Correlation between Structural and Transport Properties of Ca-Doped La Nickelates and Their Electrochemical Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Structural Characterization
2.3. Oxygen Over-Stoichiometry Studies
- (1)
- pretreatment in synthetic air (21 vol.% of oxygen in Ar), flow rate of 50 mL min−1, from 30 to 1000 °C and back, heating/cooling ramp of 10 °C min−1;
- (2)
- treatment in synthetic air, flow rate of 50 mL min−1, from 30 to 1000 °C and back, heating/cooling ramp of 10 °C min−1;
- (3)
- treatment in H2-containing atmosphere (10 vol.% of H2 in Ar), flow rate of 50 mL min−1, from 30 to 1000 °C and back, heating/cooling ramp of 10 °C min−1.
2.4. Oxygen Mobility and Surface Reactivity Studies
2.5. Electrochemical Characterization of the La2−xCaxNiO4+δ Electrodes
3. Results and Discussion
3.1. Structural Features of the La2−xCaxNiO4+δ Materials in Air at Room Temperature
3.2. Oxygen Over-Stoichiometry Studies
3.3. Structural Changes in La2−xCaxNiO4+δ under Heating in Air
3.4. Oxygen Mobility and Surface Reactivity
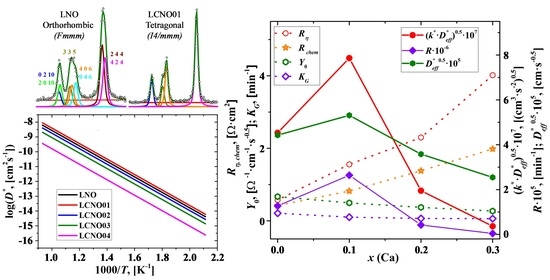
3.5. Electrochemical Study of the La2−xCaxNiO4+δ Electrodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nirala, G.; Yadav, D.; Upadhyay, S. Ruddlesden–Popper phase A2BO4 oxides: Recent studies on structure, electrical, dielectric, and optical properties. J. Adv. Ceram. 2020, 9, 129–148. [Google Scholar] [CrossRef]
- Istomin, S.Y.; Antipov, E.V. Cathode materials based on perovskite-like transition metal oxides for intermediate temperature solid oxide fuel cells. Russ. Chem. Rev. 2013, 82, 686–700. [Google Scholar] [CrossRef]
- Kilner, J.A.; Burriel, M. Materials for intermediate-temperature solid-oxide fuel cells. Ann. Rew. Mater. Res. 2014, 44, 365–393. [Google Scholar] [CrossRef]
- Guo, H.; Hu, Z.; Pi, T.-W.; Tjeng, L.H.; Komarek, A.C. Single crystal growth of pure Co3+ oxidation state material LaSrCoO4. Crystals 2016, 6, 98. [Google Scholar] [CrossRef]
- Ceretti, M.; Corallini, S.; Paulus, W. Influence of phase transformations on crystal growth of stoichiometric brownmillerite oxides: Sr2ScGaO5 and Ca2Fe2O5. Crystals 2016, 6, 146. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Q.; Sun, L. Ln2MO4 cathode materials for solid oxide fuel cells. Sci. China Chem. 2011, 54, 898–910. [Google Scholar] [CrossRef]
- Ogier, T.; Mauvy, F.; Bassat, J.M.; Laurencin, J.; Mougin, J.; Grenier, J.-C. Overstoichiometric oxides Ln2NiO4+δ (Ln = La, Pr or Nd) as oxygen anodic electrodes for solid oxide electrolysis application. Int. J. Hydrogen Energy 2015, 40, 15885–15892. [Google Scholar] [CrossRef]
- Sadykov, V.A.; Sadovskaya, E.M.; Eremeev, N.F.; Skriabin, P.I.; Krasnov, A.V.; Bespalko, Y.N.; Pavlova, S.N.; Fedorova, Y.E.; Pikalova, E.Y.; Shlyakhtina, A.V. Oxygen mobility in the materials for solid oxide fuel cells and catalytic membranes (review). Russ. J. Electrochem. 2019, 55, 701–718. [Google Scholar] [CrossRef]
- Wu, X.; Gu, C.; Cao, J.; Miao, L.; Fu, C.; Liu, W. Investigations on electrochemical performance of La2NiO4+δ cathode material doped at A site for solid oxide fuel cells. Mater. Res. Express 2020, 7, 065507. [Google Scholar] [CrossRef]
- Kaur, P.; Singh, K. Review of perovskite-structure related cathode materials for solid oxide fuel cells. Ceram. Int. 2019, 46, 5521–5535. [Google Scholar] [CrossRef]
- Badwal, S.P.S.; Foger, K. Solid oxide electrolyte fuel cell review. Ceram. Int. 1996, 22, 257–265. [Google Scholar] [CrossRef]
- Zhao, H.; Mauvy, F.; Lalanne, C.; Bassat, J.; Fourcade, S.; Grenier, J. New cathode materials for ITSOFC: Phase stability, oxygen exchange and cathode properties of La2−xNiO4+δ. Solid State Ion. 2008, 179, 2000–2005. [Google Scholar] [CrossRef]
- Tamura, H.; Hayashi, A.; Ueda, Y. Phase diagram of La2Ni04+δ (0 ≤ δ ≤ 0.18) I. Phases at room temperature and phase transition above δ = 0.15. Physica 1993, 216, 83–88. [Google Scholar] [CrossRef]
- Huang, D.P.; Xu, Q.; Chen, W.; Zhang, F.; Liu, H.X. Sintering, microstructure and conductivity of La2NiO4+δ ceramic. Ceram. Int. 2008, 34, 651–655. [Google Scholar] [CrossRef]
- Pikalova, E.Y.; Medvedev, D.A.; Khasanov, A.F. Structure, stability, and thermomechanical properties of Ca-substituted Pr2NiO4+δ. Phys. Solid State 2017, 59, 694–702. [Google Scholar] [CrossRef]
- Pikalova, E.; Kolchugin, A.; Filonova, E.; Bogdanovich, N.; Pikalov, S.; Ananyev, M.; Farlenkov, A. Validation of calcium-doped neodymium nickelates as SOFC air electrode materials. Solid State Ion. 2018, 319, 130–140. [Google Scholar] [CrossRef]
- Pikalova, E.; Kolchugin, A.; Bogdanovich, N.; Medvedev, D.; Lyagaeva, J.; Vedmid, L.; Ananyev, M.; Plaksin, S.; Farlenkov, A. Suitability of Pr2–xCaxNiO4+δ as cathode materials for electrochemical devices based on oxygen ion and proton conducting solid state electrolytes. Int. J. Hydrogen Energy 2020, 45, 13612–13624. [Google Scholar] [CrossRef]
- Nakamura, T.; Takeyama, Y.; Watanabe, S.; Yashiro, K.; Sato, K.; Hashida, T.; Mizusaki, J. Oxygen nonstoichiometry, crystal structure and mechanical properties of La2NiO4+δ. ECS Trans. 2009, 25, 2573–2580. [Google Scholar] [CrossRef]
- Kharton, V.V.; Kovalevsky, A.V.; Avdeev, M.; Tsipis, E.V.; Patrakeev, M.V.; Yaremchenko, A.A.; Naumovich, E.N.; Frade, J.R. Chemically induced expansion of La2NiO4+δ-based materials. Chem. Mater. 2007, 19, 2027–2033. [Google Scholar] [CrossRef]
- Boehm, E.; Bassat, J.; Dordor, P.; Mauvy, F.; Grenier, J.; Stevens, P. Oxygen diffusion and transport properties in non-stoichiometric Ln2-xNiO4+δ oxides. Solid State Ion. 2005, 176, 2717–2725. [Google Scholar] [CrossRef]
- Pikalova, E.Y.; Kolchugin, A.A.; Sadykov, V.A.; Sadovskaya, E.M.; Filonova, E.A.; Eremeev, N.F.; Bogdanovich, N.M. Structure, transport properties and electrochemical behavior of the layered lanthanide nickelates doped with calcium. Int. J. Hydrogen Energy 2018, 43, 17373–17386. [Google Scholar] [CrossRef]
- Li, X.; Huan, D.; Shi, N.; Yang, Y.; Wan, Y.; Xia, C.; Peng, R.; Lu, Y. Defects evolution of Ca doped La2NiO4+δ and its impact on cathode performance in proton-conducting solid oxide fuel cells. Int. J. Hydrogen Energy 2020, 45, 17736–17744. [Google Scholar] [CrossRef]
- Li, X.; Benedek, N.A. Enhancement of ionic transport in complex oxides through soft lattice modes and epitaxial strain. Chem. Mater. 2015, 27, 2647–2652. [Google Scholar] [CrossRef]
- Minervini, L.; Grimes, R.W.; Kilner, J.A.; Sickafus, K.E. Oxygen migration in La2NiO4+δ. J. Mater. Chem. 2000, 10, 2349–2354. [Google Scholar] [CrossRef]
- Medeiros, R.L.B.A.; Melo, V.R.M.; Melo, D.M.A.; Macedo, H.P.; Moure, G.T.; Adánez-Rubio, I.; Melo, M.A.F.; Adánez, J. Double perovskite (La2−xCa-Bax)NiO4 oxygen carriers for chemical looping reforming applications. Int. J. Hydrogen Energy 2020, 45, 1681–1696. [Google Scholar] [CrossRef]
- Amow, G.; Whitfield, P.S.; Davidson, I.J.; Hammond, R.P.; Munnings, C.N.; Skinner, S.J. Structural and sintering characteristics of the La2Ni1−xCoxO4+δ series. Ceram. Int. 2004, 30, 1635–1639. [Google Scholar] [CrossRef]
- Aguadero, A.; Escudero, M.J.; Pérez, M.; Alonso, J.A.; Pomjakushin, V.; Daza, L. Effect of Sr content on the crystal structure and electrical properties of the system La2−xSrxNiO4+δ (0 ≤ x ≤ 1). Dalton Trans. 2006, 36, 4377–4383. [Google Scholar] [CrossRef]
- Kolchugin, A.A.; Pikalova, E.Y.; Bogdanovich, N.M.; Bronin, D.I.; Pikalov, S.M.; Plaksin, S.V.; Ananyev, M.N.; Eremin, V.A. Structural, electrical and electrochemical properties of calcium-doped lanthanum nickelate. Solid State Ion. 2016, 288, 48–53. [Google Scholar] [CrossRef]
- Shen, Y.; Zhao, H.; Liu, X.; Xua, N. Preparation and electrical properties of Ca-doped La2NiO4+δ cathode materials for IT-SOFC. Phys. Chem. Chem. Phys. 2010, 12, 15124–15131. [Google Scholar] [CrossRef] [PubMed]
- Pikalova, E.; Bogdanovich, N.; Kolchugin, A.; Ermakova, L.; Khrustov, A.; Farlenkov, A.; Bronin, D. Methods to increase electrochemical activity of lanthanum nickelate-ferrite electrodes for intermediate and low temperature SOFCs. Int. J. Hydrogen Energy 2021. [Google Scholar] [CrossRef]
- Gilev, A.R.; Kiselev, E.A.; Zakharov, D.M.; Cherepanov, V.A. Effect of calcium and copper/iron co-doping on defect-induced properties of La2NiO4-based materials. J. Alloys Compd. 2018, 753, 491–501. [Google Scholar] [CrossRef]
- Tropin, E.S.; Ananyev, M.V.; Farlenkov, A.S.; Khodimchuk, A.V.; Berenov, A.V.; Fetisov, A.V.; Eremin, V.A.; Kolchugin, A.A. Surface defect chemistry and oxygen exchange kinetics in La2–xCaxNiO4+δ. J. Solid State Chem. 2018, 262, 199–213. [Google Scholar] [CrossRef]
- FullProf Suite. Crystallographic Tools for Rietveld, Profile Matching and Integrated-Intensity Refinements of X-ray and/or Neutron Data. Available online: https://www.ill.eu/sites/fullprof/ (accessed on 26 February 2021).
- Piminov, P.A.; Baranov, G.N.; Bogomyagkov, A.V.; Berkaev, D.E.; Borin, V.M.; Dorokhov, V.L.; Karnaev, S.E.; Kiselev, V.A.; Levichev, E.B.; Meshkov, O.I.; et al. Synchrotron radiation research and application at VEPP-4. Phys. Procedia 2016, 84, 19–26. [Google Scholar] [CrossRef]
- Aulchenko, V.M.; Evdokov, O.V.; Kutovenko, V.D.; Pirogov, B.Y.; Sharafutdinov, M.R.; Titov, V.M.; Tolochko, B.P.; Vasiljev, A.V.; Zhogin, I.A.; Zhulanov, V.V. One-coordinate X-ray detector OD-3M. Nucl. Instrum. Methods Phys. Res. 2009, 603, 76–79. [Google Scholar] [CrossRef]
- Sadykov, V.A.; Sadovskaya, E.M.; Filonova, E.A.; Eremeev, N.F.; Belyaev, V.D.; Tsvinkinberg, V.A.; Pikalova, E.Y. Oxide ionic transport features in Gd-doped La nickelates. Solid State Ion. 2020, 357, 115462. [Google Scholar] [CrossRef]
- Pikalova, E.Y.; Kalinina, E.G. Approaches to improving efficiency of solid oxide fuel cells based on ceramic membranes with mixed conductivity. Russ. Chem. Rev. 2021, 90. [Google Scholar] [CrossRef]
- Ganguly, P.; Rao, C.N.R. Crystal chemistry and magnetic properties of layered metal oxides possessing the K2NiF4 or related structures. J. Solid State Chem. 1984, 53, 193–216. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Pikalov, S.M.; Vedmid, L.B.; Filonova, E.A.; Pikalova, E.Y.; Lyagaeva, J.G.; Danilov, N.A.; Murashkina, A.A. High-temperature behavior of calcium substituted layered neodymium nickelates. J. Alloys Compd. 2019, 801, 558–567. [Google Scholar] [CrossRef]
- Sadykov, V.A.; Pikalova, E.Y.; Kolchugin, A.A.; Filonova, E.A.; Sadovskaya, E.M.; Eremeev, N.F.; Ishchenko, A.V.; Fetisov, A.V.; Pikalov, S.M. Oxygen transport properties of Ca-doped Pr2NiO4. Solid State Ion. 2018, 319, 234–243. [Google Scholar] [CrossRef]
- Shi, C.Y.; Hu, Z.B.; Hao, Y.M. Structural, magnetic and dielectric properties of La2−xCaxNiO4+δ (x = 0, 0.1, 0.2, 0.3). J. Alloys Compd. 2011, 509, 1333–1337. [Google Scholar] [CrossRef]
- Tang, J.P.; Dass, R.I.; Manthiram, A. Comparison of the crystal chemistry and electrical properties of La2−xAxNiO4 (A = Ca, Sr, and Ba). Mater. Res. Bull. 2000, 35, 411–424. [Google Scholar] [CrossRef]
- Kim, H.S.; Yoo, H.I. Effect of acceptor size and hole degeneracy on oxygen nonstoichiometry of La2NiO4+δ. Solid State Ion. 2013, 232, 129–137. [Google Scholar] [CrossRef]
- Poirot, N.; Zaghrioui, M. Synthesis and characterization of calcium-doped lanthanium nickelates La2−xCaxNiO4+δ. Solid State Sci. 2006, 8, 149–154. [Google Scholar] [CrossRef]
- Bassat, J.M.; Odier, P.; Loup, J.P. The semiconductor-to-metal transition in question in La2−xNiO4+δ (δ > 0 or δ <0). J. Solid State Chem. 1994, 110, 124–135. [Google Scholar]
- Liao, Q.; Zhuang, L.; Wei, Y.; Xue, J.; Wang, H. High oxygen permeation through A-site deficient K2NiF4+δ-type oxide hollow-fiber membrane. Ceram. Int. 2018, 44, 10852–10857. [Google Scholar] [CrossRef]
- Gilev, A.R.; Kiselev, E.A.; Chezganov, D.S.; Cherepanov, V.A. Correlation between oxygen surface exchange rate and surface structure in the La1.5Sr0.5Ni1-yCoyO4+δ ceramic membranes. Ceram. Int. 2020, 46, 17553–17560. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, J.; Yang, J.; Zong, Z.; Fu, L.; Lian, Z.; Zhang, X.; Wang, X.; Chen, C.; Ma, W.; et al. Nanoscale architecture of (La0.6Sr1.4)0.95Mn0.9B0.1O4 (B = Co, Ni, Cu) Ruddlesden–Popper oxides as efficient and durable catalysts for symmetrical solid oxide fuel cells. Renew. Energy 2020, 157, 840–850. [Google Scholar] [CrossRef]
- Sadykov, V.A.; Eremeev, N.F.; Usol’tsev, V.V.; Bobin, A.S.; Alikina, G.M.; Pelipenko, V.V.; Sadovskaya, E.M.; Muzykantov, V.S.; Bulgakov, N.N.; Uvarov, N.F. Mechanism of oxygen transfer in layered lanthanide nickelates Ln2−xNiO4+δ (Ln = La, Pr) and their nanocomposites with Ce0.9Gd0.1O2−δ and Y2(Ti0.8Zr0.2)1.6Mn0.4O7−δ solid electrolytes. Russ. J. Electrochem. 2013, 49, 645–651. [Google Scholar] [CrossRef]
- Akbari-Fakhrabadi, A.; Toledo, E.G.; Canales, J.I.; Meruane, V.; Chan, S.H.; Gracia-Pinilla, M.A. Effect of Sr2+ and Ba2+ doping on structural stability and mechanical properties of La2NiO4+δ. Ceram. Int. 2018, 44, 10551–10557. [Google Scholar] [CrossRef]
- Gilev, A.R.; Kiselev, E.A.; Cherepanov, V.A. Synthesis, oxygen nonstoichiometry and total conductivity of (La,Sr)2(Mn,Ni)O4±δ. Solid State Ion. 2015, 279, 53–59. [Google Scholar] [CrossRef]
- Kim, H.-S.; Yoo, H.-I. Isothermal Onsager matrices and acceptor size effect on mass/charge transport properties of La1.9A0.1NiO3.95+δ (A = Ca, Sr). Phys. Chem. Chem. Phys. 2014, 16, 16595–16605. [Google Scholar] [CrossRef]
- Tarutin, A.P.; Lyagaeva, J.G.; Medvedev, D.A.; Bi, L.; Yaremchenko, A.A. Recent advances in layered Ln2NiO4+δ nickelates: Fundamentals and prospects of their applications in protonic ceramic fuel and electrolysis cells. J. Mater. Chem. 2021, 9, 154–195. [Google Scholar] [CrossRef]
- Pikalova, E.Y.; Sadykov, V.A.; Filonova, E.A.; Eremeev, N.F.; Sadovskaya, E.M.; Pikalov, S.M.; Bogdanovich, N.M.; Lyagaeva, J.G.; Kolchugin, A.A.; Vedmid, L.B.; et al. Structure, oxygen transport properties and electrode performance of Ca-substituted Nd2NiO4. Solid State Ion. 2019, 335, 53–60. [Google Scholar] [CrossRef]
- Cherepanov, V.A.; Gilev, A.R.; Kiselev, E.A. Electrotransport in the La2NiO4-based solid solutions. Pure Appl. Chem. 2019, 91, 911–922. [Google Scholar] [CrossRef]
- Skinner, S.J.; Kilner, J.A. Oxygen diffusion and surface exchange in La2−xSrxNiO4+δ. Solid State Ion. 2000, 135, 709–712. [Google Scholar] [CrossRef]
- Sadykov, V.A.; Arapova, M.V.; Smal, E.A.; Pavlova, S.N.; Bobrova, L.N.; Eremeev, N.F.; Mezentseva, N.V.; Simonov, M.N. Nanocomposite Catalysts for Transformation of Biofuels into Syngas and Hydrogen: Fundamentals of Design and Performance, Application in Structured Reactors and Catalytic Membranes. In Catalysis; Spivey, J., Han, Y., Shekhawat, D., Eds.; Royal Society of Chemistry: London, UK, 2019; Volume 31, pp. 216–241. [Google Scholar]
- Gilev, A.R.; Kiselev, E.A.; Malyshkin, D.A.; Sukhanov, K.S.; Cherepanov, V.A. Hydration effect on properties of the La2−xAxNi1-yFeyO4+δ (A = Ca, Sr) cathode materials for H+-SOFCs. J. Alloys Compd. 2021, 860, 158482. [Google Scholar] [CrossRef]
- Hildenbrand, N.; Nammensma, P.; Blank, D.H.A.; Bouwmeester, H.J.M.; Boukamp, B.A. Influence of configuration and microstructure on performance of La2NiO4+δ IT-SOFC cathodes. J. Power Source 2013, 238, 442–453. [Google Scholar] [CrossRef]
- Antonova, E.P.; Khodimchuk, A.V.; Usov, G.R.; Tropin, E.S.; Farlenkov, A.S.; Khrustov, A.V.; Ananyev, M.V. EIS analysis of electrode kinetics for La2NiO4 + δ cathode in contact with Ce0.8Sm0.2O1.9 electrolyte: From DRT analysis to physical model of the electrochemical process. J. Solid State Electrochem. 2019, 23, 1279–1287. [Google Scholar] [CrossRef]
- Boukamp, B.A.; Bouwmeester, H.J.M. Interpretation of the Gerischer impedance in solid state ionics. Solid State Ion. 2003, 157, 29–33. [Google Scholar] [CrossRef]
- Ascolani-Yael, J.; Montenegro-Hernández, A.; Garcés, D.; Liu, Q.; Wang, H.; Yakal-Kremski, K.; Barnett, S.; Mogni, J. The oxygen reduction reaction in solid oxide fuel cells: From kinetic parameters measurements to electrode design. J. Phys. Energy 2020, 2, 042004. [Google Scholar] [CrossRef]
- Adler, S.B.; Lane, J.A.; Steele, B.C.H. Electrode kinetics of porous mixed-conducting oxygen electrodes. J. Electrochem. Soc. 1996, 143, 3554–3564. [Google Scholar] [CrossRef]
- Pikalova, E.Y.; Bogdanovich, N.M.; Kolchugin, A.A.; Osinkin, D.A.; Bronin, D.I. Electrical and electrochemical properties of La2NiO4+δ-based cathodes in contact with Ce0.8Sm0.2O2-δ electrolyte. Procedia Eng. 2014, 98, 105–110. [Google Scholar] [CrossRef]
- Lu, Y.; Kreller, C.; Adler, S.B. Measurement and modeling of the impedance characteristics of porous La1-xSrxCoO3-δ electrodes. J. Electrochem. Soc. 2009, 156, B513–B525. [Google Scholar] [CrossRef]
- Adler, S.B. Factors governing oxygen reduction in solid oxide fuel cell cathodes. Chem. Rev. 2004, 104, 4791–4844. [Google Scholar] [CrossRef]
- Yu, H.-C.; Adler, S.B.; Barnett, S.A.; Thornton, K. Simulation of the diffusional impedance and application to the characterization of electrodes with complex microstructures. Electrochim. Acta 2020, 354, 136534. [Google Scholar] [CrossRef]
- Sadykov, V.A.; Sadovskaya, E.M.; Eremeev, N.F.; Pikalova, E.Y.; Bogdanovich, N.M.; Filonova, E.A.; Krieger, T.A.; Fedorova, Y.E.; Krasnov, A.V.; Skriabin, P.I.; et al. Novel materials for solid oxide fuel cells cathodes and oxygen separation membranes: Fundamentals of oxygen transport and performance. Carbon Resour. Convers. 2020, 3, 112–121. [Google Scholar] [CrossRef]
- Gilev, A.R.; Kiselev, E.A.; Cherepanov, V.A. Performance of the lanthanum gallate based solid oxide fuel cells with the La2−xCaxNi1−yFeyO4+δ cathodes and Sr2Ni0.75Mg0.25MoO6−δ anode. Solid State Ion. 2019, 339, 115001. [Google Scholar] [CrossRef]
- Ananyev, M.V.; Kurumchin, E.K.; Porotnikova, N.M. Effect of oxygen nonstoichiometry on kinetics of oxygen exchange and diffusion in lanthanum-strontium cobaltites. Russ. J. Electrochem. 2010, 46, 789–797. [Google Scholar] [CrossRef]
- Sadykov, V.A.; Eremeev, N.F.; Bolotov, V.A.; Tanashev, Y.Y.; Fedorova, Y.E.; Amanbayeva, D.G.; Bobin, A.S.; Sadovskaya, E.M.; Muzykantov, V.S.; Pelipenko, V.V.; et al. The effect of microwave sintering on stability and oxygen mobility of praseodymium nickelates-cobaltites and their nanocomposites. Solid State Ion. 2016, 288, 76–81. [Google Scholar] [CrossRef]
- Boreskov, G.K.; Kasatkina, L.A.; Amerikov, V.G. Homomolecular isotope exchange of CO2 on IV period metal oxides. Kinet. Catal. 1969, 10, 102–112. [Google Scholar]
- Adler, S.B.; Chen, X.Y.; Wilson, J.R. Mechanisms and rate laws for oxygen exchange on mixed-conducting oxide surfaces. J. Catal. 2007, 245, 91–109. [Google Scholar] [CrossRef]
- Amerikov, V.G.; Kasatkina, L.A.; Popova, G.Y. Study of CO2 isotope exchange kinetics on the surface of chromium oxide. Kinet. Catal. 1968, 9, 429–432. [Google Scholar]
- Kumar Gunda, N.S.; Choi, H.-W.; Berson, A.; Kenney, B.; Karan, K.; Pharoah, J.G.; Mitra, S.K. Focused ion beam-scanning electron microscopy on solid-oxide fuel-cell electrode: Image analysis and computing effective transport properties. J. Power Source 2011, 196, 3592–3603. [Google Scholar] [CrossRef]
- Ananyev, M.V.; Farlenkov, A.S.; Eremin, V.A.; Kurumchin, E.K. Degradation kinetics of LSM-YSZ cathode materials for SOFC. Int. J. Hydrogen Energy 2018, 43, 951–959. [Google Scholar] [CrossRef]










| x | x = 0.0 | x = 0.1 | x = 0.2 | x = 0.3 | x = 0.4 | |
|---|---|---|---|---|---|---|
| t, includingδ | 0.898 | 0.897 | 0.899 | 0.899 | 0.898 | |
| structure | O | T | T | T | T | |
| a, [Å] | 5.4628(4) | 3.8557(1) | 3.8429(1) | 3.8303(1) | 3.8173(1) | |
| b, [Å] | 5.4664(4) | 3.8557(1) | 3.8429(1) | 3.8303(1) | 3.8173(1) | |
| c, [Å] | 12.6827(4) | 12.6596(3) | 12.6277(3) | 12.6032(2) | 12.5831(3) | |
| V, [Å3] | 378.74(4) | 188.21(1) | 186.49(1) | 184.90(1) | 183.36(1) | |
| ρ, [g cm−3] | 7.02 | 6.89 | 6.78 | 6.66 | 6.54 | |
| z(La/Ca) | 0.3605(1) | 0.3608(1) | 0.3613(1) | 0.3618(1) | 0.3618(1) | |
| z(O2) | 0.173(1) | 0.173(1) | 0.175(1) | 0.176(1) | 0.176(1) | |
| Bov | 0.52(4) | 0.59(3) | 0.42(4) | 0.49(4) | 0.64(3) | |
| L, [Å] | La/Ca-La/Ca | 3.538(1) | 3.525(1) | 3.504(2) | 3.483(1) | 3.477(1) |
| Ni-La/Ca | 3.254(1) | 3.247(1) | 3.233(1) | 3.220(1) | 3.211(1) | |
| Ni-Ni | 3.8641(1) | 3.8557(1) | 3.8429(1) | 3.8303(1) | 3.8173(1) | |
| Ni-O1x4 | 1.9320(1) | 1.9279(1) | 1.9215(1) | 1.9151(1) | 1.9086(1) | |
| Ni-O2x2 | 2.20(1) | 2.19(1) | 2.21(1) | 2.21(1) | 2.21(1) | |
| La/Ca-O1x4 | 2.620(1) | 2.612(1) | 2.600(1) | 2.589(1) | 2.582(1) | |
| La/Ca-O2x4 | 2.765(1) | 2.759(1) | 2.756(1) | 2.749(1) | 2.741(1) | |
| La/Ca-O2x1 | 2.38(1) | 2.38(1) | 2.35(1) | 2.35(1) | 2.34(1) | |
| Rexp | 4.40 | 4.52 | 4.25 | 4.31 | 4.24 | |
| Rp | 7.94 | 7.15 | 9.12 | 7.90 | 7.74 | |
| Rwp | 10.0 | 9.47 | 11.8 | 10.3 | 9.88 | |
| RBr | 3.75 | 3.21 | 3.13 | 2.86 | 3.34 | |
| Rf | 2.21 | 2.15 | 2.14 | 2.31 | 2.30 | |
| χ2 | 5.21 | 4.38 | 7.70 | 5.87 | 5.33 | |
| δ | 0.17(1) | 0.11(1) | 0.09(1) | 0.05(1) | 0.00(1) | |
| Tc, °C | 300 | 340 | 280 | 270 | n/a | |
| −∂δ/∂T × 105, K−1 | 12.7 | 11.8 | 10.1 | 4.1 | 1 | |
| x | R|700 K, [min−1] | ER, [kJ mole−1] | (D*|700 K)/L2, [min−1] | D*|700 °C, [cm2 s−1] | ED, [kJ mole−1] | Method | Ref. |
|---|---|---|---|---|---|---|---|
| 0 | 2.4 × 103 | 130 | 0.8 (100%) * | 2.0 × 10−9 | 100 | TPIE C18O2 | This work |
| 0 | 3.38 × 10−8 | 82 | IEDP/SIMS | [20,57] | |||
| 0 | 5.5 × 10−10 | 100 | TPIE C18O2 | [21] | |||
| 0 | 1.0 × 10−8 | 130 | IIE 18O2 | [28,32] | |||
| 0.1 | 5 × 103 | 130 | 1.2 (100%) * | 2.8 × 10−9 | 100 | TPIE C18O2 | This work |
| 0.1 | 1.8 × 10−10 | 160 | IIE 18O2 | [32] | |||
| 0.2 | 8 × 102 | 130 | 0.6 (85%) * | 1.3 × 10−9 | 100 | TPIE C18O2 | This work |
| 0.3 | 8 × 101 | 130 | 0.4 (60%) * | 6.5 × 10−10 | 100 | TPIE C18O2 | This work |
| 0.3 | 1.5 × 10−9 | 100 | TPIE C18O2 | [21] | |||
| 0.4 | Not det ** | 0.06 (5%) * | 1.1 × 10−10 | 100 | TPIE C18O2 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pikalova, E.; Sadykov, V.; Sadovskaya, E.; Yeremeev, N.; Kolchugin, A.; Shmakov, A.; Vinokurov, Z.; Mishchenko, D.; Filonova, E.; Belyaev, V. Correlation between Structural and Transport Properties of Ca-Doped La Nickelates and Their Electrochemical Performance. Crystals 2021, 11, 297. https://doi.org/10.3390/cryst11030297
Pikalova E, Sadykov V, Sadovskaya E, Yeremeev N, Kolchugin A, Shmakov A, Vinokurov Z, Mishchenko D, Filonova E, Belyaev V. Correlation between Structural and Transport Properties of Ca-Doped La Nickelates and Their Electrochemical Performance. Crystals. 2021; 11(3):297. https://doi.org/10.3390/cryst11030297
Chicago/Turabian StylePikalova, Elena, Vladislav Sadykov, Ekaterina Sadovskaya, Nikita Yeremeev, Alexander Kolchugin, Alexander Shmakov, Zakhar Vinokurov, Denis Mishchenko, Elena Filonova, and Vladimir Belyaev. 2021. "Correlation between Structural and Transport Properties of Ca-Doped La Nickelates and Their Electrochemical Performance" Crystals 11, no. 3: 297. https://doi.org/10.3390/cryst11030297
APA StylePikalova, E., Sadykov, V., Sadovskaya, E., Yeremeev, N., Kolchugin, A., Shmakov, A., Vinokurov, Z., Mishchenko, D., Filonova, E., & Belyaev, V. (2021). Correlation between Structural and Transport Properties of Ca-Doped La Nickelates and Their Electrochemical Performance. Crystals, 11(3), 297. https://doi.org/10.3390/cryst11030297