Mechanical Properties of Commercial Purity Aluminum Modified by Zirconium Micro-Additives
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
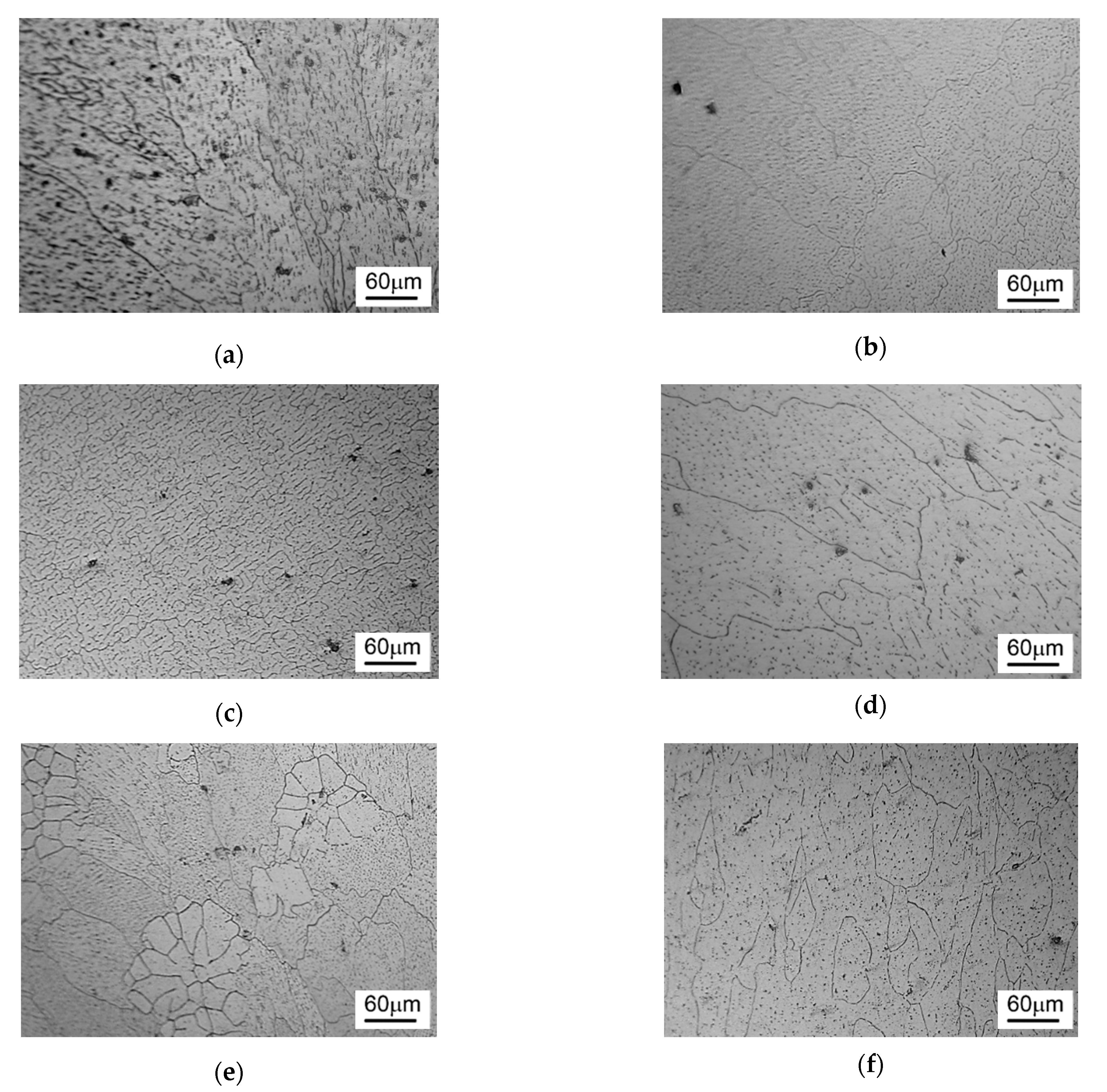
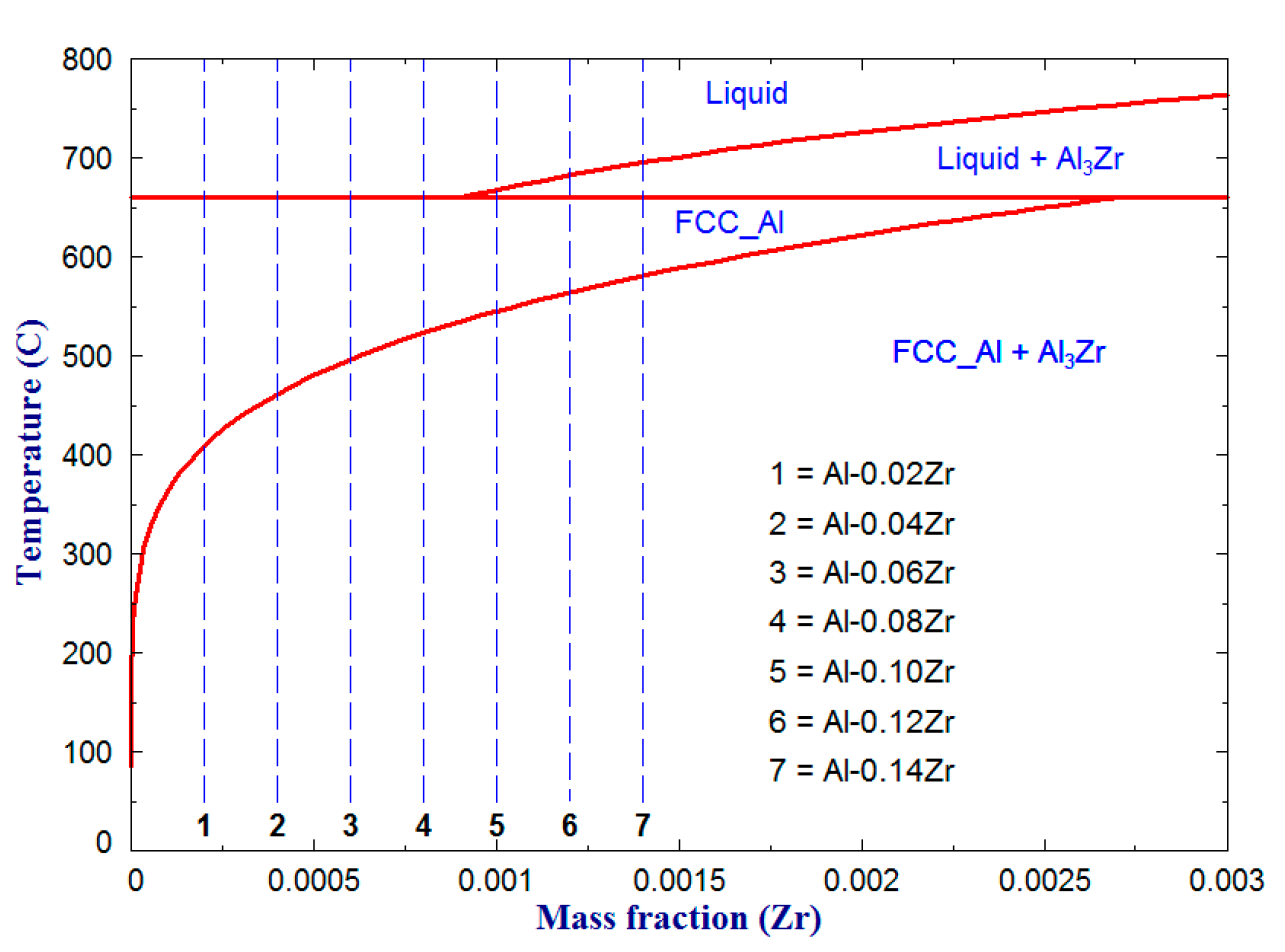
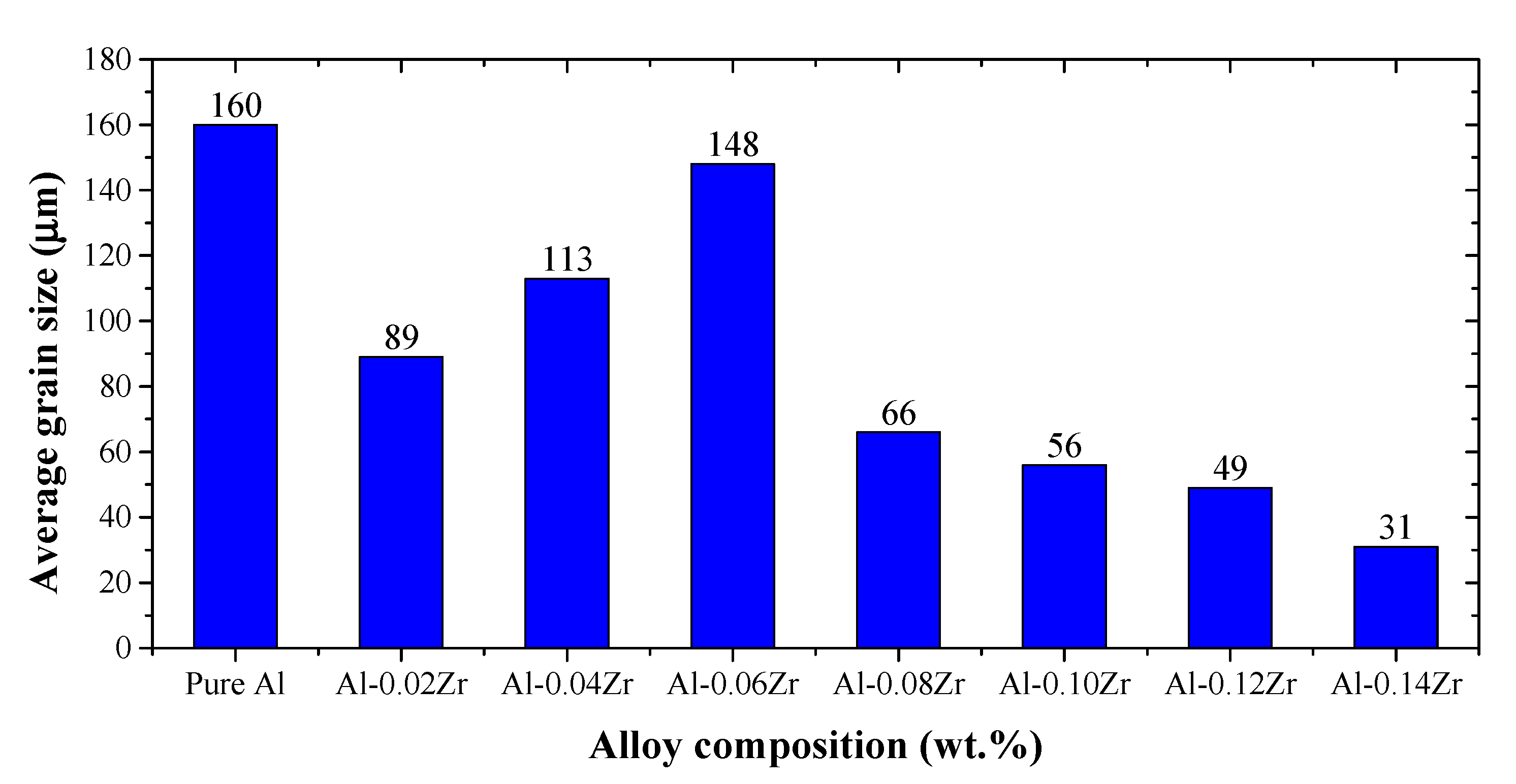
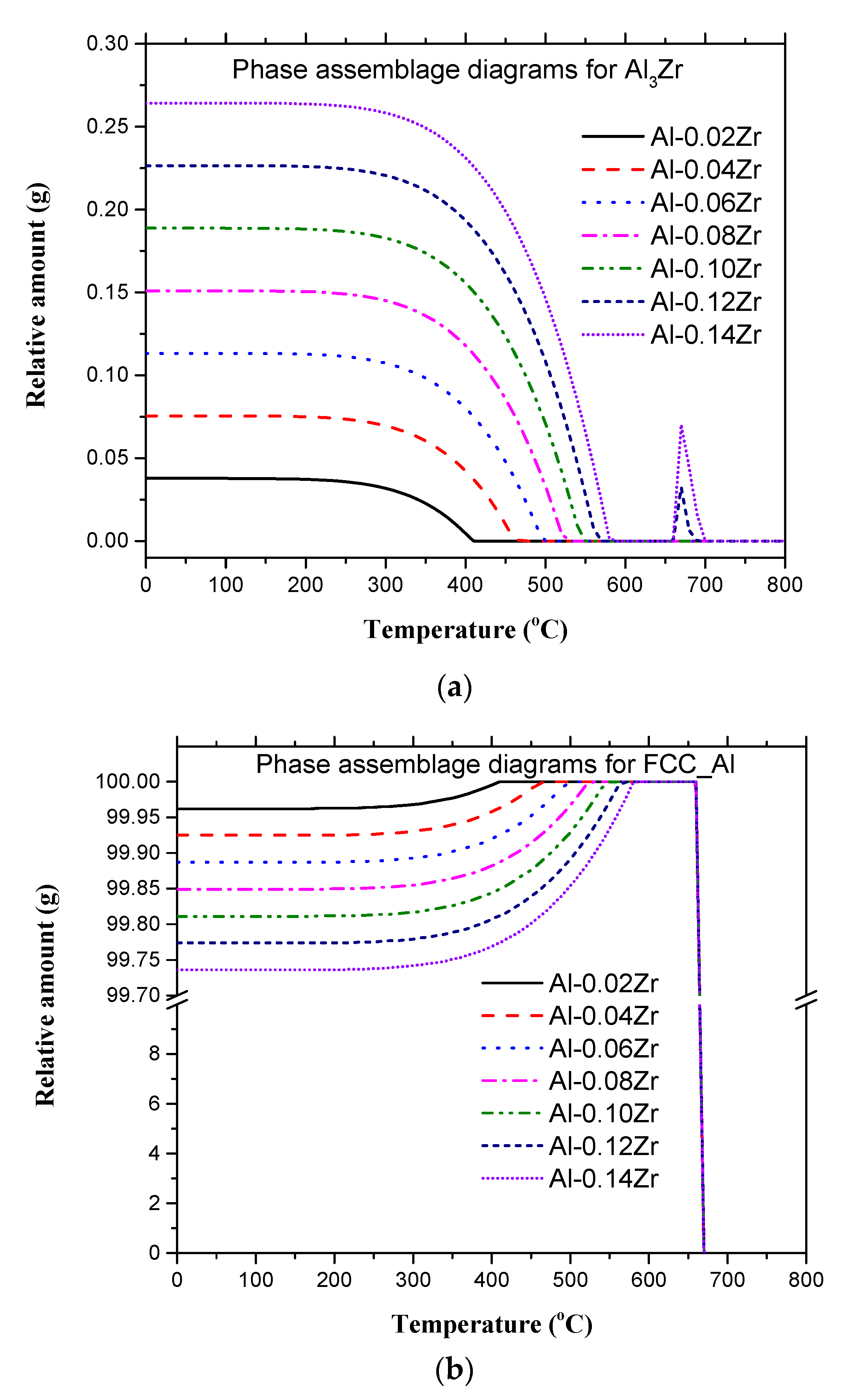
3.1. Microstructure and Grain Size Analyses
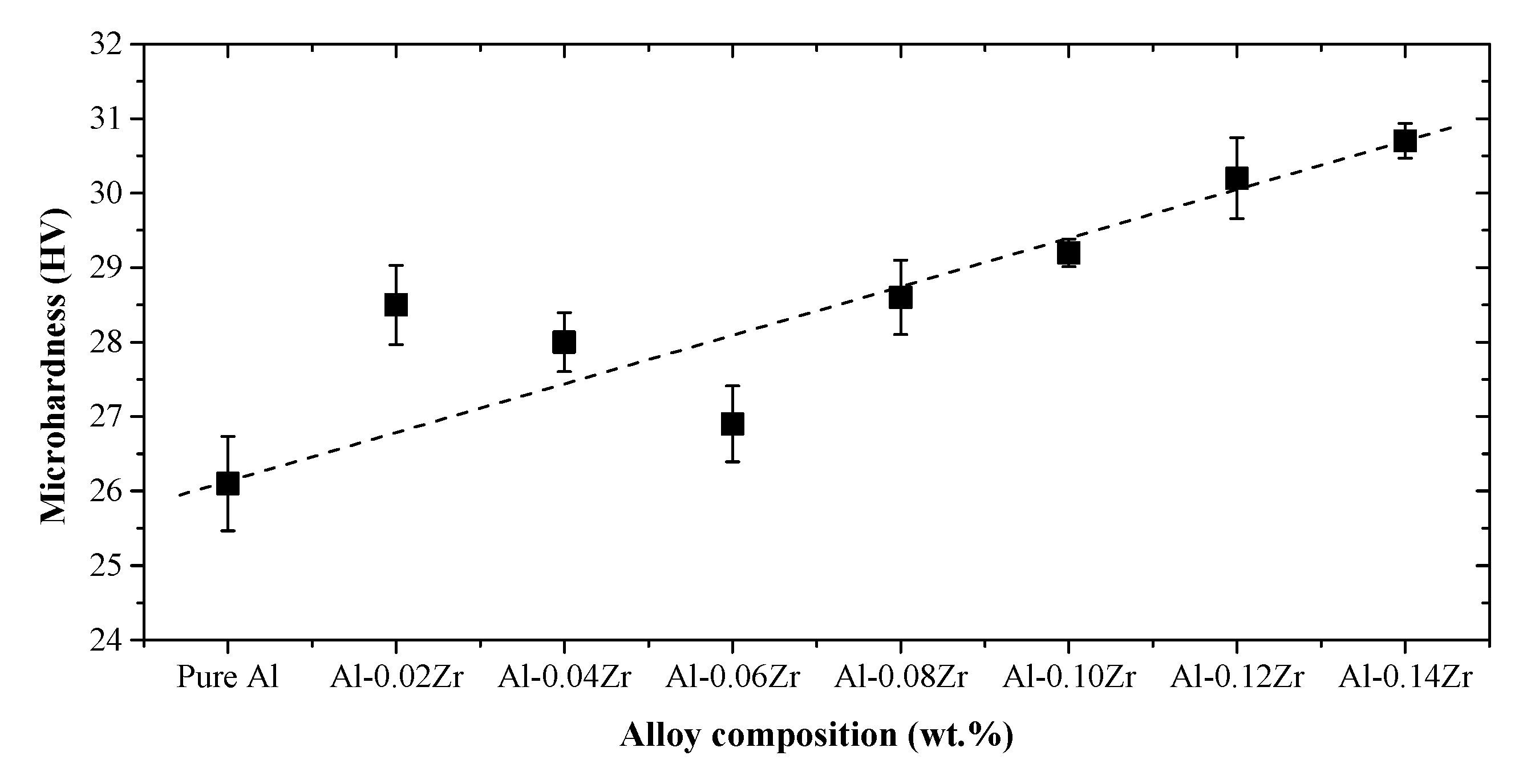
3.2. Microhardness Analysis
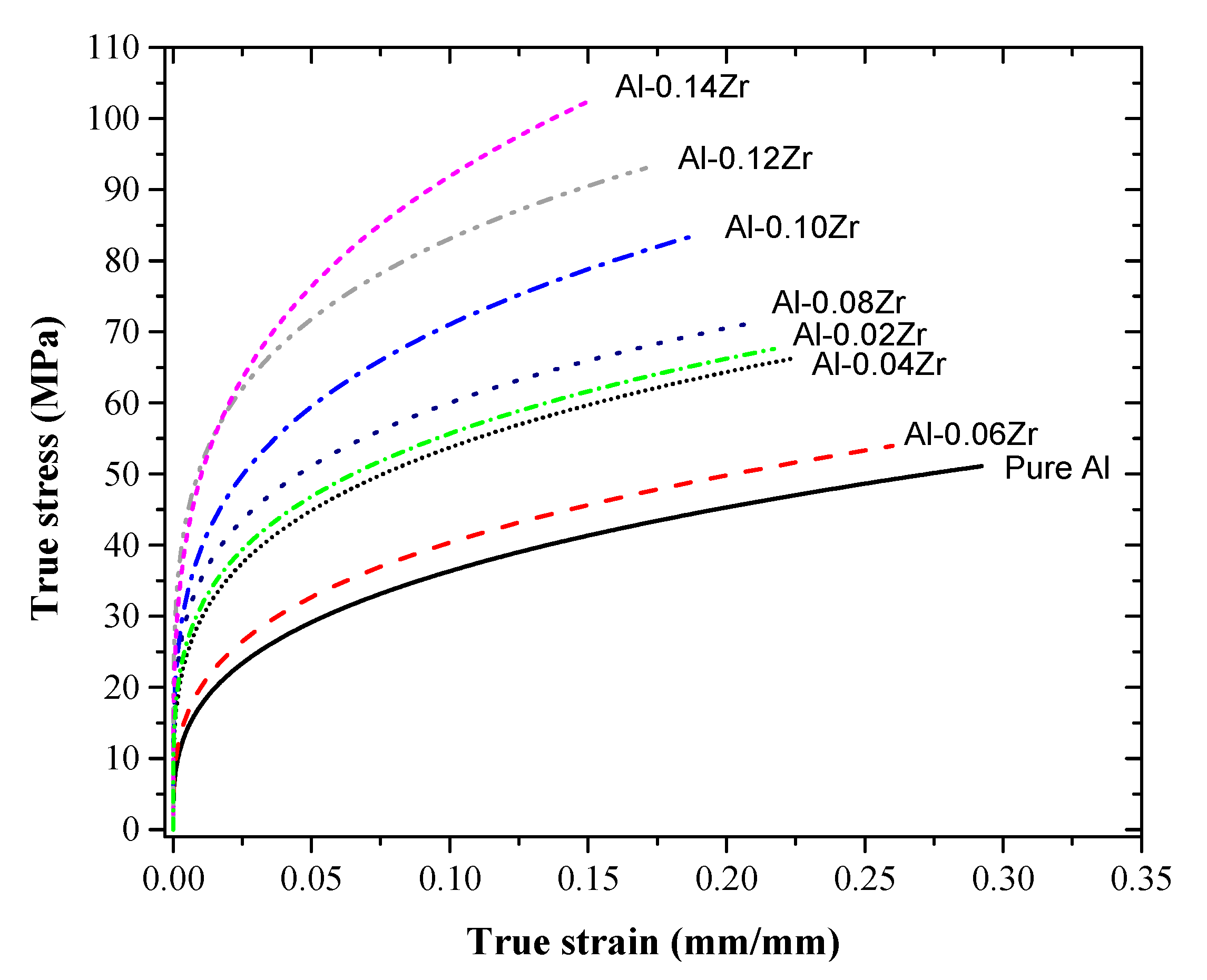
3.3. Tensile Characteristics
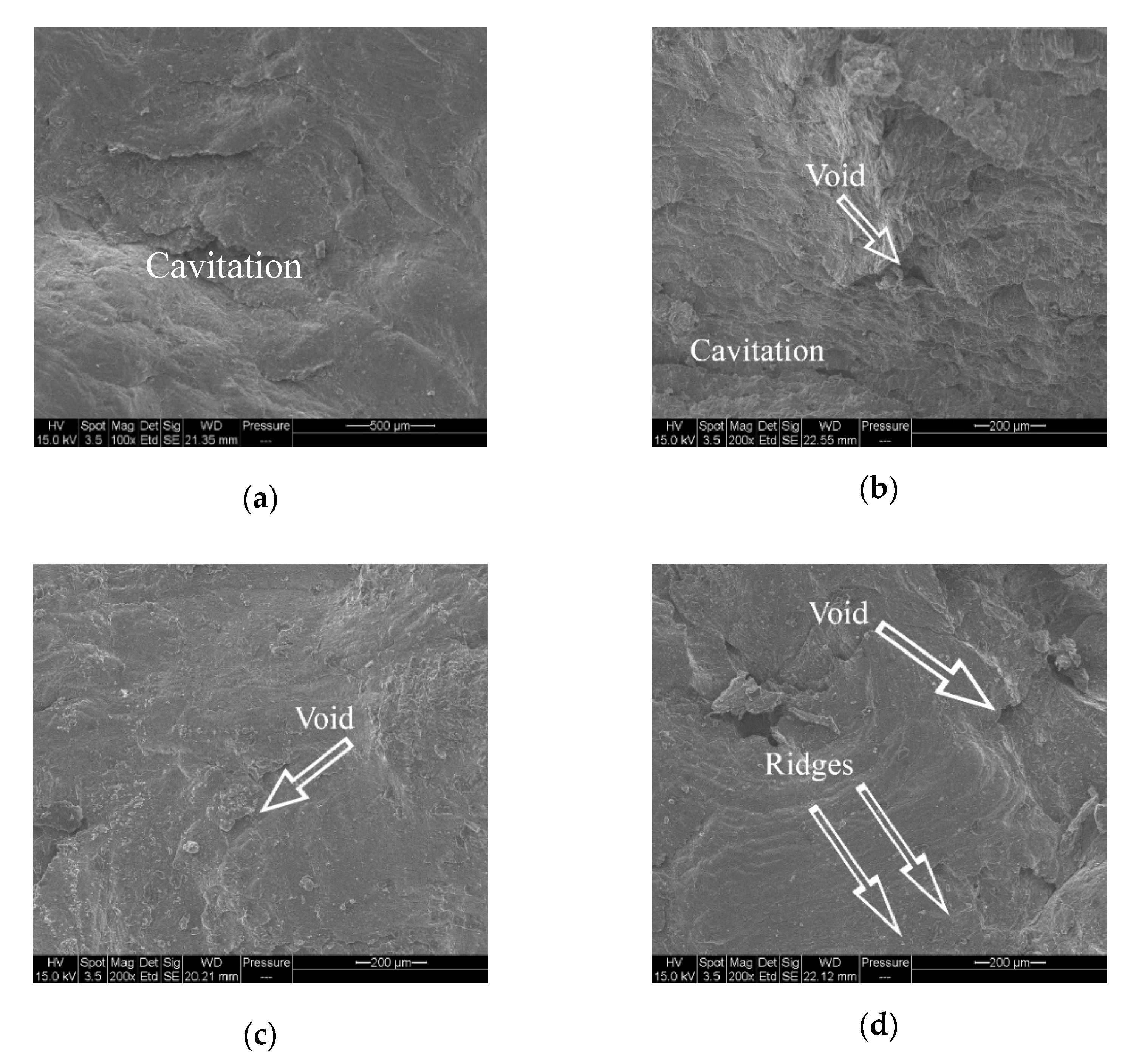
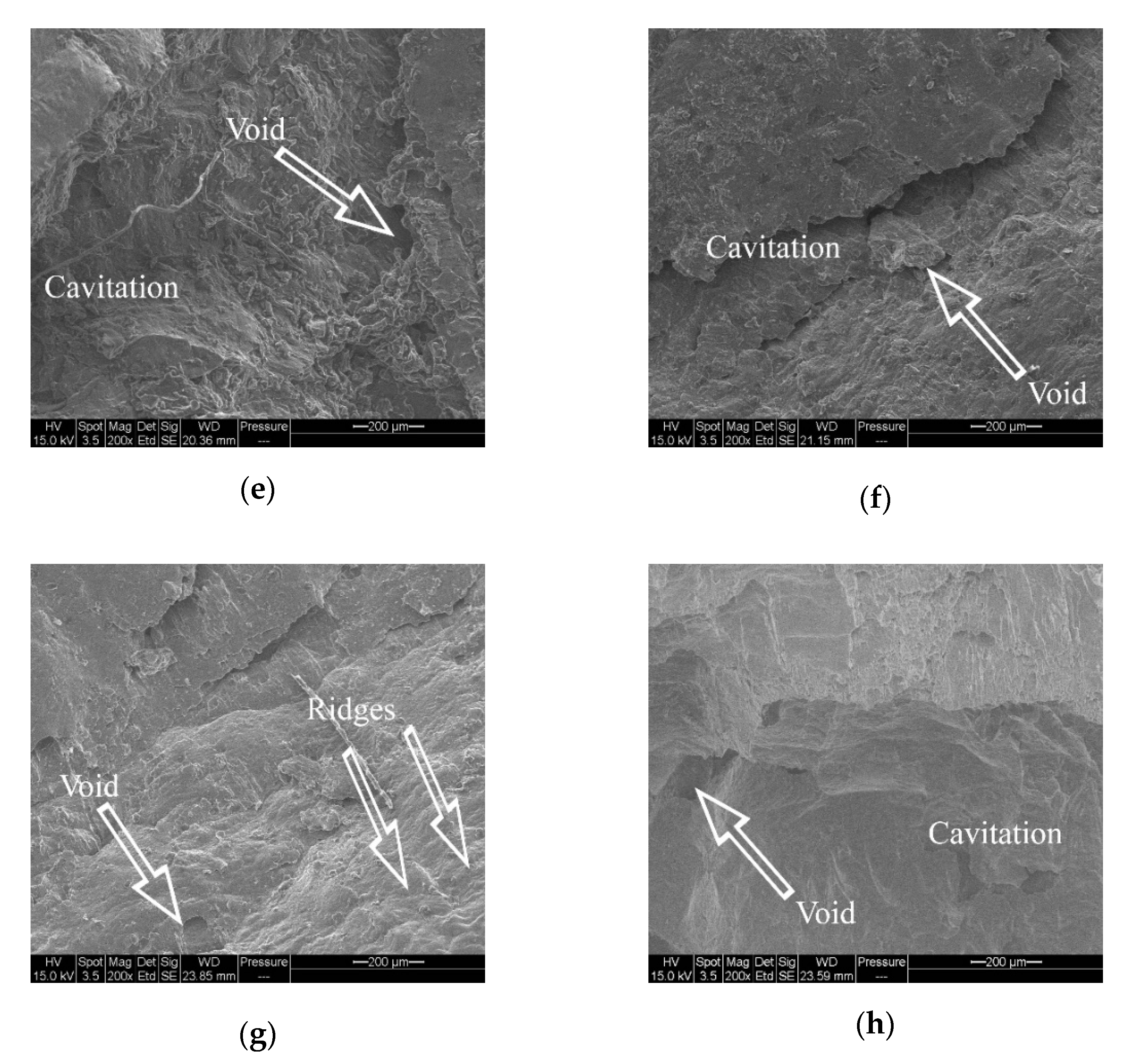
3.4. Fractography Analysis of Tensile Specimens
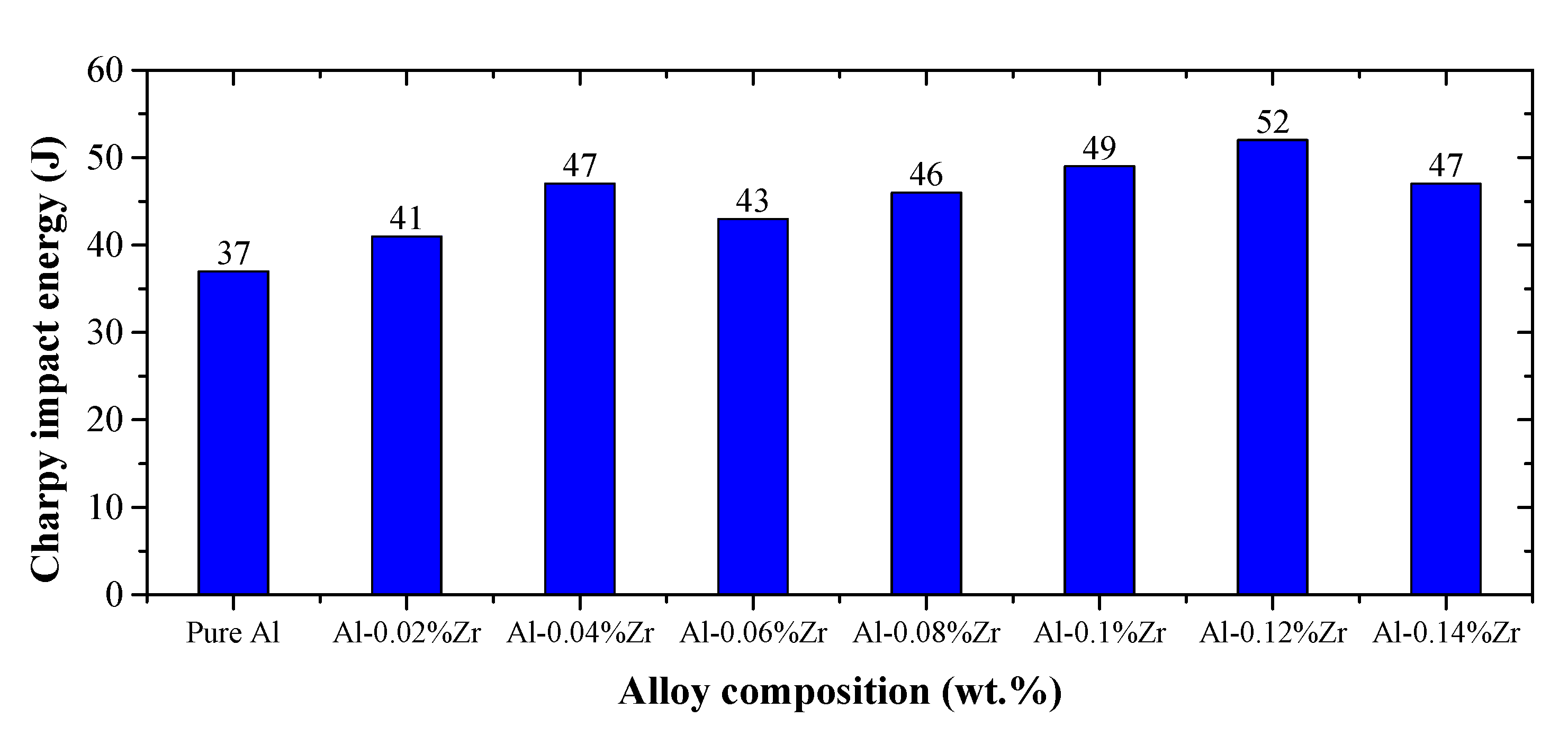
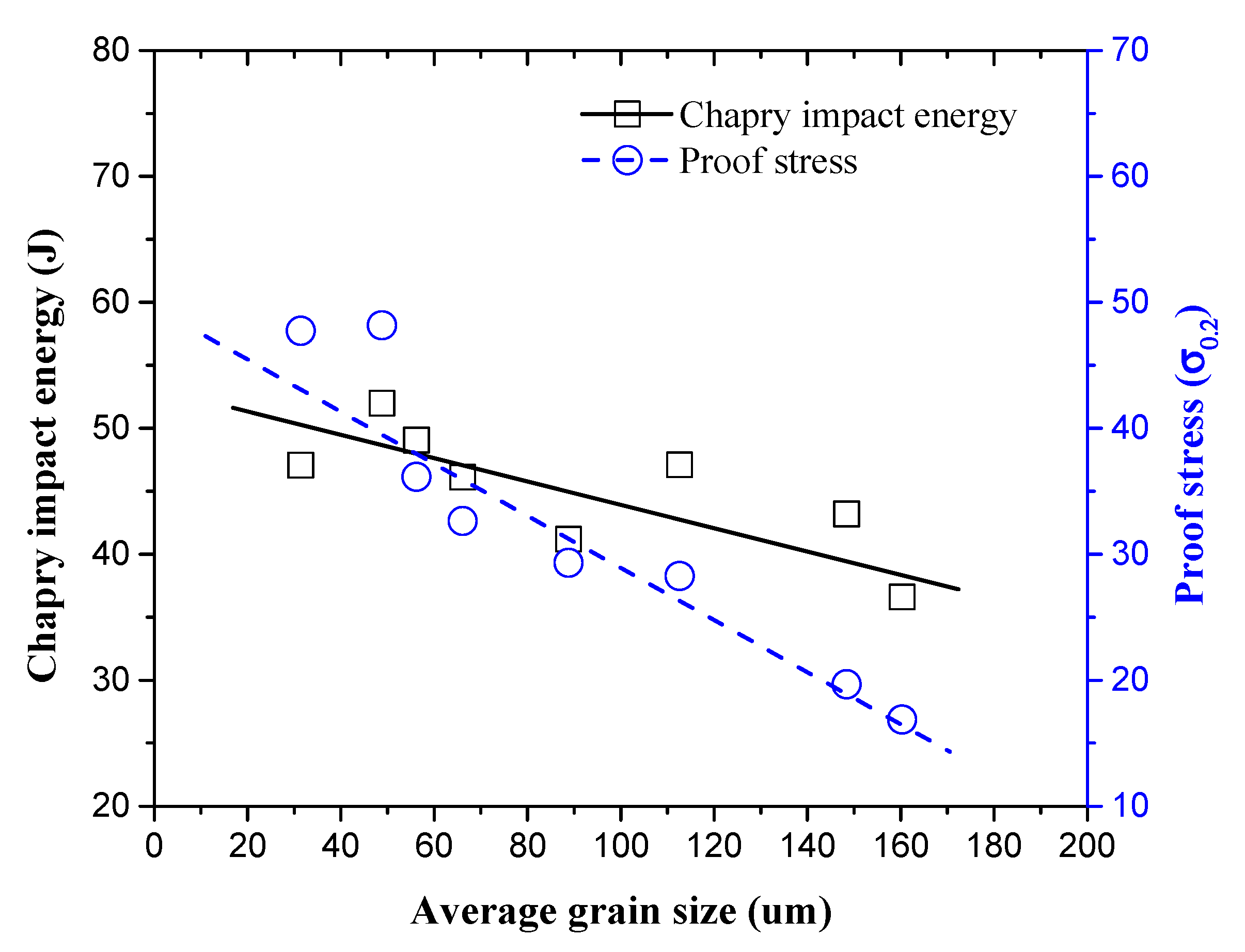
3.5. Impact Fracture Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Errandonea, D.; Burakovsky, L.; Preston, D.L.; MacLeod, S.G.; Santamaría-Perez, D.; Chen, S.; Cynn, H.; Simak, S.I.; McMahon, M.I.; Proctor, J.E.; et al. Experimental and theoretical confirmation of an orthorhombic phase transition in niobium at high pressure and temperature. Commun. Mater. 2020, 1, 60. [Google Scholar] [CrossRef]
- Straumal, B.; Korneva, A.; Kilmametov, A.; Lityńska-Dobrzyńska, L.; Gornakova, A.; Chulist, R.; Karpov, M.; Zięba, P. Structural and Mechanical Properties of Ti–Co Alloys Treated by High Pressure Torsion. Materials 2019, 12, 426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, D.; Joris, O.P.J.; Sankaran, A.; Weekes, H.E.; Bull, D.J.; Prior, T.J.; Dye, D.; Errandonea, D.; Proctor, J.E. On the high-pressure phase stability and elastic properties of β -titanium alloys. J. Phys. Condens. Matter 2017, 29, 155401. [Google Scholar] [CrossRef] [PubMed]
- Dorward, R.C.; Pritchett, T.R. Advanced aluminium alloys for aircraft and aerospace applications. Mater. Des. 1988, 9, 63–69. [Google Scholar] [CrossRef]
- Li, H.; Li, D.; Zhu, Z.; Chen, B.; Chen, X.; Yang, C.; Zhang, H.; Kang, W. Grain refinement mechanism of as-cast aluminum by hafnium. Trans. Nonferrous Met. Soc. China 2016, 26, 3059–3069. [Google Scholar] [CrossRef]
- Hall, E.O. The Deformation and Ageing of Mild Steel: III Discussion of Results. Proc. Phys. Soc. Sect. B 1951, 64, 747–753. [Google Scholar] [CrossRef]
- Petch, N.J. The cleavage strength of polycrystal. J. Iron Steel Inst. 1953, 174, 25–28. [Google Scholar]
- Zhang, Y.; Ma, N.; Yi, H.; Li, S.; Wang, H. Effect of Fe on grain refinement of commercial purity aluminum. Mater. Des. 2006, 27, 794–798. [Google Scholar] [CrossRef]
- Mohd Syukry, Z.; Ahmad Badri, I. Effect of Aging Time to the Commercial Aluminum Alloy Modified with Zirconium Addition. Mater. Sci. Forum 2015, 819, 50–56. [Google Scholar] [CrossRef]
- Zaid, A.I.O.; Mostafa, A.O. Effect of hafnium addition on wear resistance of zinc-aluminum 5 alloy: A three-dimensional presentation. Adv. Mater. Lett. 2017, 8, 910–915. [Google Scholar] [CrossRef]
- Mostafa, A.O. Mechanical Properties and Wear Behavior of Aluminum Grain Refined by Ti and Ti+B. Int. J. Surf. Eng. Interdiscip. Mater. Sci. 2019, 7, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Li, H.; Lai, Y.; Ou, Y.; Li, D. Effects of minor Zr and Er on microstructure and mechanical properties of pure aluminum. Mater. Sci. Eng. A 2013, 580, 92–98. [Google Scholar] [CrossRef]
- Toschi, S.; Balducci, E.; Ceschini, L.; Mørtsell, E.; Morri, A.; Di Sabatino, M. Effect of Zr Addition on Overaging and Tensile Behavior of 2618 Aluminum Alloy. Metals 2019, 9, 130. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Qiu, D.; Liu, Z.-L.; Taylor, J.A.; Easton, M.A.; Zhang, M.-X. The grain refinement mechanism of cast aluminium by zirconium. Acta Mater. 2013, 61, 5636–5645. [Google Scholar] [CrossRef]
- Crossley, F.A.; Mondolfo, L.F. Mechanism of Grain Refinement in Aluminum Alloys. JOM 1951, 3, 1143–1148. [Google Scholar] [CrossRef]
- Murray, J.; Peruzzi, A.; Abriata, J.P. The Al-Zr (aluminum-zirconium) system. J. Phase Equilib. 1992, 13, 277–291. [Google Scholar] [CrossRef]
- Wang, T.; Jin, Z.; Zhao, J.-C. Thermodynamic Assessment of the Al-Zr Binary System. J. Phase Equilib. 2001, 22, 544–551. [Google Scholar] [CrossRef]
- Wang, F.; Eskin, D.G.; Khvan, A.V.; Starodub, K.F.; Lim, J.J.H.; Burke, M.G.; Connolley, T.; Mi, J. On the occurrence of a eutectic-type structure in solidification of Al-Zr alloys. Scr. Mater. 2017, 133, 75–78. [Google Scholar] [CrossRef]
- Lü, X.Y.; Guo, E.J.; Rometsch, P.; Wang, L.J. Effect of one-step and two-step homogenization treatments on distribution of Al3Zr dispersoids in commercial AA7150 aluminium alloy. Trans. Nonferrous Met. Soc. China 2012, 22, 2645–2651. [Google Scholar] [CrossRef]
- Duan, Y.L.; Xu, G.F.; Peng, X.Y.; Deng, Y.; Li, Z.; Yin, Z.M. Effect of Sc and Zr additions on grain stability and superplasticity of the simple thermal-mechanical processed Al-Zn-Mg alloy sheet. Mater. Sci. Eng. A 2015, 648, 80–91. [Google Scholar] [CrossRef]
- Seyed Ebrahimi, S.H.; Emamy, M.; Pourkia, N.; Lashgari, H.R. The microstructure, hardness and tensile properties of a new super high strength aluminum alloy with Zr addition. Mater. Des. 2010, 31, 4450–4456. [Google Scholar] [CrossRef]
- Fang, H.C.; Chen, K.H.; Chen, X.; Huang, L.P.; Peng, G.S.; Huang, B.Y. Effect of Zr, Cr and Pr additions on microstructures and properties of ultra-high strength Al–Zn–Mg–Cu alloys. Mater. Sci. Eng. A 2011, 528, 7606–7615. [Google Scholar] [CrossRef]
- ASTM International. B557-15. Standard Test Methods for Tension Testing Wrought and Cast Aluminum- and Magnesium-Alloy Products. In Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- ASTM International. E23-18. Standard Test Methods for Notched Bar Impact Testing of Metallic Materials. In ASTM Book of Standards; ASTM International: West Conshohocken, PA, USA, 2018; ISBN 435493654. [Google Scholar]
- ASTM International ASTM E112-13. Standard Test Methods for Determining Average Grain Size. In ASTM Book of Standards; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- Senčič, B.; Šolić, S.; Leskovšek, V. Fracture toughness–Charpy impact test–Rockwell hardness regression based model for 51CrV4 spring steel. Mater. Sci. Technol. 2014, 30, 1500–1505. [Google Scholar] [CrossRef]
- Mahmudi, R.; Sepehrband, P.; Ghasemi, H.M. Improved properties of A319 aluminum casting alloy modified with Zr. Mater. Lett. 2006, 60, 2606–2610. [Google Scholar] [CrossRef]
- Ryum, N. Precipitation and recrystallization in an A1-0.5 wt.% Zr-alloy. Acta Metall. 1969, 17, 269–278. [Google Scholar] [CrossRef]
- Mahmoud, A.E.; Mahfouz, M.G.; Elrab, H.G.G.-; Doheim, M.A. Grain refinement of commercial pure aluminium by zirconium. J. Eng. Sci. 2014, 42, 1232–1241. [Google Scholar]
- Rohrer, G.S. The role of grain boundary energy in grain boundary complexion transitions. Curr. Opin. Solid State Mater. Sci. 2016, 20, 231–239. [Google Scholar] [CrossRef]
- Kaibyshev, O.A.; Faizova, S.N.; Hairullina, A.F. Diffusional mass transfer and superplastic deformation. Acta Mater. 2000, 48, 2093–2100. [Google Scholar] [CrossRef]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Gheribi, A.E.; Hack, K.; Jung, I.-H.; Kang, Y.-B.; Melançon, J.; et al. FactSage thermochemical software and databases, 2010–2016. Calphad 2016, 54, 35–53. [Google Scholar] [CrossRef] [Green Version]
- Souza, P.H.L.; de Oliveira, C.A.S.; do Vale Quaresma, J.M. Precipitation hardening in dilute Al–Zr alloys. J. Mater. Res. Technol. 2018, 7, 66–72. [Google Scholar] [CrossRef]
- Knipling, K.E.; Dunand, D.C.; Seidman, D.N. Nucleation and Precipitation Strengthening in Dilute Al-Ti and Al-Zr Alloys. Metall. Mater. Trans. A 2007, 38, 2552–2563. [Google Scholar] [CrossRef]
- Silva, R.; Pinto, A.; Kuznetsov, A.; Bott, I. Precipitation and Grain Size Effects on the Tensile Strain-Hardening Exponents of an API X80 Steel Pipe after High-Frequency Hot-Induction Bending. Metals 2018, 8, 168. [Google Scholar] [CrossRef] [Green Version]
- Pineau, A.; Benzerga, A.A.; Pardoen, T. Failure of metals I: Brittle and ductile fracture. Acta Mater. 2016, 107, 424–483. [Google Scholar] [CrossRef] [Green Version]
- Miyahara, N.; Mutoh, Y.; Yamaishi, K.; Uematsu, K.; Inoue, M. The Effects of Grain Size on Strength, Fracture Toughness, and Static Fatigue Crack Growth in Alumina. In Grain Boundary Controlled Properties of Fine Ceramics; Springer: Dordrecht, The Netherlands, 1992; pp. 125–136. [Google Scholar]
- Tarpani, J.R.; Spinelli, D. Grain size effects in the charpy impact energy of a thermally embrittled RPV steel. J. Mater. Sci. 2003, 38, 1493–1498. [Google Scholar] [CrossRef]
- Nakai, M.; Itoh, G. The Effect of Microstructure on Mechanical Properties of Forged 6061 Aluminum Alloy. Mater. Trans. 2014, 55, 114–119. [Google Scholar] [CrossRef] [Green Version]


| Fe | Si | Mg | Ti | B | V | Zn | Others 1 | Al | |
|---|---|---|---|---|---|---|---|---|---|
| Commercial purity Al | 0.11 | 0.05 | 0.004 | 0.004 | 0.0005 | 0.008 | 0.005 | 0.015 | Bal. |
| Microalloy | FCC_Al | Al3Zr |
|---|---|---|
| Al-0.02Zr | 99.962 | 0.037 (7) |
| Al-0.04Zr | 99.925 | 0.075 (4) |
| Al-0.06Zr | 99.887 | 0.113 (2) |
| Al-0.08Zr | 99.849 | 0.150 (9) |
| Al-0.10Zr | 99.811 | 0.188 (7) |
| Al-0.12Zr | 99.774 | 0.226 (4) |
| Al-0.14Zr | 99.736 | 0.264 (2) |
| Material | Proof Stress (σ0.2) MPa | Strain Hardening Exponent (n) | Strength Coefficient (k) MPa |
|---|---|---|---|
| Pure Al | 16.87 ± 9.6% | 0.318 | 75.60 |
| Al-0.02Zr | 29.32 ± 5.1% | 0.251 | 99.04 |
| Al-0.04Zr | 28.27 ± 7.0% | 0.260 | 97.79 |
| Al-0.06Zr | 19.67 ± 6.1% | 0.304 | 81.26 |
| Al-0.08Zr | 32.63 ± 8.4% | 0.232 | 102.42 |
| Al-0.10Zr | 36.14 ± 7.3% | 0.256 | 128.08 |
| Al-0.12Zr | 48.18 ± 4.6% | 0.211 | 135.08 |
| Al-0.14Zr | 47.74 ± 3.3% | 0.267 | 169.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mostafa, A.; Adaileh, W.; Awad, A.; Kilani, A. Mechanical Properties of Commercial Purity Aluminum Modified by Zirconium Micro-Additives. Crystals 2021, 11, 270. https://doi.org/10.3390/cryst11030270
Mostafa A, Adaileh W, Awad A, Kilani A. Mechanical Properties of Commercial Purity Aluminum Modified by Zirconium Micro-Additives. Crystals. 2021; 11(3):270. https://doi.org/10.3390/cryst11030270
Chicago/Turabian StyleMostafa, Ahmad, Wail Adaileh, Alaa Awad, and Adnan Kilani. 2021. "Mechanical Properties of Commercial Purity Aluminum Modified by Zirconium Micro-Additives" Crystals 11, no. 3: 270. https://doi.org/10.3390/cryst11030270
APA StyleMostafa, A., Adaileh, W., Awad, A., & Kilani, A. (2021). Mechanical Properties of Commercial Purity Aluminum Modified by Zirconium Micro-Additives. Crystals, 11(3), 270. https://doi.org/10.3390/cryst11030270







