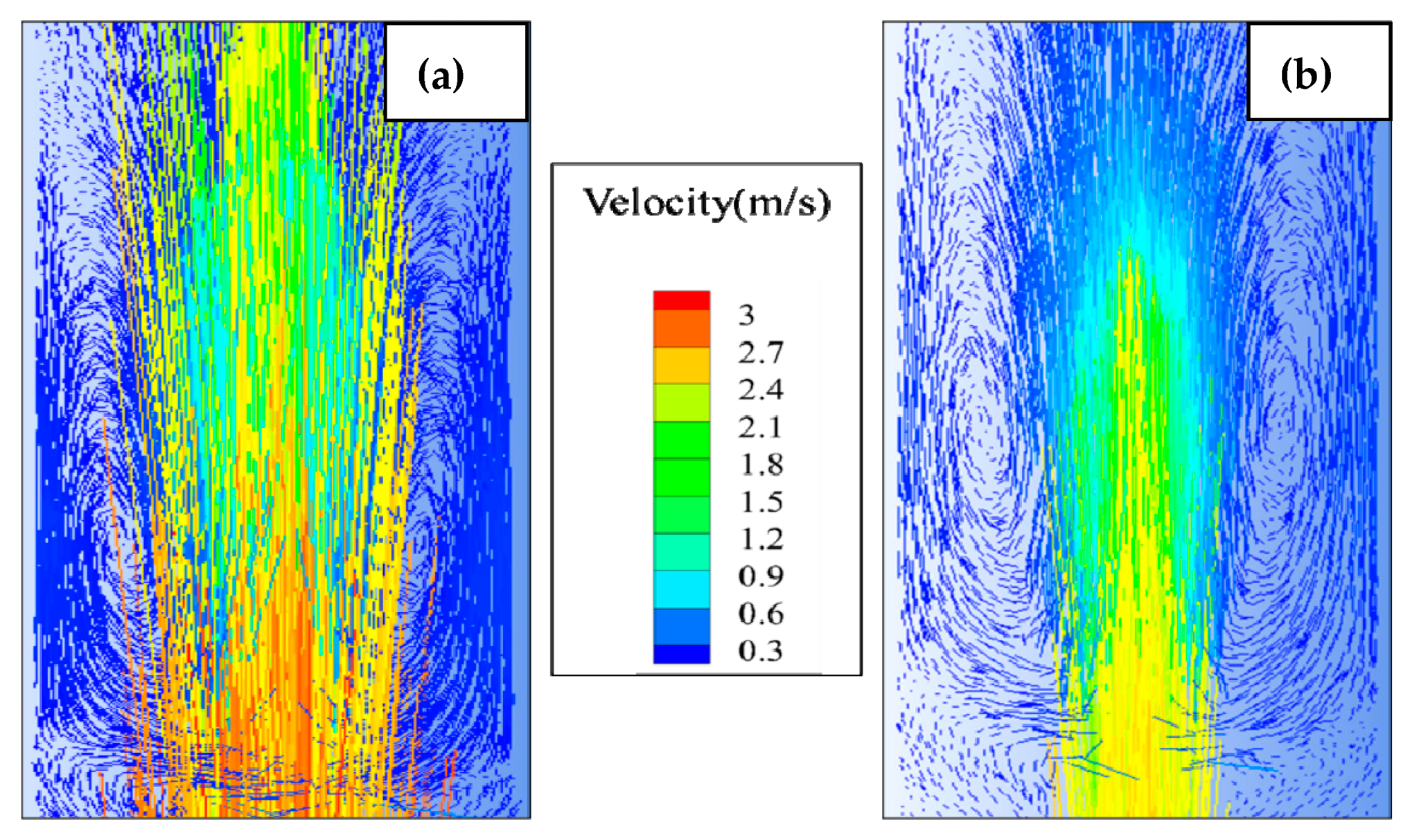
2.1.3. Hydrodynamics
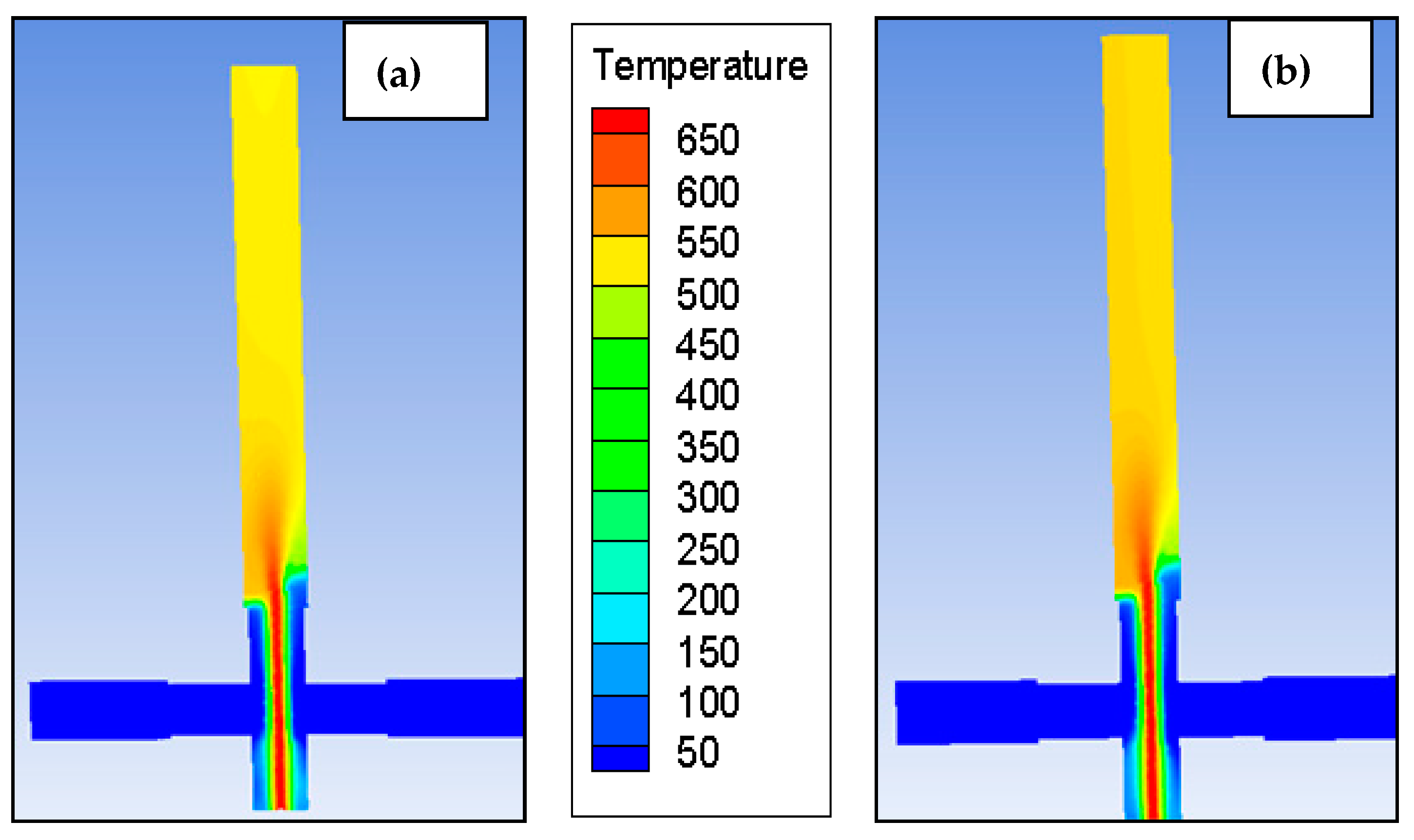
Energy Balance Equation
The Turbulence Model
2.1.4. Mixing and Reaction Model
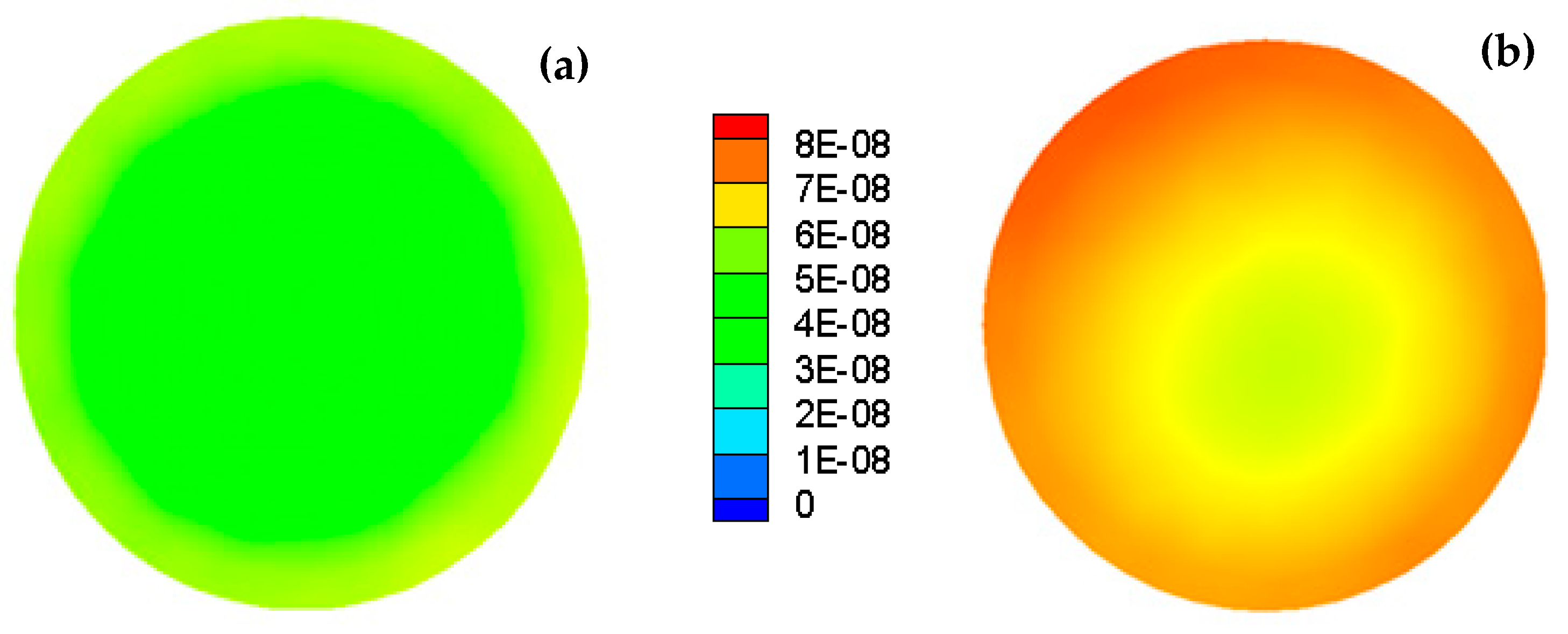
2.1.5. Population Balance Model
2.2. Numerical Procedure
3. Comparison and Validation
4. Result and Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Darr, J.A.; Zhang, J.; Makwana, N.M.; Weng, X. Continuous Hydrothermal Synthesis of Inorganic Nanoparticles: Applications and Future Directions. Chem. Rev. 2017, 117, 11125–11238. [Google Scholar] [CrossRef] [Green Version]
- Adschiri, T.; Hakuta, Y.; Arai, K. Hydrothermal Synthesis of Metal Oxide Fine Particles at Supercritical Conditions. Ind. Eng. Chem. Res. 2000, 39, 4901–4907. [Google Scholar] [CrossRef]
- Adschiri, T.; Kanazawa, K.; Arai, K. Rapid and Continuous Hydrothermal Crystallization of Metal Oxide Particles in Supercritical Water. J. Am. Ceramic Soc. 1992, 75, 1019–1022. [Google Scholar] [CrossRef]
- Darr, J.A.; Poliakoff, M. New Directions in Inorganic and Metal-Organic. Co-ordination Chemistry in Supercritical Fluids. Chem. Rev. 1999, 99, 495–541. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.Y.; Tighe, C.; Gruar, R.I.; Mahmud, T.; Darr, J.A.; Wang, X.Z. Numerical modelling of hydrothermal fluid flow and heat transfer in a tubular heat exchanger under near critical conditions. J. Supercrit. Fluids 2011, 57, 236–246. [Google Scholar] [CrossRef]
- Demoisson, F.; Ariane, M.; Leybros, A.; Muhr, H.; Bernard, F. Design of a reactor operating in supercritical water conditions using CFD simulations. Examples of synthesized nanomaterials. J. Supercrit. Fluids 2011, 58, 371–377. [Google Scholar] [CrossRef]
- Kawasaki, S.-I.; Sue, K.; Ookawara, R.; Wakashima, Y.; Suzuki, A.; Hakuta, Y.; Arai, K. Engineering study of continuous supercritical hydrothermal method using a T-shaped mixer: Experimental synthesis of NiO nanoparticles and CFD simulation. J. Supercrit. Fluids 2010, 54, 96–102. [Google Scholar] [CrossRef]
- Chen, M.; Ma, C.Y.; Mahmud, T.; Darr, J.A.; Wang, X.Z. Modelling and simulation of continuous hydrothermal flow synthesis process for nano-materials manufacture. J. Supercrit. Fluids 2011, 59, 131–139. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, S.; Xu, D.; Guo, Y. Impact of Mixing for the Production of CuO Nanoparticles in Supercritical Hydrothermal Synthesis. Ind. Eng. Chem. Res. 2013, 53, 481–493. [Google Scholar] [CrossRef]
- Leybros, A.; Piolet, R.; Ariane, M.; Muhr, H.; Bernard, F.; Demoisson, F. CFD simulation of ZnO nanoparticle precipitation in a supercritical water synthesis reactor. J. Supercrit. Fluids 2012, 70, 17–26. [Google Scholar] [CrossRef]
- Ma, C.Y.; Chen, M.; Wang, X.Z. Modelling and simulation of counter-current and confined jet reactors for hydrothermal synthesis of nano-materials. Chem. Eng. Sci. 2014, 109, 26–37. [Google Scholar] [CrossRef]
- Kawasaki, S.-I.; Xiuyi, Y.; Sue, K.; Hakuta, Y.; Suzuki, A.; Arai, K. Continuous supercritical hydrothermal synthesis of controlled size and highly crystalline anatase TiO2 nanoparticles. J. Supercrit. Fluids 2009, 50, 276–282. [Google Scholar] [CrossRef]
- Erriguible, A.; Marias, F.; Cansell, F.; Aymonier, C. Monodisperse model to predict the growth of inorganic nanostructured particles in supercritical fluids through a coalescence and aggregation mechanism. J. Supercrit. Fluids 2009, 48, 79–84. [Google Scholar] [CrossRef]
- Bhutani, G.; Brito-Parada, P.R.; Cilliers, J.J. Polydispersed flow modelling using population balances in an adaptive mesh finite element framework. Comput. Chem. Eng. 2016, 87, 208–225. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.Y.; Mahmud, T.; Wang, X.Z.; Tighe, C.J.; I Gruar, R.; A Darr, J. Numerical Simulation of Fluid Flow and Heat Transfer in a Counter-Current Reactor System for Nanomaterial Production. Chem. Prod. Process. Model. 2011, 6. [Google Scholar] [CrossRef]
- Tighe, C.J.; Gruar, R.I.; Ma, C.Y.; Mahmud, T.; Wang, X.Z.; Darr, J.A. Investigation of counter-current mixing in a continuous hydrothermal flow reactor. J. Supercrit. Fluids 2012, 62, 165–172. [Google Scholar] [CrossRef] [Green Version]
- Launder, B.E.; Reece, G.J.; Rodi, W. Peogress in the development of a Reynolds-stress turbulence closure. J. Fluid Mech. 1975, 68, 537–566. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.Y.; Wang, X.Z.; Tighe, C.J.; Darr, J.A.; Tighe, C. Modelling and Simulation of Counter-Current and Confined Jet Reactors for Continuous Hydrothermal Flow Synthesis of Nano-materials. In Proceedings of the 8th IFAC International Symposium on Advanced Control of Chemical Processes, Singapore, 10–13 July 2012. [Google Scholar]
- Tompson, R.V.; Loyalka, S.K. Chapman–Enskog solution for diffusion: Pidduck’s equation for arbitrary mass ratio. Phys. Fluids 1987, 30, 2073–2075. [Google Scholar] [CrossRef]
- Adschiri, T. Supercritical Hydrothermal Synthesis of Organic–Inorganic Hybrid Nanoparticles. Chem. Letters 2007, 36, 1188–1193. [Google Scholar] [CrossRef]
- Courtecuisse, V.; Bocquet, J.; Chhor, K.; Pommier, C. Modeling of a continuous reactor for TiO2 powder synthesis in a supercritical fluid—experimental validation. J. Supercrit. Fluids 1996, 9, 222–226. [Google Scholar] [CrossRef]
- Hounslow, M.J.; Ryall, R.L.; Marshall, V.R. A discretized population balance for nucleation, growth, and aggregation. AIChE J. 1988, 34, 1821–1832. [Google Scholar] [CrossRef]
- Lister, J.D.; Smit, D.J.; Hounslow, M.J. Adjustable discretized population balance for growth and aggregation. AIChE J. 1995, 41, 591–603. [Google Scholar] [CrossRef]
- Randolph, A.D.; Larson, M.A. Theory and Particulate Processes: Analysis and Techniques of Continuous Crystallization; Randolph, A.D., Larson, M.A., Eds.; Academic Press: New York, NY, USA, 1971. [Google Scholar]
- Marchisio, D.; Pikturna, J.T.; Fox, R.O.; Vigil, R.D.; A Barresi, A. Quadrature method of moments for population-balance equations. AIChE J. 2003, 49, 1266–1276. [Google Scholar] [CrossRef]
- McGraw, R. Description of Aerosol Dynamics by the Quadrature Method of Moments. Aerosol Sci. Technol. 1997, 27, 255–265. [Google Scholar] [CrossRef]
- Alonso, E.; Montequi, I.; Lucas, S.; Cocero, M.; Cocero, M.J. Synthesis of titanium oxide particles in supercritical CO2: Effect of operational variables in the characteristics of the final product. J. Supercrit. Fluids 2007, 39, 453–461. [Google Scholar] [CrossRef]
- Chen, M.; Ma, C.Y.; Mahmud, T.; Lin, T.; Wang, X.Z. Hydrothermal Synthesis of TIO2 Nanoparticles: Process Modelling and Experimental Validation. In Particulate Materials: Synthesis, Characterisation, Processing and Modelling; Wu, C.Y., Ge, W., Eds.; The Royal Society of Chemistry (RSC): Cambridge, UK, 2012; pp. 28–33. [Google Scholar]
- Chen, M.; Ma, C.Y.; Mahmud, T.; Lin, T.; Wang, X.Z. Population Balance Modelling and Experimental Validation for Synthesis of TiO2 Nanoparticles Using Continuous Hydrothermal Process. Adv. Mater. Res. 2012, 508, 175–179. [Google Scholar] [CrossRef]
- Hakuta, Y.; Hayashi, H.; Arai, K. Hydrothermal synthesis of photocatalyst potassium hexatitanate nanowires under supercritical conditions. J. Mater. Sci. 2004, 39, 4977–4980. [Google Scholar] [CrossRef]
- Hayashi, H.; Torii, K. Hydrothermal synthesis of titania photocatalyst under subcritical and supercritical water conditions. J. Mater. Chem. 2002, 12, 3671–3676. [Google Scholar] [CrossRef]
- Luo, H. Coalescence, Breakup and Liquid Circulation in Bubble Column Reactors; Norwegian Institute of Technology: Trondheim, Norway, 1993. [Google Scholar]
- Higashitani, K.; Yamauchi, K.; Matsuno, Y.; Hosokawa, G. Turbulent coagulation of particles dispersed in a viscous fluid. J. Chem. Eng. Jpn. 1983, 16, 299–304. [Google Scholar] [CrossRef] [Green Version]
- Saffman, P.G.; Turner, J.S. On the collision of drops in turbulent clouds. J. Fluid Mech. 1956, 1, 16. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.K.; Friedlander, S.K. Enhanced Power-Law Agglomerate Growth in the Free-Molecule Regime. J. Aerosol. Sci. J. Aerosol Sci. 1993, 24, 273–282. [Google Scholar] [CrossRef]
- Testino, A.; Buscaglia, V.; Buscaglia, M.T.; Viviani, A.M.; Nanni, P. Kinetic Modeling of Aqueous and Hydrothermal Synthesis of Barium Titanate (BaTiO3). Chem. Mater. 2005, 17, 5346–5356. [Google Scholar] [CrossRef]
- Peukert, W.; Schwarzer, H.-C.; Stenger, F. Control of aggregation in production and handling of nanoparticles. Chem. Eng. Process. 2005, 44, 245–252. [Google Scholar] [CrossRef]
- Weng, X.; Zeng, Q.; Zhang, Y.; Dong, F.; Wu, Z. A Facile Approach for the Syntheses of Ultrafine TiO2 Nano-crystallites with Defects and C Heterojunction for Photocatalytic Water Splitting. ACS Sustain. Chem. Eng. 2016, 4, 4314–4320. [Google Scholar] [CrossRef]
- Ginter, D.M.; Loyalka, S.K. Apparent size-dependent growth in aggregating crystallizers. Chem. Eng. Sci. 1996, 51, 3685–3695. [Google Scholar] [CrossRef]


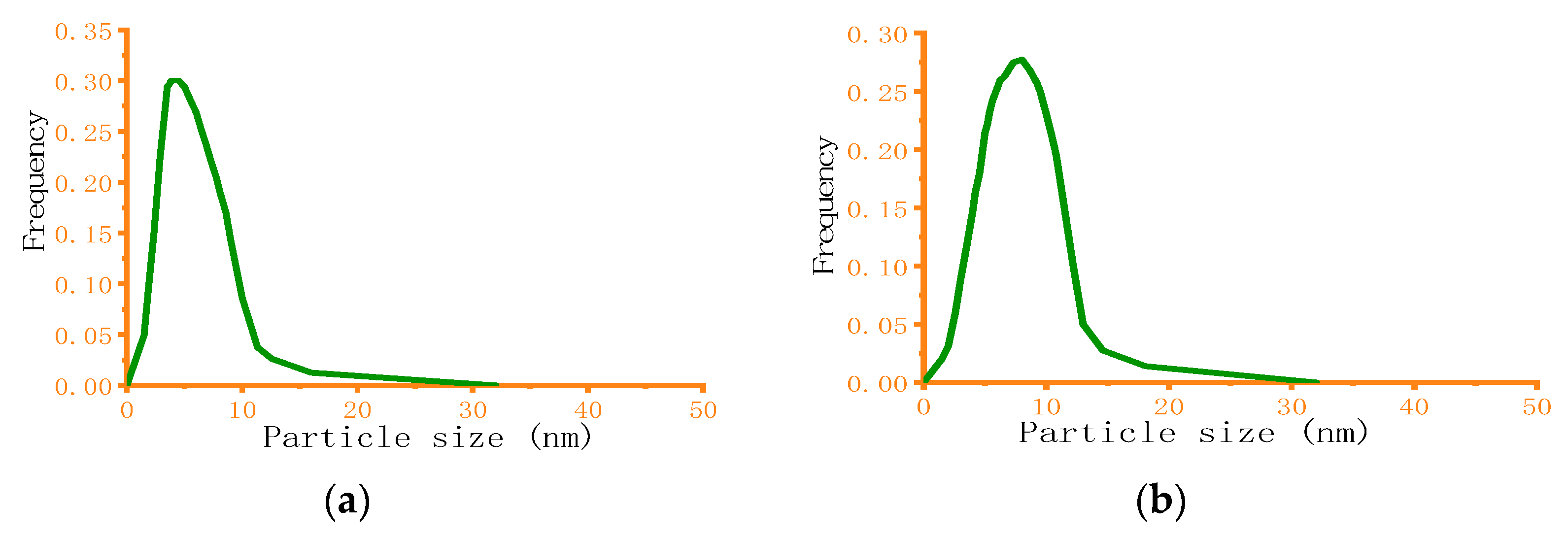
 ] represents experimental data and solid line [
] represents experimental data and solid line [  ] represents simulation data).
] represents simulation data).
 ] represents experimental data and solid line [
] represents experimental data and solid line [  ] represents simulation data).
] represents simulation data).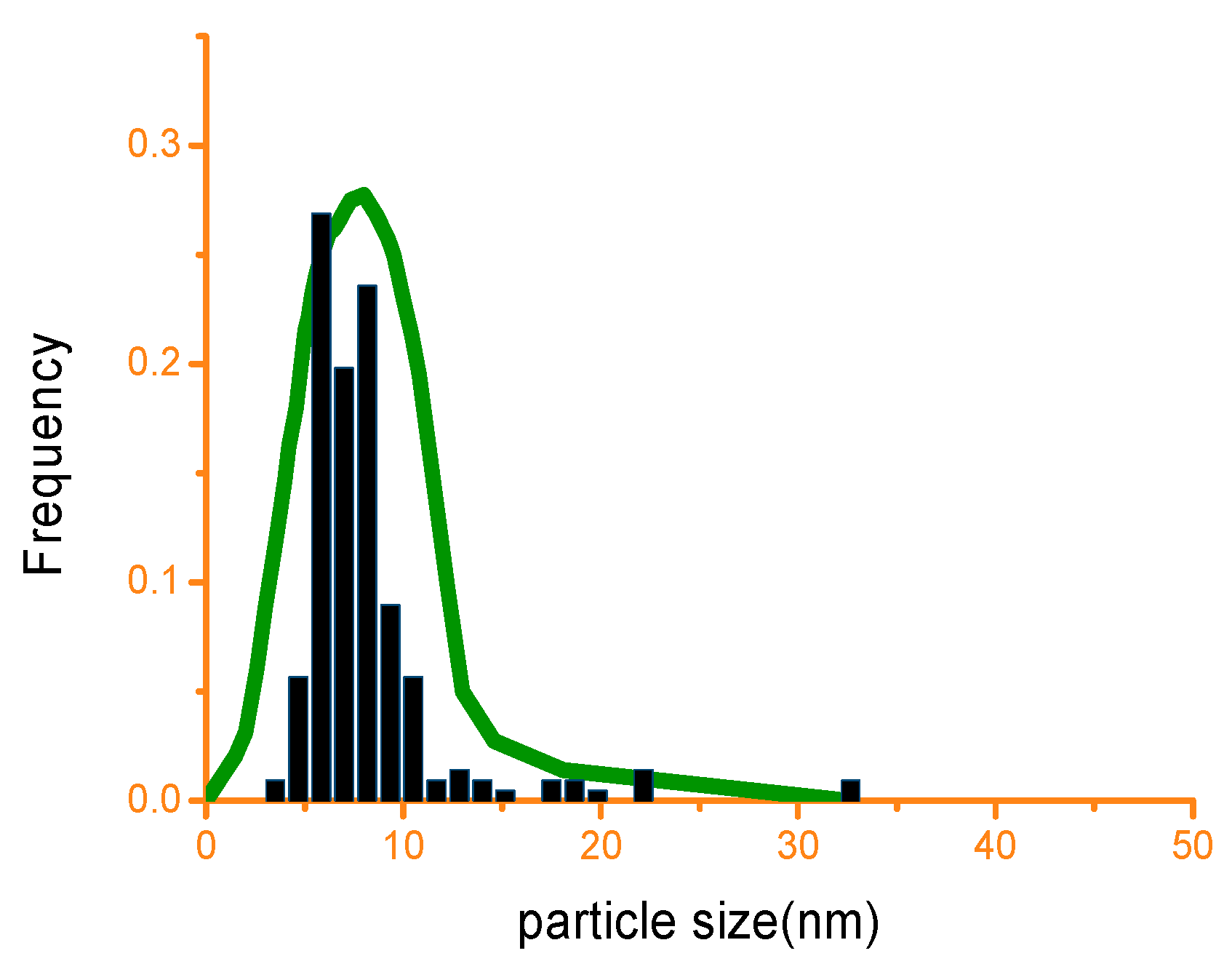
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.Y.; Wang, X.Z. Population Balance and CFD Simulation of Particle Aggregation and Growth in a Continuous Confined Jet Mixer for Hydrothermal Synthesis of Nanocrystals. Crystals 2021, 11, 144. https://doi.org/10.3390/cryst11020144
Li QY, Wang XZ. Population Balance and CFD Simulation of Particle Aggregation and Growth in a Continuous Confined Jet Mixer for Hydrothermal Synthesis of Nanocrystals. Crystals. 2021; 11(2):144. https://doi.org/10.3390/cryst11020144
Chicago/Turabian StyleLi, Qing Yun, and Xue Zhong Wang. 2021. "Population Balance and CFD Simulation of Particle Aggregation and Growth in a Continuous Confined Jet Mixer for Hydrothermal Synthesis of Nanocrystals" Crystals 11, no. 2: 144. https://doi.org/10.3390/cryst11020144



