X-Ray Photoelectron Spectroscopy Depth Profiling of As-Grown and Annealed Titanium Nitride Thin Films
Abstract
:1. Introduction
2. Experimental Details
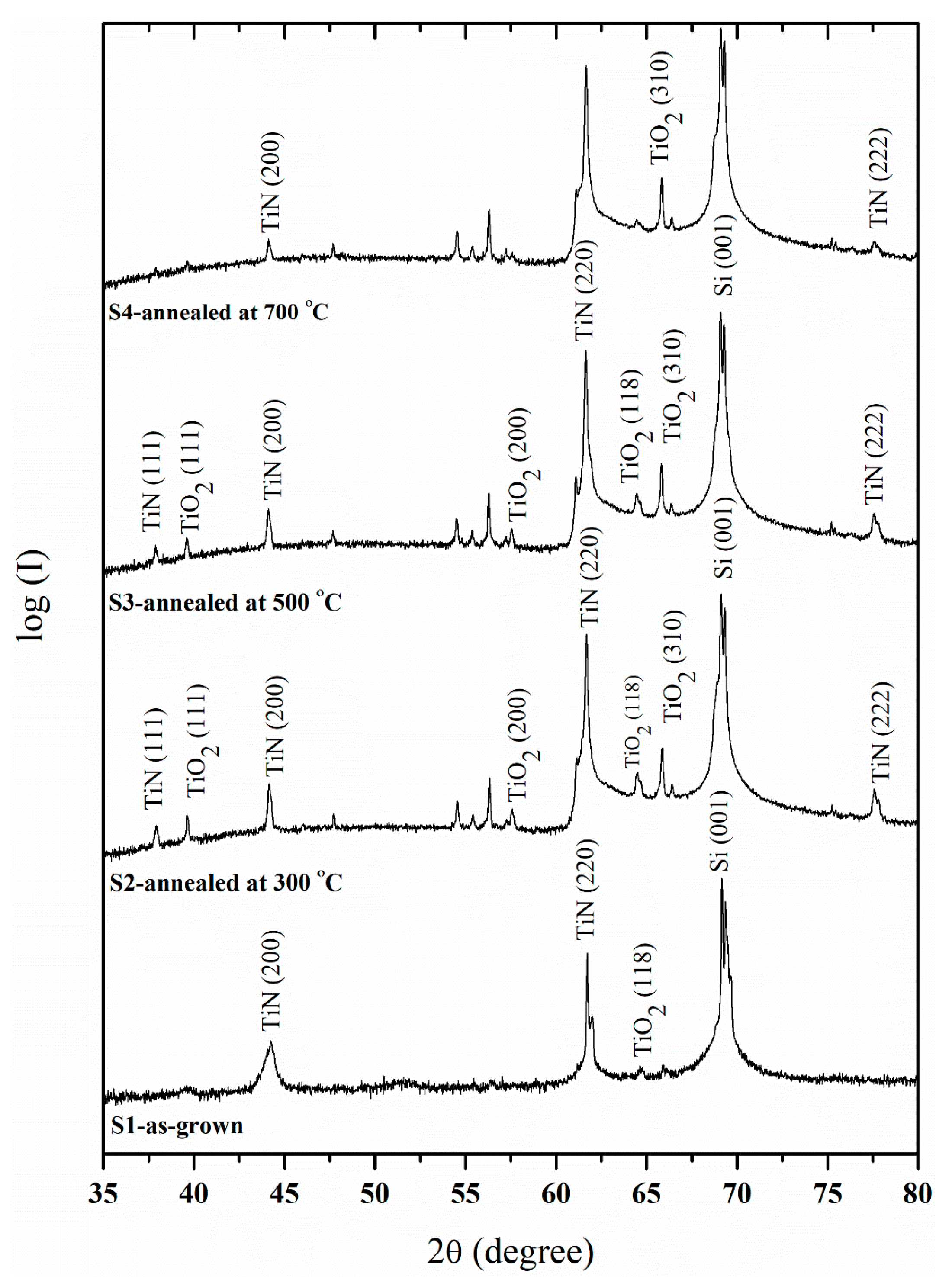
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lemme, M.C.; Efavi, J.; Mollenhauer, T.; Schmidt, M.; Gottlob, H.; Wahlbrink, T.; Kurz, H. Nanoscale TiN metal gate technology for CMOS integration. Microelectron. Eng. 2006, 83, 1551–1554. [Google Scholar] [CrossRef]
- Fillot, F.; Morel, T.; Minoret, S.; Matko, I.; Maîtrejean, S.; Guillaumot, B.; Chenevier, B.; Billon, T. Investigations of titanium nitride as metal gate material, elaborated by metal organic atomic layer deposition using TDMAT and NH3. Microelectron. Eng. 2005, 82, 248–253. [Google Scholar] [CrossRef]
- Achour, A.; Chaker, M.; Achour, H.; Arman, A.; Islam, M.; Mardani, M.; Boujtita, M.; Le Brizoual, L.; Djouadi, M.; Brousse, T. Role of nitrogen doping at the surface of titanium nitride thin films towards capacitive charge storage enhancement. J. Power Sources 2017, 359, 349–354. [Google Scholar] [CrossRef]
- Lukosius, M.; Walczyk, C.; Fraschke, M.; Wolansky, D.; Richter, H.; Wenger, C. High performance metal–insulator–metal capacitors with atomic vapor deposited HfO2 dielectrics. Thin Solid Films 2010, 518, 4380–4384. [Google Scholar] [CrossRef]
- Sundgren, J.-E. Structure and properties of TiN coatings. Thin Solid Films 1985, 128, 21–44. [Google Scholar] [CrossRef]
- Sedira, S.; Achour, S.; Avci, A.; Eskizeybek, V. Physical deposition of carbon doped titanium nitride film by DC magnetron sputtering for metallic implant coating use. Appl. Surf. Sci. 2014, 295, 81–85. [Google Scholar] [CrossRef]
- Sabitzer, C.; Steinkellner, C.; Koller, C.; Polcik, P.; Rachbauer, R.; Mayrhofer, P. Diffusion behavior of C, Cr, and Fe in arc evaporated TiN- and CrN-based coatings and their influence on thermal stability and hardness. Surf. Coat. Technol. 2015, 275, 185–192. [Google Scholar] [CrossRef]
- Chung, K.; Liu, G.; Duh, J.; Wang, J. Biocompatibility of a titanium–aluminum nitride film coating on a dental alloy. Surf. Coat. Technol. 2004, 188–189, 745–749. [Google Scholar] [CrossRef] [Green Version]
- Franks, W.; Schenker, I.; Schmutz, P.; Hierlemann, A. Impedance Characterization and Modeling of Electrodes for Biomedical Applications. IEEE Trans. Biomed. Eng. 2005, 52, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Jeyachandran, Y.; Narayandass, S.; Mangalaraj, D.; Areva, S.; Mielczarski, J. Properties of titanium nitride films prepared by direct current magnetron sputtering. Mater. Sci. Eng. A 2007, 445, 223–236. [Google Scholar] [CrossRef]
- Yu, S.; Frapper, G.; Zhang, L.; Zeng, Q.; Oganov, A.R. Phase stability, chemical bonding and mechanical properties of titanium nitrides: A first-principles study. Phys. Chem. Chem. Phys. 2015, 17, 11763–11769. [Google Scholar] [CrossRef] [Green Version]
- Zhao, G.; Zhang, T.; Zhang, T.; Wang, J.; Han, G. Electrical and optical properties of titanium nitride coatings prepared by atmospheric pressure chemical vapor deposition. J. Non-Cryst. Solids 2008, 354, 1272–1275. [Google Scholar] [CrossRef]
- Cheng, H.-E.; Wen, Y.-W. Correlation between process parameters, microstructure and hardness of titanium nitride films by chemical vapor deposition. Surf. Coat. Technol. 2004, 179, 103–109. [Google Scholar] [CrossRef]
- Guo, H.; Chen, W.; Shan, Y.; Wang, W.; Zhang, Z.; Jia, J. Microstructures and properties of titanium nitride films prepared by pulsed laser deposition at different substrate temperatures. Appl. Surf. Sci. 2015, 357, 473–478. [Google Scholar] [CrossRef]
- Xiang, W.; Liu, Y.; Zhang, J. Influence of Microstructure on the Electrical Properties of Heteroepitaxial TiN Films. Electron. Mater. Lett. 2018, 14, 314–318. [Google Scholar] [CrossRef]
- Mucha, N.R.; Som, J.; Shaji, S.; Fialkova, S.; Apte, P.R.; Balasubramanian, B.; Shield, J.E.; Anderson, M.; Kumar, D. Electrical and optical properties of titanium oxynitride thin films. J. Mater. Sci. 2020, 55, 5123–5134. [Google Scholar] [CrossRef]
- Solovan, M.N.; Brus, V.V.; Maistruk, E.V.; Maryanchuk, P.D. Electrical and optical properties of TiN thin films. Inorg. Mater. 2013, 50, 40–45. [Google Scholar] [CrossRef]
- Kavitha, A.; Kannan, R.; Reddy, P.S.; Rajashabala, S. The effect of annealing on the structural, optical and electrical properties of Titanium Nitride (TiN) thin films prepared by DC magnetron sputtering with supported discharge. J. Mater. Sci. Mater. Electron. 2016, 27, 10427–10434. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, T.P.; Li, X.D.; Liu, Y.C.; Liu, Y.; Yang, H.Y. Investigation of localized surface plasmon resonance of TiN nanoparticles in TiN_xO_y thin films. Opt. Mater. Express 2016, 6, 2422–2433. [Google Scholar] [CrossRef]
- Ponon, N.K.; Appleby, D.J.; Arac, E.; King, P.; Ganti, S.; Kwa, K.S.; O’Neill, A. Effect of deposition conditions and post deposition anneal on reactively sputtered titanium nitride thin films. Thin Solid Films 2015, 578, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Wang, X.; Liu, W.; Ji, X.; Wang, C. Ohmic contact formation mechanisms of TiN film on 4H–SiC. Ceram. Int. 2020, 46, 7142–7148. [Google Scholar] [CrossRef]
- Krockenberger, Y.; Karimoto, S.-I.; Yamamoto, H.; Semba, K. Coherent growth of superconducting TiN thin films by plasma enhanced molecular beam epitaxy. J. Appl. Phys. 2012, 112, 083920. [Google Scholar] [CrossRef]
- Maurya, K.C.; Shalaev, V.M.; Boltasseva, A.; Saha, B. Reduced optical losses in refractory plasmonic titanium nitride thin films deposited with molecular beam epitaxy. Opt. Mater. Express 2020, 10, 2679–2692. [Google Scholar] [CrossRef]
- Yokota, K.; Nakamura, K.; Kasuya, T.; Ohnishi, M. Phase composition and crystalline structure of titanium nitride deposited on silicon by an ion-beam assisted deposition technique. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2003, 206, 386–389. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Li, F.S.; Rong, H.; Tong, L.; Zhao, Y.F.; Yang, S.; Jian, Z.; An, D.S.; Yun, G. Remote plasma-enhanced atomic layer deposition of metallic TiN films with low work function and high uniformity. J. Vac. Sci. Technol. A 2018, 36, 041501. [Google Scholar] [CrossRef]
- Andrievski, R.A.; Dashevsky, Z.M.; Kalinnikov, G.V. Conductivity and the Hall coefficient of nanostructured titanium nitride films. Tech. Phys. Lett. 2004, 30, 930–932. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Open Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Haider, M.B. XPS Depth Profile Analysis of Zn3N2 Thin Films Grown at Different N2/Ar Gas Flow Rates by RF Magnetron Sputtering. Nanoscale Res. Lett. 2017, 12, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Pauw, L.J. A method of measuring specific resistivity and Hall effect of discs of arbitrary shape. Philips Res. Rep. 1958, 13, 1–9. [Google Scholar]
- Solís-Pomar, F.; Nápoles, O.; Robaina, O.V.; Gutierrez-Lazos, C.; Fundora, A.; Colin, A.; Pérez-Tijerina, E.; Melendrez, M. Preparation and characterization of nanostructured titanium nitride thin films at room temperature. Ceram. Int. 2016, 42, 7571–7575. [Google Scholar] [CrossRef]
- Ghobadi, N.; Ganji, M.; Luna, C.; Arman, A.; Ahmadpourian, A. Effects of substrate temperature on the properties of sputtered TiN thin films. J. Mater. Sci. Mater. Electron. 2015, 27, 2800–2808. [Google Scholar] [CrossRef]
- Subramanian, B.; Ananthakumar, R.; Jayachandran, M. Structural and tribological properties of DC reactive magnetron sputtered titanium/titanium nitride (Ti/TiN) multilayered coatings. Surf. Coat. Technol. 2011, 205, 3485–3492. [Google Scholar] [CrossRef]
- Liang, H.; Xu, J.; Zhou, D.; Sun, X.; Chu, S.; Bai, Y. Thickness dependent microstructural and electrical properties of TiN thin films prepared by DC reactive magnetron sputtering. Ceram. Int. 2016, 42, 2642–2647. [Google Scholar] [CrossRef]
- Dean, J.A. Lang’s Handbook of Chemistry; McGraw Hill: New York, NY, USA, 1999; Volume 42, p. 120. [Google Scholar]







| Sample | Annealing (°C) | RMS Surface Roughness (nm) | Average Grain Size (nm) | Band Gap (eV) | Carrier Concentration (cm−3) | Resistivity (Ω.cm) |
|---|---|---|---|---|---|---|
| S1 | As-grown | 0.505 | 11.20 | 3.67 | ||
| S2 | 300 | 0.506 | 11.22 | 3.56 | ||
| S3 | 500 | 0.821 | 9.245 | 3.53 | ||
| S4 | 700 | 1.648 | 9.418 | 3.52 |
| Sample | Etching Time (s) | Ti-O2 (eV) | Ti-O-N (eV) | Ti-N (eV) | Ti (eV) |
|---|---|---|---|---|---|
| S1 | 30 | 459.0 | 457.6 | 456.5 | |
| S2 | 459.0 | 458.2 | 456.8 | ||
| S3 | 458.6 | 457.5 | 456.3 | ||
| S4 | 459.2 | 458.5 | 457.0 | ||
| S1 | 90 | 458.6 | 457.0 | 455.6 | |
| S2 | 458.5 | 456.8 | 455.3 | ||
| S3 | 458.0 | 456.5 | 455.2 | ||
| S4 | 458.3 | 456.6 | 455.1 | ||
| S1 | 150 | 458.3 | 456.6 | 455.2 | |
| S2 | 457.6 | 456.0 | 454.8 | ||
| S3 | 457.3 | 455.9 | 454.6 | ||
| S4 | 457.9 | 456.1 | 454.7 | ||
| S1 | 210 | 458.1 | 456.3 | 454.5 | |
| S2 | 457.6 | 455.9 | 454.7 | ||
| S3 | 457.3 | 455.9 | 454.5 | ||
| S4 | 457.8 | 456.0 | 454.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maarouf, M.; Haider, M.B.; Drmosh, Q.A.; Mekki, M.B. X-Ray Photoelectron Spectroscopy Depth Profiling of As-Grown and Annealed Titanium Nitride Thin Films. Crystals 2021, 11, 239. https://doi.org/10.3390/cryst11030239
Maarouf M, Haider MB, Drmosh QA, Mekki MB. X-Ray Photoelectron Spectroscopy Depth Profiling of As-Grown and Annealed Titanium Nitride Thin Films. Crystals. 2021; 11(3):239. https://doi.org/10.3390/cryst11030239
Chicago/Turabian StyleMaarouf, Monzer, Muhammad Baseer Haider, Qasem Ahmed Drmosh, and Mogtaba B. Mekki. 2021. "X-Ray Photoelectron Spectroscopy Depth Profiling of As-Grown and Annealed Titanium Nitride Thin Films" Crystals 11, no. 3: 239. https://doi.org/10.3390/cryst11030239






