Simple and Acid-Free Hydrothermal Synthesis of Bioactive Glass 58SiO2-33CaO-9P2O5 (wt%)
Abstract
1. Introduction
2. Materials and Methods
2.1. Acid-Free Hydrothermal Synthesis
2.2. In Vitro Experiment in SBF Fluid
2.3. In Vitro Assay Within the Cellular Medium
2.4. Characterization
3. Results and Discussion
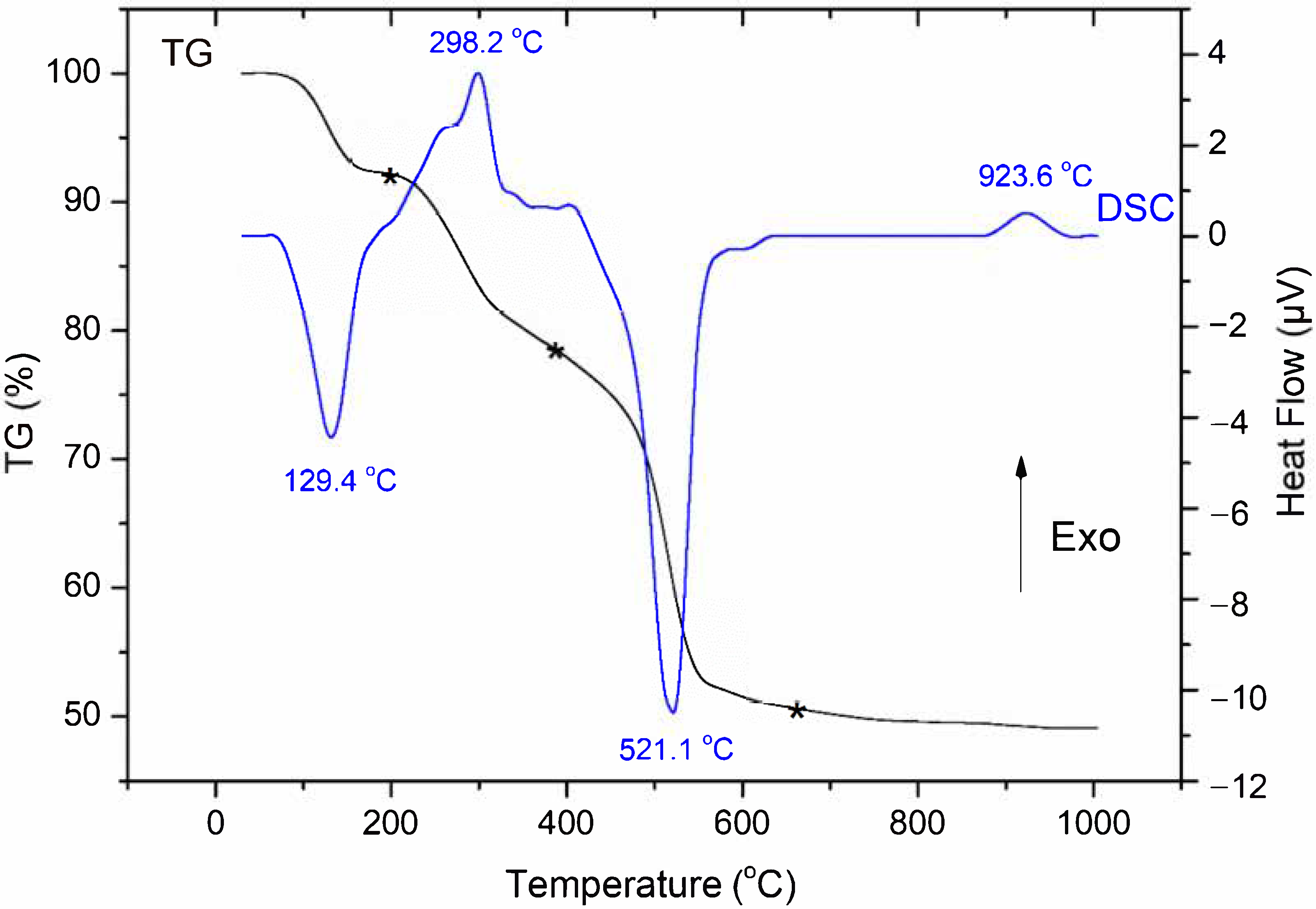
3.1. Thermal Behavior
3.2. Textural Analysis
3.3. Bioactivity Evaluation
3.3.1. XRD Analysis
3.3.2. FTIR Analysis
3.3.3. SEM–EDX Analysis
3.3.4. ICP-OES Analysis
3.4. Biocompatibility Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rahaman, M.N.; Day, D.E.; Bal, B.S.; Fu, Q.; Jung, S.B.; Bonewald, L.F.; Tomsia, A.P. Bioactive glass in tissue engineering. Acta Biomater. 2011, 7, 2355–2373. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. The story of Bioglass®. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Julian, R.J. Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar]
- Baino, F.; Novajra, G.; Pacheco, M.P.; Boccaccini, A.R.; Brovarone, C.V. Bioactive glasses: Special applications outside the skeletal system. J. Non. Cryst. Solids. 2016, 432, 15–30. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Erol, M.; Stark, W. Polymer/bioactive glass nanocomposites for biomedical applications: A review. Compos. Sci. Technol. 2010, 70, 1764–1776. [Google Scholar] [CrossRef]
- Zheng, K.; Boccaccini, A.R. Sol-gel processing of bioactive glass nanoparticles: A review. Adv. Colloid Interface Sci. 2017, 249, 363–373. [Google Scholar] [CrossRef]
- Sharifianjazi, F.; Parvin, N.; Tahriri, M.J. Synthesis and characteristics of sol-gel bioactive SiO2-P2O5-CaO-Ag2O glasses. Non Cryst. Solids. 2017, 476, 108–113. [Google Scholar] [CrossRef]
- Vulpoi, A.; Gruian, C.; Vanea, E.; Baia, L.; Simon, S.; Steinhoff, H.J.; Goller, G.; Simon, V. Silver effect on the structure of SiO2-CaO-P2O5 ternary system. Mater. Sci. Eng. C 2012, 32, 178–183. [Google Scholar] [CrossRef]
- Balamurugan, A.; Balossier, G.; Kannan, S.; Michel, J.; Rebelo, A.H.S.; Ferreira, J.M.F. Development and in vitro characterization of sol–gel derived CaO–P2O5–SiO2–ZnO bioglass. Acta Biomater. 2007, 3, 255–262. [Google Scholar] [CrossRef]
- Salman, S.; Salama, S.; Abo-Mosallam, H. The role of strontium and potassium on crystallization and bioactivity of Na2O–CaO–P2O5–SiO2 glasses. Ceram. Int. 2012, 38, 55–63. [Google Scholar] [CrossRef]
- El-Kheshen, A.A.; Khaliafaa, F.A.; Saad, E.A.; Elwana, R.L. Effect of Al2O3 addition on bioactivity, thermal and mechanical properties of some bioactive glasses. Ceram. Int. 2008, 34, 1667–1673. [Google Scholar] [CrossRef]
- Baino, F.; Fiume, E.; Miola, M.; Leone, F.; Onida, B.; Verné, E. Fe-doped bioactive glass-derived scaffolds produced by sol-gel foaming. Mater. Lett. 2019, 235, 207–211. [Google Scholar] [CrossRef]
- Bari, A.; Bloise, N.; Fiorilli, S.; Novajra, G.; Vallet-Regí, M.; Bruni, G.; Torres-Pardo, A.; González-Calbet, J.M.; Visai, L.; Vitale-Brovarone, C. Copper-containing mesoporous bioactive glass nanoparticles as multifunctional agent for bone regeneration. Acta Biomater. 2017, 55, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Bini, M.; Grandi, S.; Capsoni, D.; Mustarelli, P.; Saino, E.; Visai, L.J. SiO2− P2O5− CaO glasses and glass-ceramics with and without ZnO: Relationships among composition, microstructure, and bioactivity. Phys. Chem. C 2009, 113, 8821–8828. [Google Scholar] [CrossRef]
- Bejarano, J.; Caviedes, P.; Palzal, H. Sol–gel synthesis and in vitro bioactivity of copper and zinc-doped silicate bioactive glasses and glass-ceramics. Biomed. Mater. 2015, 10, 025001. [Google Scholar] [CrossRef]
- Bui, X.V.; Dang, T.H. Bioactive glass 58S prepared using an innovation sol-gel process. Process. App. Ceram. 2019, 13, 98–103. [Google Scholar] [CrossRef]
- Sepulveda, P.; Jones, J.R.; Hench, L.L. Characterization of melt-derived 45S5 and sol-gel–derived 58S bioactive glasses. J. Biomed. Mater. Res. 2001, 58, 734–740. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Arends, I.W.C.E.; Hanefeld, U. Green Chemistry and Catalysis; Wiley VCH: Weinheim, Germany, 2007. [Google Scholar]
- Clark, J.H.; Macquarrie, D.J. Green Chemistry and Technology; Abingdon: Nashville, TN, USA, 2008. [Google Scholar]
- Ben-Arfa, B.A.E.; Fernandes, H.R.; Salvado, I.M.M.; Ferreira, J.M.F.; Pullar, R.C. Effects of catalysts on polymerization and microstructure of sol-gel derived bioglasses. J. Am. Ceram. Soc. 2018, 101, 2831–2839. [Google Scholar] [CrossRef]
- Hoa, B.T.; Hoa, H.T.T.; Tien, N.A.; Khang, N.H.D.; Guseva, E.V.; Tuan, T.A.; Vuong, B.X. Green synthesis of bioactive glass 70SiO2-30CaO by hydrothermal method. Mater. Lett. 2020, 274, 128032. [Google Scholar] [CrossRef]
- Tuan, T.A.; Guseva, E.V.; Phuc, L.H.; Hien, N.Q.; Long, N.V.; Vuong, B.X. Acid-free Hydrothermal Process for Synthesis of Bioactive Glasses 70SiO2–(30-x)CaO–xZnO (x = 1, 3, 5 mol.%). Proceed. 2020, 62, 1–12. [Google Scholar]
- Fernandes, H.R.; Gaddam, A.; Rebelo, A.; Brazete, D.; Stan, G.E.; Ferreira, J.M.F. Bioactive glasses and glass-ceramics for healthcare applications in bone regeneration and tissue engineering. Materials 2018, 11, 2530. [Google Scholar] [CrossRef]
- Wallace, K.E.; Hill, R.G.; Pembroke, J.T.; Brown, C.J.; Hatton, P.V. Influence of sodium oxide content on bioactive glass properties. J. Mater. Sci. Mater. Med. 1999, 10, 697–701. [Google Scholar] [CrossRef]
- Kansal, I.; Reddy, A.; Muñoz, F.; Choi, S.; Kim, H.; Tulyaganov, D.U.; Ferreira, J.M.F. Structure, biodegradation behavior and cytotoxicity of alkali-containing alkaline-earth phosphosilicate glasses. Mater. Sci. Eng. C 2014, 44, 159–165. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T.J. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. Immunol. Meth. 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Saboori, A.; Rabiee, M.; Moztarzadeh, F.; Sheikhi, M.; Tahriri, M.; Karimi, M. Synthesis, characterization and in vitro bioactivity of sol-gel-derived SiO2–CaO–P2O5–MgO bioglass. Mater. Sci. Eng. C 2009, 29, 335–340. [Google Scholar] [CrossRef]
- El-Kady, A.M.; Ali, A.F. Fabrication and characterization of ZnO modified bioactive glass nanoparticles. Ceram. Int. 2012, 38, 1195–1204. [Google Scholar] [CrossRef]
- Delben, J.R.J.; Pereira, K.; Oliveira, S.L.; Alencar, L.D.S.; Hernandes, A.C.; Delben, A.A.S.T. Bioactive glass prepared by sol–gel emulsion. J. Non-Cryst. Solids. 2013, 361, 119–123. [Google Scholar] [CrossRef]
- Román, J.; Padilla, S.; Vallet-Regí, M. Sol− gel glasses as precursors of bioactive glass ceramics. Chem. Mater. 2003, 15, 798–806. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Aina, V.; Malavasi, G.; Pla, A.F.; Munaron, L.; Morterra, C. Zinc-containing bioactive glasses: Surface reactivity and behaviour towards endothelial cells. Acta Biomater. 2009, 5, 1211–1222. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.Y.; Kawachi, G.; Kikuta, K.; Cho, S.B.; Kamitakahara, M.; Ohtsuki, C.J. Preparation of bioactive spherical particles in the CaO–SiO2 system through sol–gel processing under coexistence of poly (ethylene glycol). Eur. Ceram. Soc. 2008, 28, 1595–1602. [Google Scholar] [CrossRef]
- Innocenzi, P.J. Infrared spectroscopy of sol–gel derived silica-based films: A spectra-microstructure overview. Non-Cryst. Solids 2003, 316, 309–319. [Google Scholar] [CrossRef]
- Ding, J.; Chen, Y.; Chen, W.; Hu, L.; Boulon, G. Effect of P2O5 addition on the structural and spectroscopic properties of sodium aluminosilicate glass. Chin. Opt. Lett. 2012, 10, 071602. [Google Scholar] [CrossRef]
- Chen, X.F.; Lei, B.; Wang, Y.J.; Zhao, N.J. Morphological control and in vitro bioactivity of nanoscale bioactive glasses. Non-Cryst. Solids. 2009, 355, 791–796. [Google Scholar] [CrossRef]
- Hong, Z.; Liu, A.; Chen, L.; Chen, X.; Jing, X.J. Preparation of bioactive glass ceramic nanoparticles by combination of sol–gel and coprecipitation method. Non-Cryst. Solids 2009, 355, 368–372. [Google Scholar] [CrossRef]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef]
- Silver, I.A.; Deas, J.; Hska, M.E. Interactions of bioactive glasses with osteoblasts in vitro: Effects of 45S5 Bioglass®, and 58S and 77S bioactive glasses on metabolism, intracellular ion concentrations and cell viability. Biomaterials 2001, 22, 175–185. [Google Scholar] [CrossRef]






| Composition | Na+ | K+ | Ca2+ | Mg2+ | Cl− | HCO3− | HPO42− |
|---|---|---|---|---|---|---|---|
| SBF | 142.0 | 5.0 | 2.5 | 1.5 | 148.0 | 4.2 | 1.0 |
| Plasma | 142.0 | 5.0 | 2.5 | 1.5 | 103.0 | 27.0 | 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anh Tuan, T.; V. Guseva, E.; Anh Tien, N.; Tan Dat, H.; Vuong, B.X. Simple and Acid-Free Hydrothermal Synthesis of Bioactive Glass 58SiO2-33CaO-9P2O5 (wt%). Crystals 2021, 11, 283. https://doi.org/10.3390/cryst11030283
Anh Tuan T, V. Guseva E, Anh Tien N, Tan Dat H, Vuong BX. Simple and Acid-Free Hydrothermal Synthesis of Bioactive Glass 58SiO2-33CaO-9P2O5 (wt%). Crystals. 2021; 11(3):283. https://doi.org/10.3390/cryst11030283
Chicago/Turabian StyleAnh Tuan, Ta, Elena V. Guseva, Nguyen Anh Tien, Ho Tan Dat, and Bui Xuan Vuong. 2021. "Simple and Acid-Free Hydrothermal Synthesis of Bioactive Glass 58SiO2-33CaO-9P2O5 (wt%)" Crystals 11, no. 3: 283. https://doi.org/10.3390/cryst11030283
APA StyleAnh Tuan, T., V. Guseva, E., Anh Tien, N., Tan Dat, H., & Vuong, B. X. (2021). Simple and Acid-Free Hydrothermal Synthesis of Bioactive Glass 58SiO2-33CaO-9P2O5 (wt%). Crystals, 11(3), 283. https://doi.org/10.3390/cryst11030283






